Cross-Linked Regulation of Coral-Associated Dinoflagellates and Bacteria in Pocillopora sp. during High-Temperature Stress and Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coral Sample Collection and Recovery in the Laboratory
2.2. Heat Treatment of Samples
2.3. Determination of Maximum Photochemical Efficiency
2.4. Photosynthetic Pigment Analysis
2.5. Genomic DNA Extraction and Symbiodiniaceae Subclade Analysis
2.6. Determination of Symbiodiniaceae Density
2.7. Community Composition Analysis and qPCR-Based Quantification of Associated Bacteria
2.8. Statistical Analyses
2.9. Ethical Approval and Consent to Participate
3. Results
3.1. Surface Morphology of Corals under Heat Treatment
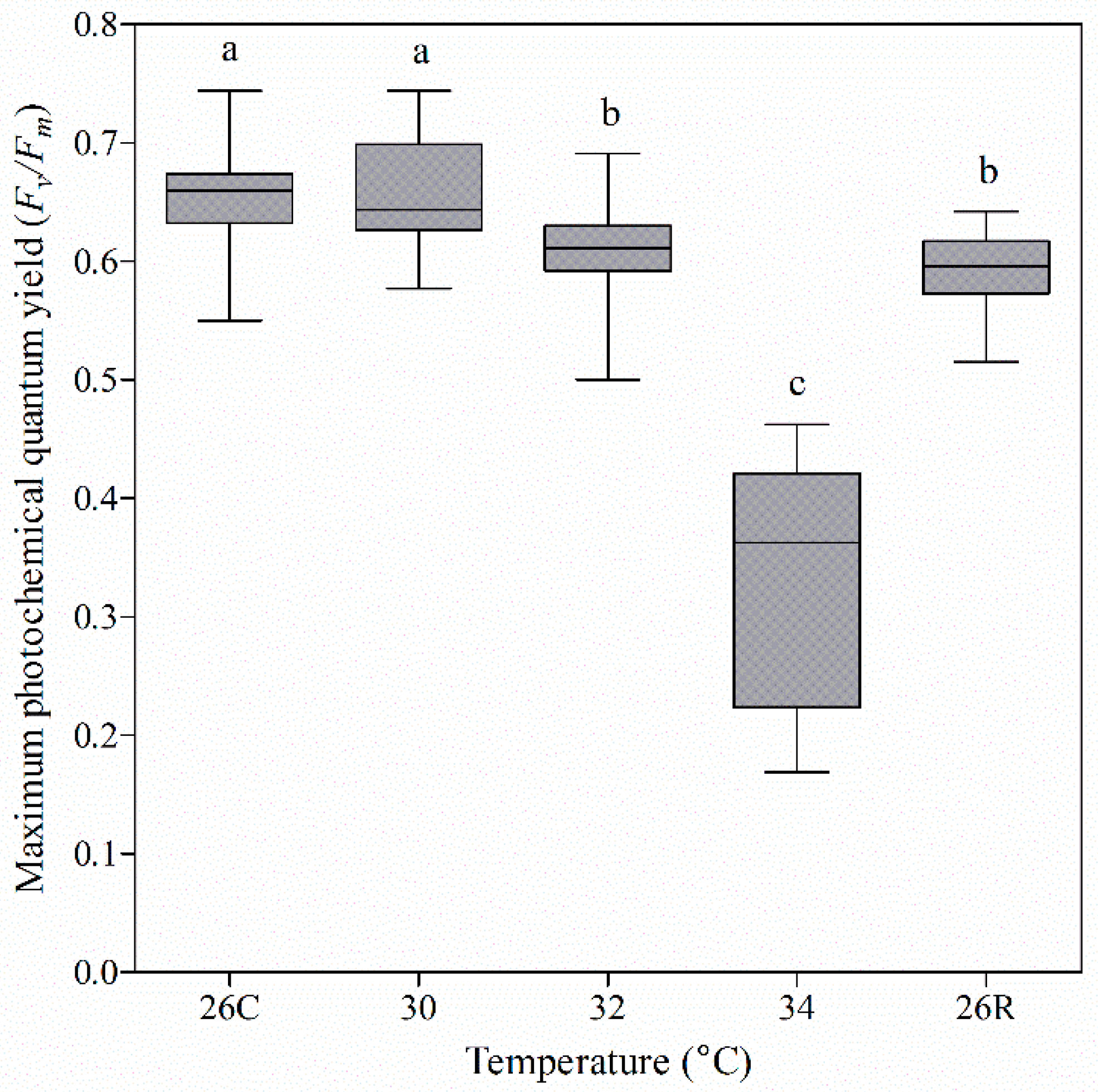
3.2. Photochemical Efficiency of Coral Nubbins under Heat Treatment
3.3. Photosynthetic Pigment Contents
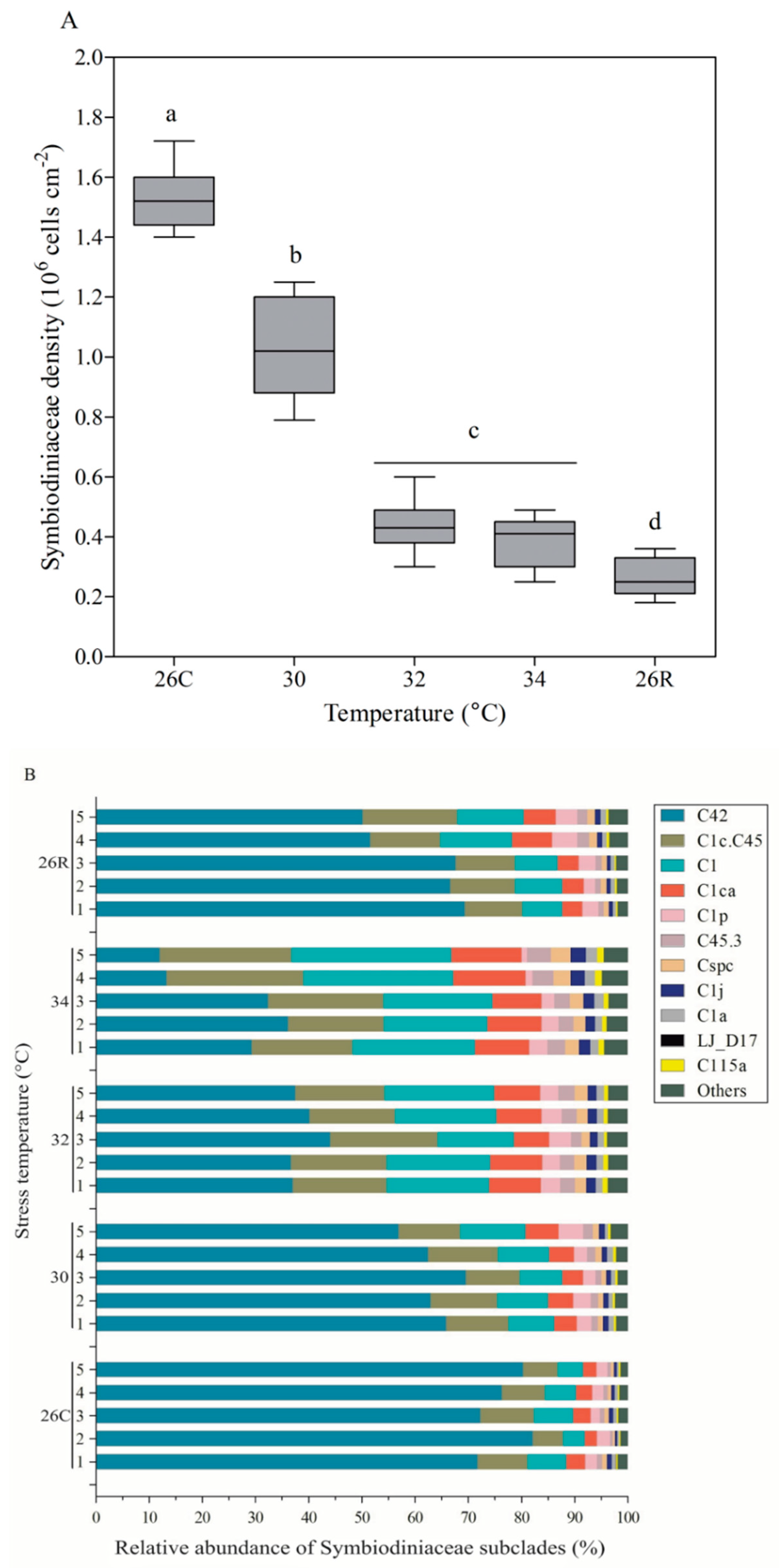
3.4. Symbiodiniaceae Density and Subclade Composition
3.5. Community Composition and Absolute Number of Photosynthetic Bacteria
4. Discussion
4.1. Changes in Photosynthetic Pigments and Fv/Fm Were Inconsistent with Changes in Symbiodiniaceae Density and Subclade Composition
4.2. Potential Regulatory Role of Coral-Associated Bacteria under Heat Stress
4.3. Model of the Coordinated Response of the Coral Holobiont during Heat Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef] [Green Version]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [Green Version]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Meissner, K.J.; Lippmann, T.; Sen Gupta, A. Large-scale stress factors affecting coral reefs: Open ocean sea surface temperature and surface seawater aragonite saturation over the next 400 years. Coral Reefs 2012, 31, 309–319. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkelmans, R.; De’ath, G.; Kininmonth, S.; Skirving, W.J. A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: Spatial correlation, patterns, and predictions. Coral Reefs 2004, 23, 74–83. [Google Scholar] [CrossRef]
- Edwards, A.J.; Clark, S.; Zahir, H.; Rajasuriya, A.; Naseer, A.; Rubens, J. Coral bleaching and mortality on artificial and natural reefs in Maldives in 1998, sea surface temperature anomalies and initial recovery. Mar. Pollut. Bull. 2001, 42, 7–15. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Alvarez-Noriega, M.; Alvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reefs 1997, 16, S129–S138. [Google Scholar] [CrossRef]
- Rowan, R.; Knowlton, N.; Baker, A.; Jara, J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 1997, 388, 265–269. [Google Scholar] [CrossRef]
- Rowan, R. Coral bleaching—Thermal adaptation in reef coral symbionts. Nature 2004, 430, 742. [Google Scholar] [CrossRef] [PubMed]
- Torda, G.; Donelson, J.M.; Aranda, M.; Barshis, D.J.; Bay, L.; Berumen, M.L.; Bourne, D.G.; Cantin, N.; Foret, S.; Matz, M.; et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Chang. 2017, 7, 627–636. [Google Scholar] [CrossRef]
- Baker, A.C.; Starger, C.J.; McClanahan, T.R.; Glynn, P.W. Corals’ adaptive response to climate change. Nature 2004, 430, 741. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Tseng, C.H.; Chen, C.A.; Tang, S.L. The dynamics of microbial partnerships in the coral Isopora palifera. ISME J. 2011, 5, 728–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceh, J.; Raina, J.B.; Soo, R.M.; van Keulen, M.; Bourne, D.G. Coral-Bacterial Communities before and after a Coral Mass Spawning Event on Ningaloo Reef. PLoS ONE 2012, 7, e36920. [Google Scholar] [CrossRef]
- Howells, E.J.; Beltran, V.H.; Larsen, N.W.; Bay, L.K.; Willis, B.L.; van Oppen, M.J.H. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Chang. 2012, 2, 116–120. [Google Scholar] [CrossRef]
- Nunez-Pons, L.; Bertocci, I.; Baghdasarian, G. Symbiont dynamics during thermal acclimation using cnidarian-dinoflagellate model holobionts. Mar. Environ. Res. 2017, 130, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Meron, D.; Rodolfo-Metalpa, R.; Cunning, R.; Baker, A.C.; Fine, M.; Banin, E. Changes in coral microbial communities in response to a natural pH gradient. ISME J. 2012, 6, 1775–1785. [Google Scholar] [CrossRef] [Green Version]
- Sampayo, E.M.; Ridgway, T.; Bongaerts, P.; Hoegh-Guldberg, O. Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc. Natl. Acad. Sci. USA 2008, 105, 10444–10449. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, R.N.; Cunning, R.; Baker, A.C. Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob. Chang. Biol. 2015, 21, 236–249. [Google Scholar] [CrossRef]
- Ziegler, M.; Seneca, F.O.; Yum, L.K.; Palumbi, S.R.; Voolstra, C.R. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 2017, 8, 14213. [Google Scholar] [CrossRef] [Green Version]
- Meron, D.; Maor-Landaw, K.; Eyal, G.; Elifantz, H.; Banin, E.; Loya, Y.; Levy, O. The Complexity of the Holobiont in the Red Sea Coral Euphyllia paradivisa under Heat Stress. Microorganisms 2020, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Muscatine, L.; Mccloskey, L.R.; Marian, R.E. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol. Oceanogr. 1981, 26, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, P.G.; Dubinsky, Z.; Muscatine, L.; Porter, J.W. Light and the bioenergetics of a symbiotic coral. BioScience 1984, 34, 705–709. [Google Scholar] [CrossRef]
- Kemp, D.W.; Hernandez-Pech, X.; Iglesias-Prieto, R.; Fitt, W.K.; Schmidt, G.W. Community dynamics and physiology of Symbiodinium spp. before, during, and after a coral bleaching event. Limnol. Oceanogr. 2014, 59, 788–797. [Google Scholar] [CrossRef]
- Xu, L.J.; Yu, K.F.; Li, S.; Liu, G.H.; Tao, S.C.; Shi, Q.; Chen, T.R.; Zhang, H.L. Interseasonal and interspecies diversities of Symbiodinium density and effective photochemical efficiency in five dominant reef coral species from Luhuitou fringing reef, northern South China Sea. Coral Reefs 2017, 36, 477–487. [Google Scholar] [CrossRef]
- Tong, H.Y.; Cai, L.; Zhou, G.W.; Yuan, T.; Zhang, W.P.; Tian, R.M.; Huang, H.; Qian, P.Y. Temperature shapes coral-algal symbiosis in the South China Sea. Sci. Rep. 2017, 7, 40118. [Google Scholar] [CrossRef] [Green Version]
- De Castro, A.P.; Araujo, S.D.; Reis, A.M.M.; Moura, R.L.; Francini, R.B.; Pappas, G.; Rodrigues, T.B.; Thompson, F.L.; Kruger, R.H. Bacterial Community Associated with Healthy and Diseased Reef Coral Mussismilia hispida from Eastern Brazil. Microb. Ecol. 2010, 59, 658–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, E.; Koren, O.; Reshef, L.; Efrony, R.; Zilber-Rosenberg, I. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 2007, 5, 355–362. [Google Scholar] [CrossRef]
- Sweet, M.J.; Croquer, A.; Bythell, J.C. Bacterial assemblages differ between compartments within the coral holobiont. Coral Reefs 2011, 30, 39–52. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Long, L.J.; Dong, J.D.; Yang, J.; Zhang, S. Bacterial dynamics within the mucus, tissue and skeleton of the coral Porites lutea during different seasons. Sci. Rep. 2014, 4, 7320. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.Y.; Yu, K.F.; Wang, Y.H.; Huang, X.Y.; Huang, W.; Qin, Z.J.; Pan, Z.L.; Yao, Q.C.; Wang, W.H.; Wu, Z.C. Distinct Bacterial Communities Associated with Massive and Branching Scleractinian Corals and Potential Linkages to Coral Susceptibility to Thermal or Cold Stress. Front. Microbiol. 2017, 8, 979. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.B. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Prog. Ser. 2006, 322, 1–14. [Google Scholar] [CrossRef]
- Bourne, D.; Iida, Y.; Uthicke, S.; Smith-Keune, C. Changes in coral-associated microbial communities during a bleaching event. ISME J. 2008, 2, 350–363. [Google Scholar] [CrossRef]
- Garren, M.; Azam, F. Corals shed bacteria as a potential mechanism of resilience to organic matter enrichment. ISME J. 2012, 6, 1159–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meron, D.; Atias, E.; Kruh, L.I.; Elifantz, H.; Minz, D.; Fine, M.; Banin, E. The impact of reduced pH on the microbial community of the coral Acropora eurystoma. ISME J. 2011, 5, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littman, R.; Willis, B.L.; Bourne, D.G. Metagenomic analysis of the coral holobiont during a natural bleaching event on the Great Barrier Reef. Environ. Microbiol. Rep. 2011, 3, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Tout, J.; Siboni, N.; Messer, L.F.; Garren, M.; Stocker, R.; Webster, N.S.; Ralph, P.J.; Seymour, J.R. Increased seawater temperature increases the abundance and alters the structure of natural Vibrio populations associated with the coral Pocillopora damicomis. Front. Microbiol. 2015, 6, 432. [Google Scholar] [CrossRef] [Green Version]
- Thurber, R.V.; Willner-Hall, D.; Rodriguez-Mueller, B.; Desnues, C.; Edwards, R.A.; Angly, F.; Dinsdale, E.; Kelly, L.; Rohwer, F. Metagenomic analysis of stressed coral holobionts. Environ. Microbiol. 2009, 11, 2148–2163. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Jensen, A. Chorophylls and Carotenoids; Cambridge University Press: New York, NY, USA, 1978. [Google Scholar]
- LaJeunesse, T.C.; Trench, R.K. Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt). Biol. Bull. 2000, 199, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, A.W.; Suarez, A.; Goff, L.J. Molecular delineation of species and syngens in Volvocacean green algae (Chlorophyta). J. Phycol. 1994, 30, 80–90. [Google Scholar] [CrossRef]
- Chen, B.; Yu, K.F.; Liang, J.Y.; Huang, W.; Wang, G.H.; Su, H.F.; Qin, Z.J.; Huang, X.Y.; Pan, Z.L.; Luo, W.W.; et al. Latitudinal Variation in the Molecular Diversity and Community Composition of Symbiodiniaceae in Coral from the South China Sea. Front. Microbiol. 2019, 10, 1278. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.J.; Yu, K.F.; Chen, B.; Wang, Y.H.; Liang, J.Y.; Luo, W.W.; Xu, L.J.; Huang, X.Y. Diversity of Symbiodiniaceae in 15 Coral Species from the Southern South China Sea: Potential Relationship with Coral Thermal Adaptability. Front. Microbiol. 2019, 10, 2343. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Tan, G.C.; Wang, H.Y.; Gai, X.P. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and Experimental Application of a Novel Non-Degenerate Universal Primer Set that Amplifies Prokaryotic 16S rRNA Genes with a Low Possibility to Amplify Eukaryotic rRNA Genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- Qin, Z.J.; Yu, K.F.; Liang, J.Y.; Yao, Q.C.; Chen, B. Significant Changes in Microbial Communities Associated With Reef Corals in the Southern South China Sea During the 2015/2016 Global-Scale Coral Bleaching Event. J. Geophys. Res. Oceans 2020, 125, e2019JC015579. [Google Scholar] [CrossRef]
- Schloss, P.D.; Gevers, D.; Westcott, S.L. Reducing the Effects of PCR Amplification and Sequencing Artifacts on 16S rRNA-Based Studies. PLoS ONE 2011, 6, e27310. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.P.; Yu, K.F.; Huang, W.; Liang, J.Y.; Qin, Z.J.; Chen, B.; Yao, Q.C.; Liao, Z.H. Thermal acclimation increases heat tolerance of the scleractinian coral Acropora pruinosa. Sci. Total Environ. 2020, 733, 139319. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Lee, W.C.; Kim, H.C.; Hong, S. Photosynthetic characteristics of Pyropia yezoensis (Ueda) Hwang & Choi measured using Diving-PAM in the Jindo-Haenam region on the southwestern coast of the Korean Peninsula. J. Appl. Phycol. 2020, 32, 2631–2640. [Google Scholar] [CrossRef]
- Poquita-Du, R.C.; Goh, Y.L.; Huang, D.W.; Chou, L.M.; Todd, P.A. Gene Expression and Photophysiological Changes in Pocillopora acuta Coral Holobiont Following Heat Stress and Recovery. Microorganisms 2020, 8, 1227. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, K.E.; Dias, D.A.; Lutz, A.; Roessner, U.; Davy, S.K. Mapping carbon fate during bleaching in a model cnidarian symbiosis: The application of C-13 metabolomics. New Phytol. 2017, 214, 1551–1562. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Lanetty, M.; Chang, S.J.; Song, J.I. Specificity of two temperate dinoflagellate-anthozoan associations from the north-western Pacific Ocean. Mar. Biol. 2003, 143, 1193–1199. [Google Scholar] [CrossRef]
- Seneca, F.O.; Palumbi, S.R. The role of transcriptome resilience in resistance of corals to bleaching. Mol. Ecol. 2015, 24, 1467–1484. [Google Scholar] [CrossRef]
- Sogin, E.M.; Putnam, H.M.; Anderson, P.E.; Gates, R.D. Metabolomic signatures of increases in temperature and ocean acidification from the reef-building coral, Pocillopora damicornis. Metabolomics 2016, 12, 71. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Deng, C.; Yu, K.; Ge, R.; Xu, Y.; Qin, Z.; Chen, B.; Wang, Y.; Su, H.; Huang, X.; et al. Cross-Linked Regulation of Coral-Associated Dinoflagellates and Bacteria in Pocillopora sp. during High-Temperature Stress and Recovery. Microorganisms 2021, 9, 1972. https://doi.org/10.3390/microorganisms9091972
Liang J, Deng C, Yu K, Ge R, Xu Y, Qin Z, Chen B, Wang Y, Su H, Huang X, et al. Cross-Linked Regulation of Coral-Associated Dinoflagellates and Bacteria in Pocillopora sp. during High-Temperature Stress and Recovery. Microorganisms. 2021; 9(9):1972. https://doi.org/10.3390/microorganisms9091972
Chicago/Turabian StyleLiang, Jiayuan, Chuanqi Deng, Kefu Yu, Ruiqi Ge, Yongqian Xu, Zhenjun Qin, Biao Chen, Yinghui Wang, Hongfei Su, Xueyong Huang, and et al. 2021. "Cross-Linked Regulation of Coral-Associated Dinoflagellates and Bacteria in Pocillopora sp. during High-Temperature Stress and Recovery" Microorganisms 9, no. 9: 1972. https://doi.org/10.3390/microorganisms9091972
APA StyleLiang, J., Deng, C., Yu, K., Ge, R., Xu, Y., Qin, Z., Chen, B., Wang, Y., Su, H., Huang, X., Huang, W., Wang, G., & Gong, S. (2021). Cross-Linked Regulation of Coral-Associated Dinoflagellates and Bacteria in Pocillopora sp. during High-Temperature Stress and Recovery. Microorganisms, 9(9), 1972. https://doi.org/10.3390/microorganisms9091972





