Phylogeny and Optimization of Trichoderma harzianum for Chitinase Production: Evaluation of Their Antifungal Behaviour against the Prominent Soil Borne Phyto-Pathogens of Temperate India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Trichoderma spp.
2.2. Chitinase Activity
2.3. Optimization of Chitinase Production
2.3.1. Colloidal Chitin Concentration
2.3.2. Effect of pH and Temperature
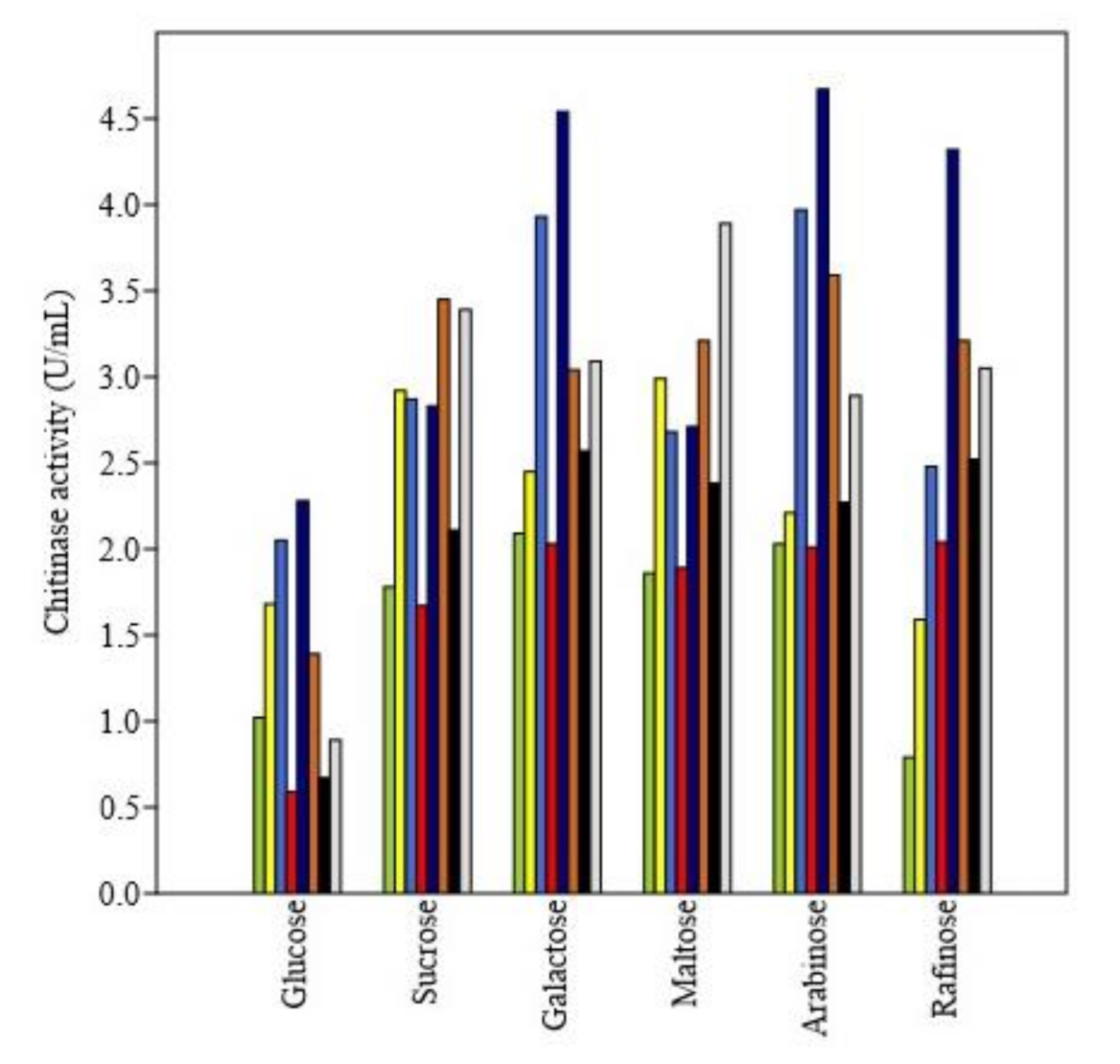
2.3.3. Effect of Nitrogen and Carbon Sources
2.3.4. Effect of Metal Ions
2.4. Molecular Characterization
2.4.1. Genomic DNA Extraction
2.4.2. PCR Amplification
2.4.3. Sequencing and Phylogenetic Analysis
2.5. Antagonistic Activity of Cell-Free Culture Filtrates against Soil Borne Pathogens
2.6. Statistical Analysis
3. Results
3.1. Screening and Morphological Identification of Trichoderma Isolates
3.2. Screening for Chitinase Activity
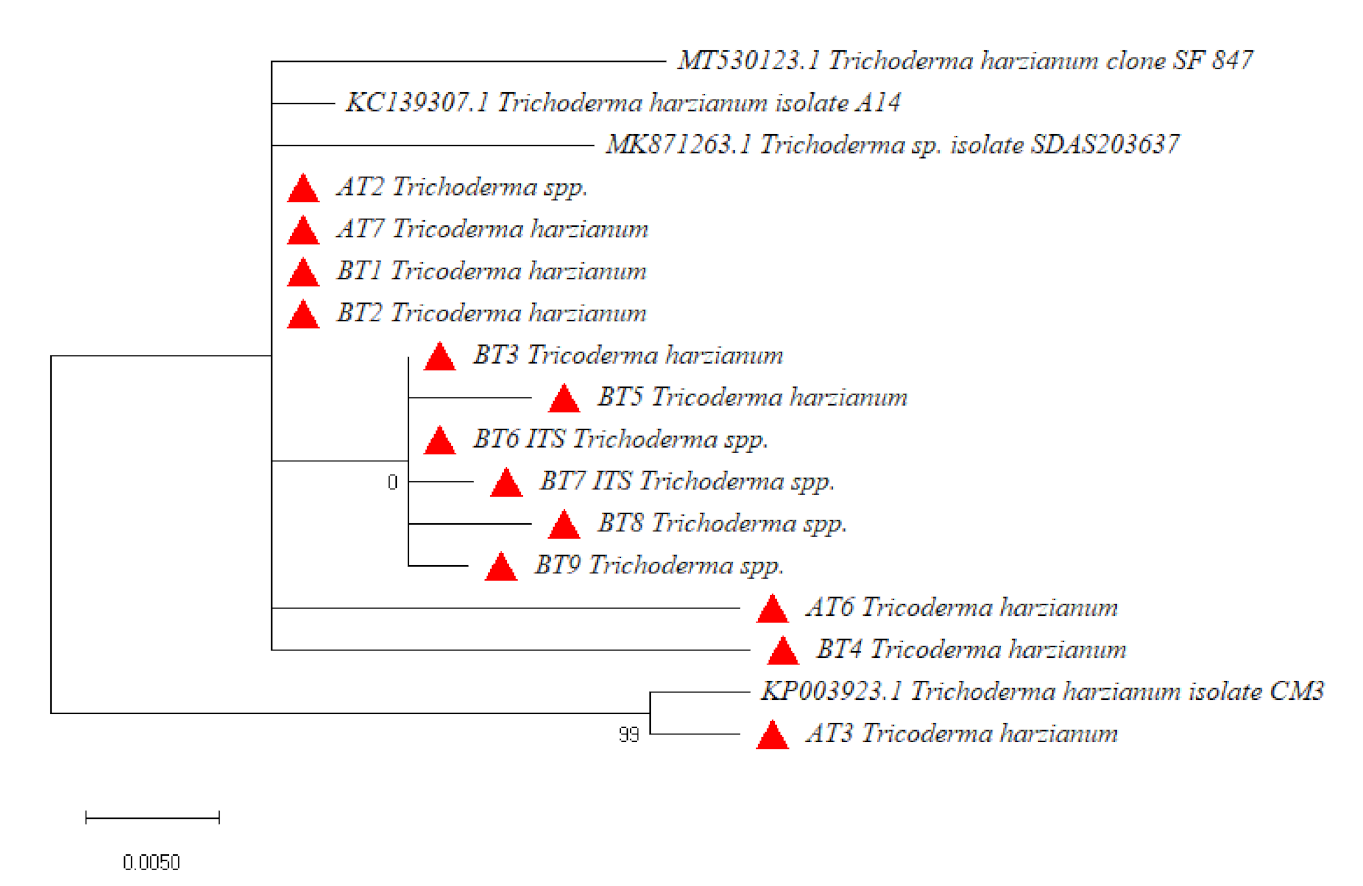
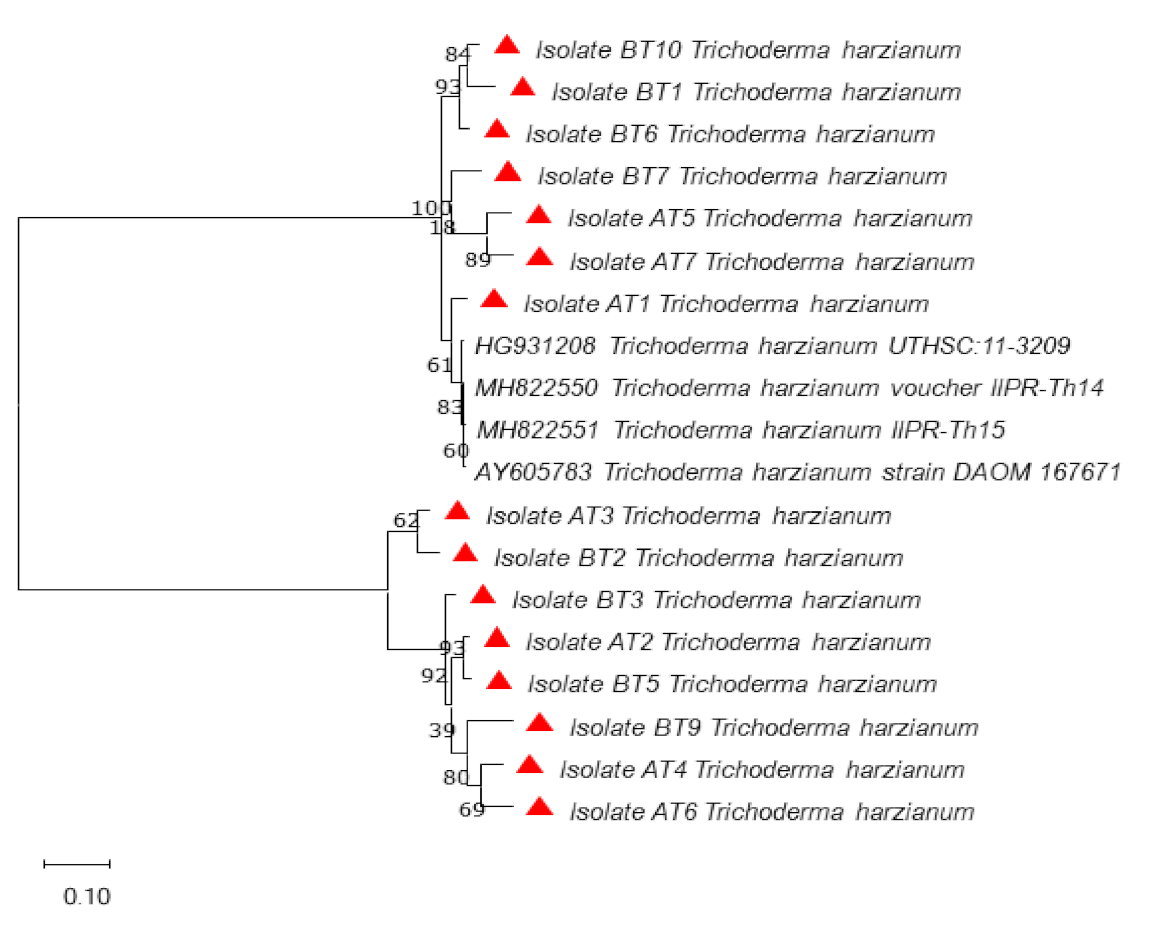
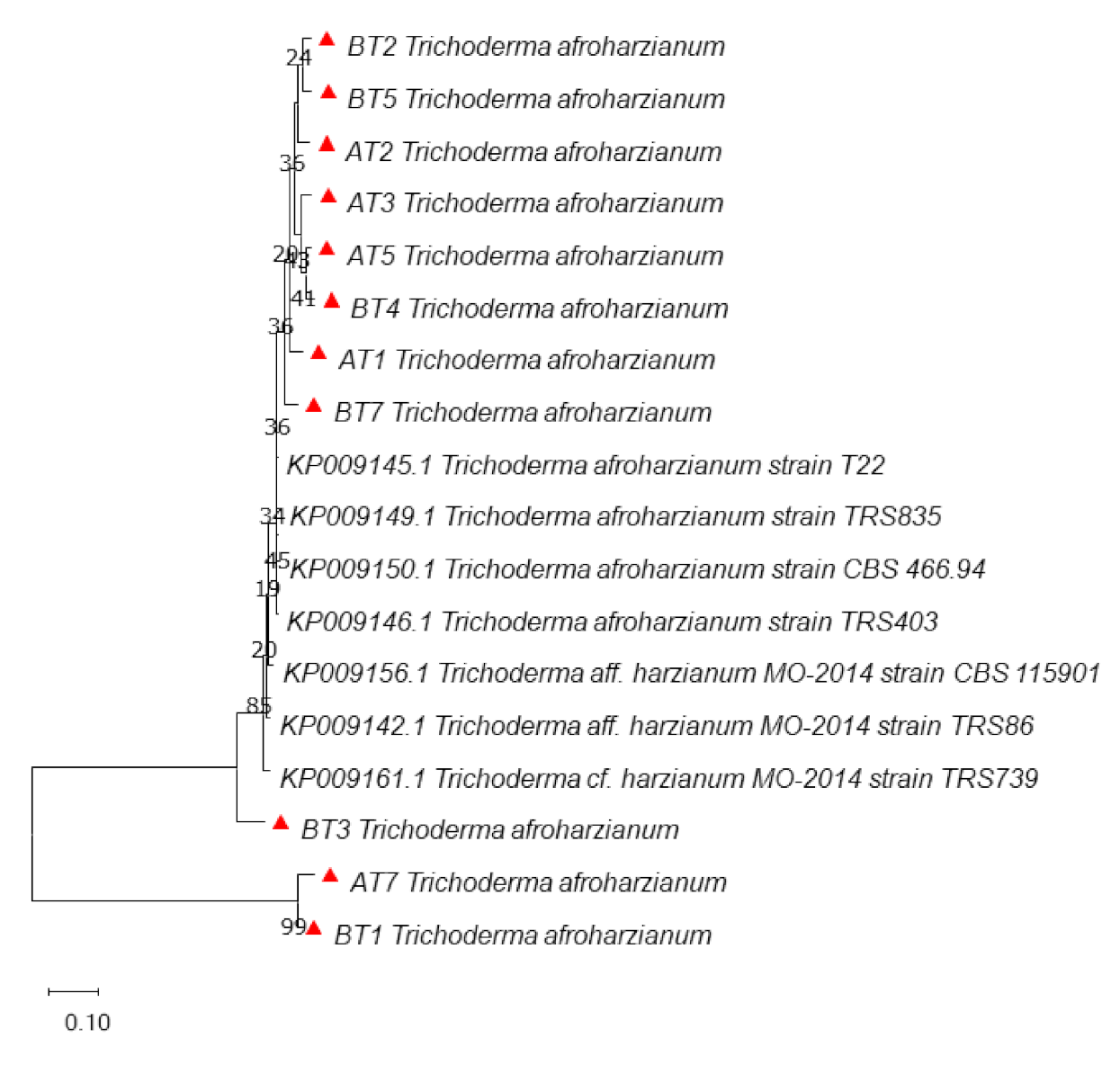
3.3. Molecular Identification and Phylogenetic Analysis
3.4. Optimization of Chitinase Producing Trichoderma Isolates
3.4.1. Colloidal Chitin Concentration
3.4.2. Effect of pH and Temperature
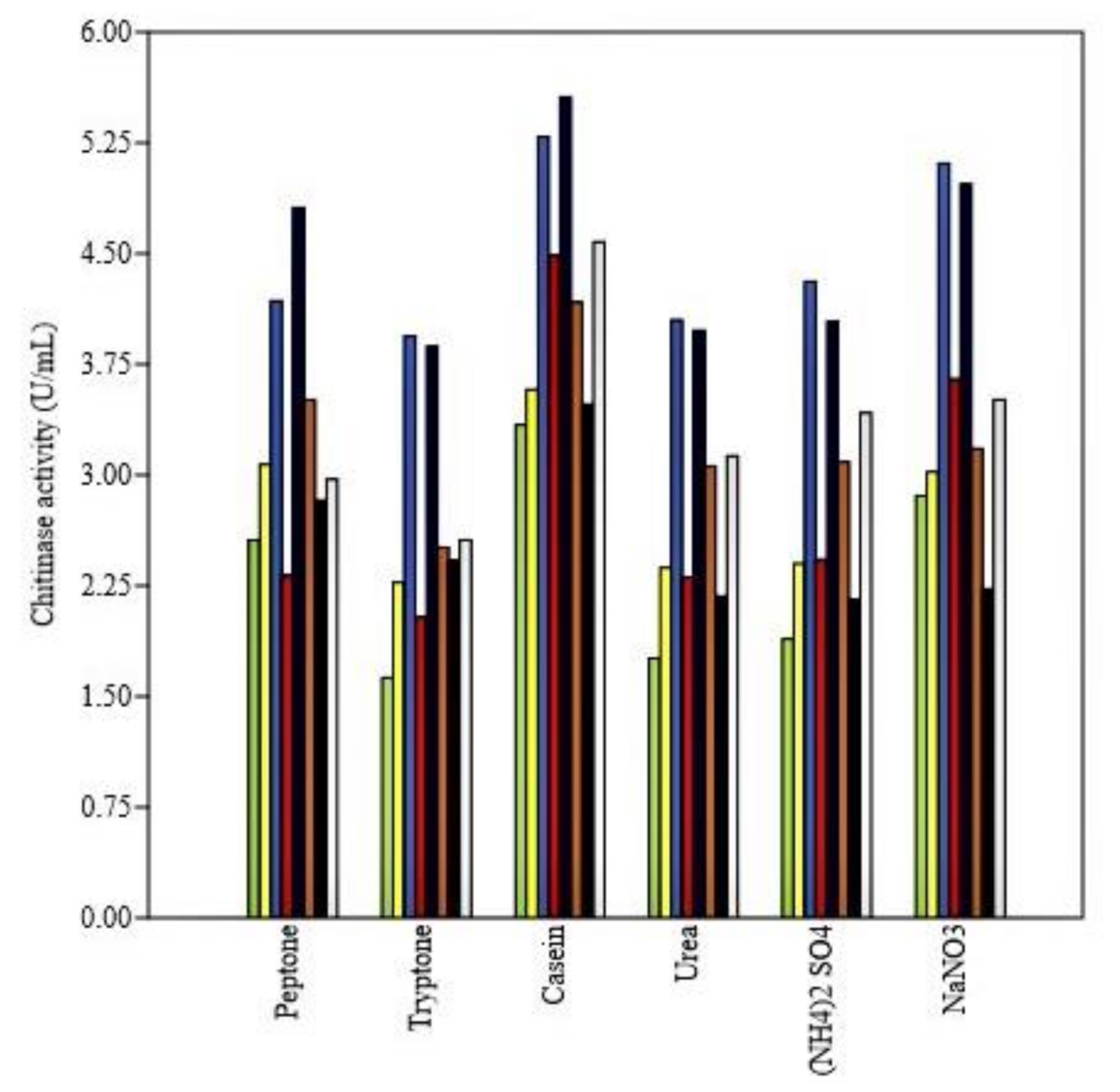
3.4.3. Effect of Nitrogen and Carbon Sources
3.4.4. Effect of Metal Ions on Chitinase Activity of Trichoderma Species
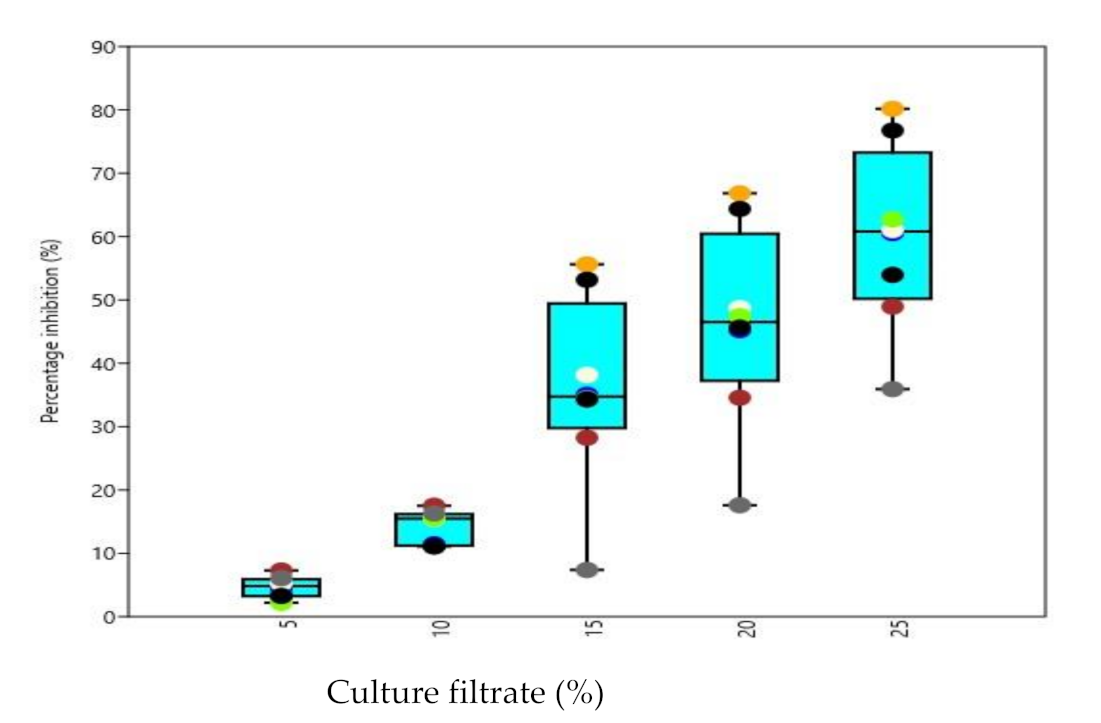
3.5. Biocontrol Activity of Culture Filtrates under In Vitro Conditions
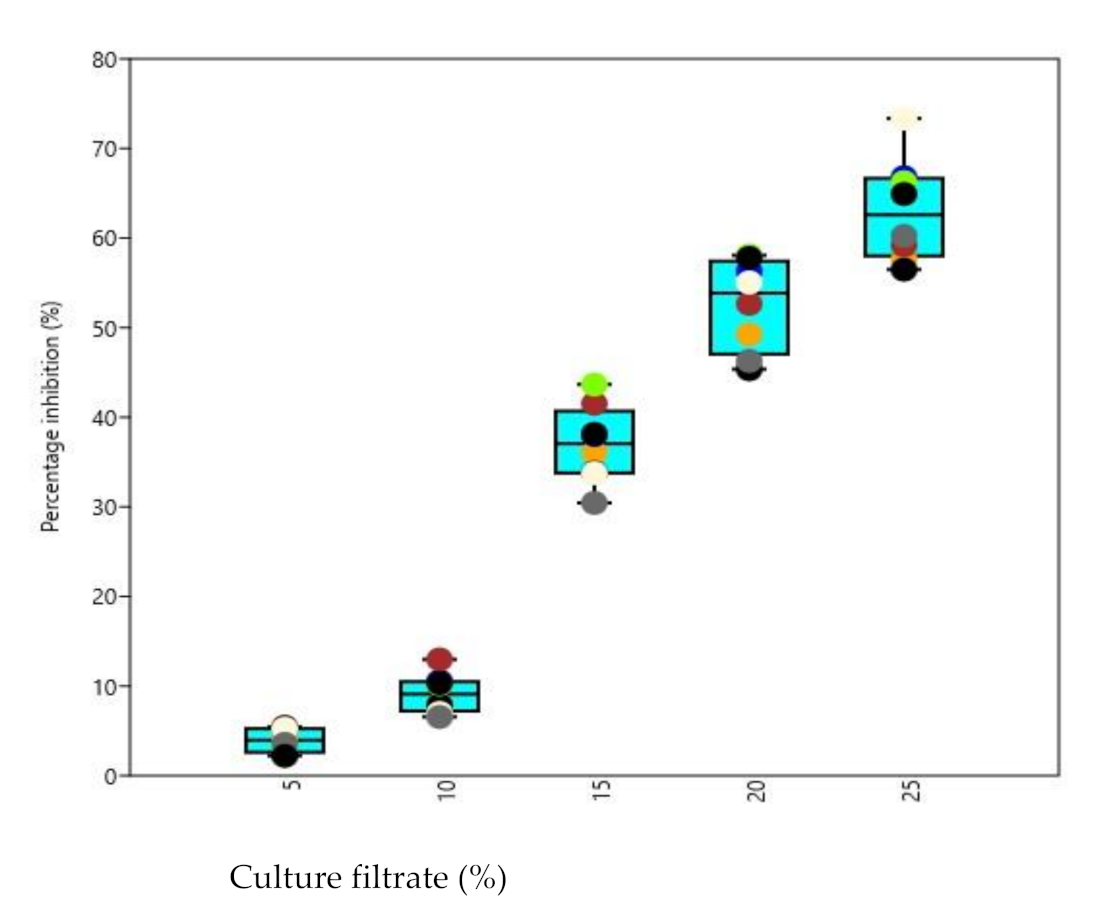
3.6. Biocontrol Activity of Culture Filtrates under In Vivo Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohiddin, F.A.; Khan, M.R.; Khan, S.M. Why Trichoderma is considered super hero (super fungus) against the evil parasites? Plant Pathol. J. 2010, 9, 92–102. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Ruangwong, O.-U.; Pornsuriya, C.; Pitija, K.; Sunpapao, A. Biocontrol Mechanisms of Trichoderma koningiopsis PSU3-2 against Postharvest Anthracnose of Chili Pepper. J. Fungi 2021, 7, 276. [Google Scholar] [CrossRef]
- Sanchez-Montesinos, B.; Diánez, F.; Moreno-Gavíra, A.; Gea, F.J.; Santos, M. Role of Trichoderma aggressivum f. europaeum as Plant-Growth Promoter in Horticulture. Agronomy 2020, 10, 1004. [Google Scholar]
- Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.; Matsui, K.; Arikit, S.; Sunpapao, A. Role of volatiles from the endophytic fungus Trichoderma asperelloides PSU-P1 in biocontrol potential and in promoting the plant growth of Arabidopsis thaliana. J. Fungi 2020, 6, 341. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-pentyl-α-pyrone and plant biopolymer formulations modulate plant metabolism and fruit quality of plum tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J. Glucosinolates profile of Arabidopsis thaliana modified root colonization of Trichoderma species. Biol. Control 2021, 155, 104522. [Google Scholar] [CrossRef]
- Thirunavukkarasu, N.; Dhinamala, K.; Moses, R. Production of Chitin from Two Marine Stomato pods Oratosquilla spp. (Crustacea). J. Chem. Pharm. Res. 2011, 3, 353–359. [Google Scholar]
- Flach, J.; Pilet, P.E.; Jolles, P. What’s New in Chitinase Research. Experientia 1992, 48, 701–716. [Google Scholar] [CrossRef]
- Loc, N.H.; Huy, N.D.; Quang, H.T.; Lan, T.T.; Ha, T.T.T. Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34. Mycology 2020, 11, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priya, C.; Jagannathan, N.; Kalaichelvan, P. Production of Chitinase by Streptomyces hygroscopicus vmch2 by Optimization of Cultural Conditions. Intern. J. Pharma. Bio. Sci. 2011, 11, 210–219. [Google Scholar]
- Moon, C.; Seo, D.J.; Song, Y.S.; Hong, S.H.; Choi, S.H.; Jung, W.J. Antifungal activity and patterns of N-acetyl-chitooligosaccharide degradation via chitinase produced from Serratia marcescens PRNK-1. Microb. Pathog. 2017, 113, 218–224. [Google Scholar] [CrossRef]
- Oyeleye, A.; Norm, Y.M. Chitinase: Diversity, limitations, and trends in engineering for suitable applications. Biosci. Rep. 2018, 38, BSR2018032300. [Google Scholar] [CrossRef]
- Hamed, I.; Ozogul, J.; Regenstein, M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Langner, T.; Gohre, V. Fungal chitinases: Function, regulation, and potential roles in plant/pathogen interactions. Curr. Genet. 2016, 62, 243–254. [Google Scholar] [CrossRef]
- Natarajan, K.; Murthy, V.R. Optimization of Chitinase Production from Serratiamarcescens. Biol. Segm. 2010, 1, 1510. [Google Scholar]
- Hayes, M.; Carney, B.; Slater, J.W.; Bruck, W.M. Mining marine shellfish waste for bioactive molecules: Chitin and chitosan, Part B: Applications. Biotechnol. J. 2008, 3, 878–889. [Google Scholar] [CrossRef]
- Narasimhan, A.; Shivakumar, S. Optimization of Chitinase Produced by a biocontrol strain of Bacillus subtilis using Plackett-Burman design. Eur. J. Exp. Biol. 2012, 2, 861–865. [Google Scholar]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159. [Google Scholar] [CrossRef]
- Poveda, J. Biological control of Fusarium oxysporum f. sp. ciceri and Ascochyta rabiei infecting protected geographical indication Fuentesaúco-Chickpea by Trichoderma species. Eur. J. Plant Pathol. 2021, 160, 825–840. [Google Scholar] [CrossRef]
- Agarwal, T.; Kotasthane, A.S. A Simple Medium for Screening Chitinase Activity of Trichoderma spp. Methods Mol. Identif. Lab Protoc. 2009. Available online: http://www.isth.info/methods/method.php.
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Jaklitsch, W.M.; Komon, M.; Kubicek, C.P.; Druzhinina, I.S. Hypocreavoglmayrii sp. nov.from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia 2005, 97, 1365–1378. [Google Scholar] [CrossRef]
- Nabi, S.U.; Baranwal, V.K.; Yadav, M.K. Association of Apple necrotic mosaic virus (ApNMV) with mosaic disease in commercially grown cultivars of apple (Malus domestica Borkh) in India. 3 Biotech 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Glen, S.; Michael, L.; Christina, K.; Koichiro, T. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Behzad, H.; Mousa, T.; Mohammad, R.M.; Mahdi, D. Biological Potential of Some Iranian Trichoderma Isolates in the Control of Soil Borne Plant Pathogenic Fungi. Afr. J. Biotecnol. 2008, 7, 967–972. [Google Scholar]
- Meng, X.; Miao, Y.; Liu, Q.; Ma, L.; Guo, K.; Liu, D.; Ran, W.; Shen, Q. TgSWO from Trichoderma guizhouense NJAU4742 promotes growth in cucumber plants by modifying the root morphology and the cell wall architecture. Microb. Cell Factories 2019, 18, 148. [Google Scholar] [CrossRef] [Green Version]
- Hammad, M.; Guillemette, T.; Alem, M. First report of three species of Trichoderma isolated from the rhizosphere in Algeria and the high antagonistic effect of Trichoderma brevicompactum to control grey mould disease of tomato. Egypt. J. Biol. Pest Control 2021, 31, 85. [Google Scholar] [CrossRef]
- Gunaratna, L.N.R.; Deshappriya, N.; Jayaratne, D.L.; Rajapaksha, R.G.S.A.S. Damping-off disease of big onion (Allium cepa L.) in Sri Lanka and evaluation of Trichoderma asperellum and Trichoderma virens for its control. Trop. Plant Res. 2019, 62, 275–292. [Google Scholar] [CrossRef]
- Khatri, D.K.; Tiwari, D.N.; Bariya, H.S. Chitinolytic efficacy and secretion of cell wall-degrading enzymes from Trichoderma spp. in response to phytopathological fungi. J. App. Biol. Biotech. 2017, 5, 1–8. [Google Scholar]
- Xie, X.H.; Fu, X.; Yan, X.Y.; Peng, W.F.; Kang, L.X. A Broad-Specificity Chitinase from Penicillium oxalicum k10 Exhibits Antifungal Activity and Biodegradation Properties of Chitin. Mar. Drugs 2021, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.; Chet, I.; Herrera-Estrella, A. Genetic diversity and vegetative compatibility among Trichoderma harzianum isolates. Mol. Gen. Genet. 1997, 256, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kullnig-Gradinger, C.; Szakacs, G.; Kubicek, C.P. Phylogeny and evolution of the genus Trichoderma: A multigene approach. Mycol. Res. 2002, 106, 757–767. [Google Scholar] [CrossRef]
- Chaverri, P.; Castlebury, L.A.; Samuels, G.J.; Geiser, D.M. Multilocus phylogenetic structure within the Trichoderma harzianum/Hypocrealixii complex. Mol. Phylogenet. Evol. 2003, 23, 302–313. [Google Scholar] [CrossRef]
- Naeimi, S.; Khodaparast, S.A.; Javan-Nikkhah, M.; Vagvolgyi, C.; Kredics, L. Species pattern and phylogenetic relationships of Trichoderma strains in rice fields of Southern Caspian sea, Iran. Cereal Res. Commun. 2011, 39, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Ulhoa, C.J.; Peberdy, J.F. Regulation of Chitinase Synthesis in Trichoderma harzianum. J. Gen. Microbiol. 1991, 137, 2163–2169. [Google Scholar] [CrossRef] [Green Version]
- Aida, F.M.; Al-Nusarie, S.; Taghreed, S. Production, optimization, characterization and antifungal activity of chitinase produced by Aspergillus terrus. Afr. J. Biotechnol. 2014, 13, 1567–1578. [Google Scholar]
- Sharaf, E.F. A Potent Chitinolytic Activity of Alternaria alternate Isolated from Egyptian Black Sand. Pol. J. Microbiol. 2005, 54, 145–151. [Google Scholar]
- Bghagat, S.; Pan, S. Variability in production of extracellular hydrolytic enzymes by Trichoderma spp. and induction of resistance in Gram (Cicer arietinum). J. Biol. Control. 2008, 22, 57–67. [Google Scholar]
- Cheba, B.A.; Zaghloul, T.A.; El-Massry, M. Effect of pH and Temperature on Bacillus sp. R2 Chitinase Activity and Stability. Procedia Technol. 2016, 22, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Ekundayo, E.A.; Ekundayo, F.O.; Bamidele, F. Production, partial purification and optimization of a chitinase produced from Trichoderma viride, an isolate of maize cob. Mycosphere 2016, 7, 786–793. [Google Scholar] [CrossRef]
- Nizamudeen, S.; Bajaj, B.K. A Novel Thermo-Alkali tolerant Endoglucanase Production Using Cost-Effective Agricultural Residues As Substrates By A Newly Isolated Bacillus sp. NZ. Food Technol. Biotecnol. 2009, 47, 435–440. [Google Scholar]
- Sandhya, C.; Adapa, L.L.; Nampoothiri, K.M.; Binod, P.; Szakacs, G.; Pandy, A. Extracellular Chitinase Production by Trichodermaharzianum in Submerged Fermentation. J. Basic Microbiol. 2004, 44, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, B.; Gomathi, D.; Kalaiselvi, M.; Ravikumar, G.; Arulraj, C.; Uma, C. Production and Purification of Chitinase by Streptomyces sp. from Soil. J. Adv. Sci. Res. 2012, 3, 25–29. [Google Scholar]
- Gueye, N.; Kumar, G.K.; Ndiaye, M.; Sall, S.Y.D.; Ndiaye, M.A.F.; Diop, T.A.; Ram, M.R. Factors affecting the chitinase activity of Trichoderma asperellum isolated from agriculture field soils. J. Appl. Biol. Biotechnol. 2020, 8, 41–44. [Google Scholar]
- Donzelle, Z.G.G.; Lorito, M.; Scala, F.; Harman, G.E. Cloning Sequence and Structure of a Gene Encoding an Artificial Glucan 1,3-glucosidase from Trichoderma atroviride. Gene 2001, 277, 199–208. [Google Scholar] [CrossRef]
- Zarei, M.; Aminzadeh, S.; Zolgharnein, H.; Safahieh, A.; Daliri, M.; Noghabi, K.A.; Ghoroghi, A.; Motallebi, A. Characterization of a chitinase with antifungal activity from a native Serratia marcescens B4A. Braz. J. Microbiol. 2011, 42, 1017–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajola, A.O.; Alasoadura, S.O. Antagonistic effects of Trichoderma harzianum on Pythium amphanidermatumcausing the Damping-off disease of Tobacco in Nigeria. Mycopathologia 1975, 57, 47–52. [Google Scholar] [CrossRef]


| S. No. | Isolate | Conidiophore Branching | Conidial Color | Species Identified | Chitinase Activity (Units/mL ± SD) |
|---|---|---|---|---|---|
| 1. | AT1 | Highly branched regular | Green | Trichoderma harzianum | ND * |
| 2. | AT2 | Highly branched regular | Green | T. harzianum | 1.13 ± 0.04 g |
| 3. | AT3 | Highly branched regular | Green | T. harzianum | 2.06 ± 0.10 d |
| 4. | AT4 | Highly branched regular | Green | T. harzianum | ND |
| 5. | AT5 | Highly branched regular | Green | T. harzianum | ND |
| 6. | AT6 | Highly branched regular | Green | T. harzianum | ND |
| 7. | AT7 | Highly branched regular | Green | T. harzianum | 3.26 ± 0.13 b |
| 8. | BT1 | Highly branched regular | Green | T. harzianum | 1.19 ± 0.08 g |
| 9. | BT2 | Highly branched regular | Green | T. harzianum | ND |
| 10. | BT3 | Highly branched regular | Green | T. harzianum | 3.38 ± 0.22 a |
| 11. | BT4 | Highly branched regular | Green | T. harzianum | 2.07 ± 0.13 c |
| 12. | BT5 | Highly branched regular | Green | T. harzianum | ND |
| 13. | BT6 | Highly branched regular | Green | T. harzianum | ND |
| 14. | BT7 | Highly branched regular | Green | T. harzianum | 1.71 ± 0.14 f |
| 15. | BT8 | Highly branched regular | Green | T. harzianum | 2.04 ± 0.08 e |
| 16. | BT9 | Highly branched regular | Green | T. harzianum | ND |
| 17. | BT10 | Highly branched regular | Green | T. harzianum | ND |
| Isolates | Chitin Concentration (%) | ||||
|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | |
| AT2 | 1.33 ± 0.12 h | 1.47 ± 0.04 h1 | 2.53 ± 0.11 f2 | 2.10 ± 0.06 h3 | 1.93 ± 0.03 h3 |
| AT3 | 2.11 ± 0.08 e | 2.48 ± 0.14 g1 | 2.94 ± 0.04 d2 | 3.03 ± 0.03 e3 | 2.38 ± 0.04 h4 |
| AT7 | 3.51 ± 0.13 a | 3.67 ± 0.10 b1 | 3.89 ± 0.09 a2 | 3.11 ± 0.09 d3 | 2.74 ± 0.04 d4 |
| BT1 | 1.57 ± 0.18 g | 2.05 ± 0.04 f1 | 2.33 ± 0.03 h2 | 2.89 ± 0.05 f3 | 2.31 ± 0.04 f4 |
| BT3 | 3.46 ± 0.14 b | 3.69 ± 0.14 a1 | 3.82 ± 0.03 b2 | 4.32 ± 0.06 a3 | 3.75 ± 0.02 b4 |
| BT4 | 2.42 ± 0.14 d | 2.65 ± 0.29 d1 | 2.91 ± 0.06 e2 | 3.48 ± 0.6 c3 | 2.79 ± 0.08 c4 |
| BT7 | 1.93 ± 0.05 f | 2.05 ± 0.04 e1 | 2.45 ± 0.09 g2 | 2.12 ± 0.03 g3 | 2.03 ± 0.05 g4 |
| BT8 | 2.84 ± 0.11 c | 3.42 ± 0.2 c1 | 3.69 ± 0.04 c2 | 4.21 ± 0.06 b3 | 3.91 ± 0.06 a4 |
| Isolates | (a) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3.0 | 3.5 | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 6.5 | 7.0 | |
| AT2 | 0.57 ± 0.12 f | 0.73 ± 0.06 g1 | 0.99 ± 0.03h2 | 1.23 ± 1.23h3 | 1.72 ± 0.05 g4 | 1.93 ± 0.04ah5 | 2.04 ± 0.05 h6 | 1.94 ± 0.06 h7 | 1.82 ± 0.04 g8 |
| AT3 | 1.07 ± 0.06 e | 1.37 ± 0.08 e1 | 1.84 ± 0.02 e2 | 1.97 ± 1.97 e3 | 2.37 ± 0.06 f4 | 2.79 ± 0.05 f5 | 3.07 ± 0.03f6 | 2.81 ± 0.05 f7 | 2.38 ± 0.03 f8 |
| AT7 | 2.15 ± 0.61b | 2.69 ± 0.04 b1 | 2.95 ± 0.03b2 | 3.28 ± 3.28 a3 | 3.67 ± 0.05a4 | 4.01 ± 0.03 a5 | 5.18 ± 0.05a6 | 4.11 ± 0.06 b7 | 4.11 ± 0.02 a8 |
| BT1 | 0.32 ± 0.05 h | 0.71 ± 0.02 h1 | 1.03 ± 0.04 g2 | 1.41 ± 1.41g3 | 1.73 ± 0.09 h4 | 1.98 ± 0.03 ag5 | 2.31 ± 0.04g6 | 2.43 ± 0.04 g7 | 2.14 ± 0.03 e8 |
| BT3 | 2.28 ± 0.51a | 2.81 ± 0.07 a1 | 3.05 ± 0.02a2 | 3.22 ± 3.22 b3 | 3.59 ± 0.06 b4 | 3.97 ± 0.04 b5 | 4.67 ± 0.05 b6 | 4.05 ± 0.05 c7 | 3.62 ± 0.05b8 |
| BT4 | 1.29 ± 0.09 d | 1.43 ± 0.09 d1 | 1.93 ± 0.04 d2 | 2.12 ± 2.12d3 | 2.39 ± 0.04 e4 | 2.83 ± 0.05 e5 | 3.35 ± 0.05 d6 | 2.93 ± 0.04 e7 | 2.67 ± 0.05 d8 |
| BT7 | 0.45 ± 0.09 g | 0.87 ± 0.16 f1 | 1.32 ± 0.04 f2 | 1.89 ± 1.89 f3 | 2.41 ± 0.04 d4 | 2.91 ± 0.05 d5 | 3.21 ± 0.05 e6 | 3.04 ± 0.03 d7 | 2.93 ± 0.05 d8 |
| BT8 | 2.11 ± 0.61 c | 2.46 ± 0.04c1 | 2.67 ± 0.07 c2 | 2.82 ± 2.82c3 | 3.05 ± 0.06c4 | 3.47 ± 0.04 c5 | 3.98 ± 0.03c6 | 4.12 ± 0.05 a7 | 3.37 ± 0.06 c8 |
| Isolates | 15 | 20 | 25 | 30 | 35 | 40 | 45 |
|---|---|---|---|---|---|---|---|
| AT2 | 0.37 ± 0.13h | 0.93 ± 0.10 g1 | 1.46 ± 0.13 g2 | 1.59 ± 0.28 g3 | 0.88 ± 0.12 h4 | 0.62 ± 0.05 h5 | 0.39 ± 0.06h6 |
| AT3 | 1.29 ± 0.29 b | 1.69 ± 0.29 d1 | 2.28 ± 0.24 d2 | 2.89 ± 0.06 c3 | 2.39 ± 0.12 b4 | 2.11 ± 0.10 b5 | 1.95 ± 0.04 b6 |
| AT7 | 2.59 ± 0.28 a | 2.94 ± 0.08 a1 | 3.37 ± 0.26 b2 | 3.59 ± 0.28 a3 | 3.01 ± 0.01 a4 | 2.43 ± 0.09 a5 | 2.04 ± 0.04 a6 |
| BT1 | 0.67 ± 0.11g | 0.92 ± 0.09 h1 | 1.27 ± 0.13 h2 | 2.22 ± 0.19 f3 | 1.66 ± 0.28 g4 | 1.23 ± 0.11 g5 | 0.94 ± 0.10 e6 |
| BT3 | 2.22 ± 0.18 c | 2.91 ± 0.25 b1 | 3.46 ± 0.17 a2 | 2.89 ± 0.06 c3 | 1.67 ± 0.29 f4 | 1.47 ± 0.13 e5 | 1.38 ± 0.09 c6 |
| BT4 | 1.06 ± 0.08f | 1.16 ± 0.14 f1 | 2.22 ± 0.18 e2 | 2.87 ± 0.06d3 | 2.16 ± 0.11 c4 | 1.69 ± 0.29 c5 | 1.51 ± 0.20 d6 |
| BT7 | 1.47 ± 0.21 d | 1.72 ± 0.20 c1 | 1.89 ± 0.13 f2 | 2.42 ± 0.04 e3 | 1.82 ± 0.10 e4 | 1.26 ± 0.12f5 | 0.59 ± 0.06 f6 |
| BT8 | 1.35 ± 0.21 e | 1.51 ± 0.50 e1 | 2.44 ± 0.04 c2 | 2.97 ± 0.01 b3 | 1.96 ± 0.10 d4 | 1.35 ± 0.32 d5 | 0.55 ± 0.05 g6 |
| Isolates | Relative Activity (%) ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | KCl | NaCl | AgNO3 | CaCl2 | FeSO4 | HgCl2 | MgCl2 | Zn SO4 | |
| AT2 | 100 | 97.32 ± 1.59 d | 98.31 ± 2.05 a1 | 78.07 ± 3.01 e2 | 128.67 ± 2.15 g3 | 84.48 ± 0.72 c4 | 71.21 ± 1.71 a5 | 135.99 ± 3.85 g6 | 93.53 ± 1.47 a7 |
| AT3 | 100 | 89.05 ± 0.06 h | 105.23 ± 1.21a1 | 76.69 ± 3.28f2 | 156.23 ± 1.33 c3 | 79.28 ± 1.03 e4 | 52.02 ± 0.92 b5 | 161.33 ± 2.71c6 | 63.36 ± 3.17 b8 |
| AT7 | 100 | 98.02 ± 1.61c | 98.89 ± 1.96 a1 | 81.72 ± 2.84c2 | 134.67 ± 1.00 e3 | 81.41 ± 1.39 d4 | 37.79 ± 0.91 f5 | 147.72 ± 2.10 d6 | 46.35 ± 2.06 f8 |
| BT1 | 100 | 98.89 ± 1.96a | 104.61 ± 1.21 a1 | 75.45 ± 3.46h2 | 156.32 ± 1.54 b3 | 76.92 ± 1.00 g4 | 50.29 ± 2.41 c5 | 162.33 ± 2.35 b6 | 59.63 ± 3.18 d8 |
| BT3 | 100 | 93.54 ± 1.31 f | 102.20 ± 1.20 a1 | 80.58 ± 2.07d2 | 134.35 ± 2.51 f3 | 84.72 ± 0.74 b4 | 21.47 ± 3.12 h5 | 137.12 ± 2.51 f6 | 31.45 ± 4.16 i8 |
| BT4 | 100 | 97.10 ± 0.65 e | 98.83 ± 1.97 a1 | 84.38 ± 1.53b2 | 117.22 ± 1.33 g3 | 89.33 ± 1.13 a4 | 49.39 ± 2.06 d5 | 125.31 ± 2.69 h6 | 61.73 ± 2.54 c8 |
| BT7 | 100 | 98.38 ± 2.05b | 101.25 ± 1.00 a1 | 85.08 ± 1.00a2 | 159.59 ± 2.04a3 | 77.18 ± 1.82 f4 | 25.61 ± 2.08 g5 | 173.44 ± 3.49a6 | 42.04 ± 2.73 g8 |
| BT8 | 100 | 92.34 ± 1.46 g | 107.05 ± 0.23 a1 | 75.56 ± 0.83g2 | 152.29 ± 1.71 d3 | 71.61 ± 1.48 h4 | 39.77 ± 0.87 e5 | 136.18 ± 3.46 e6 | 51.78 ± 3.32 e8 |
| Isolates | Wilt Incidence (%) | Disease Control (%) | |||
|---|---|---|---|---|---|
| Flowering | Fruit Formation | Ripening | Mean | ||
| AT1 | 4.20 ± 0.21 q | 24.35 ± 1.34 n1 | 44.26 ± 1.85 p2 | 24.27 p3 | 39.97 c4 |
| AT2 | 5.80 ± 0.52 g | 26.32 ± 1.15 h1 | 50.32 ± 1.26 h2 | 27.48 i3 | 32.03 k4 |
| AT3 | 6.80 ± 0.36 c | 27.31 ± 1.35 g1 | 52.16 ± 0.89 d2 | 28.73 c4 | 28.86 q4 |
| AT4 | 5.20 ± 0.14 m | 25.68 ± 0.88 k1 | 47.56 ± 1.02 m2 | 26.15 k3 | 35.32 h4 |
| AT5 | 5.30 ± 0.21 l | 22.13 ± 1.63 p1 | 40.45 ± 1.08 q2 | 22.62 q3 | 44.05 b4 |
| AT6 | 4.60 ± 0.28 o | 25.00 ± 0.87 l1 | 47.80 ± 0.71 l2 | 25.80 m3 | 36.18 f4 |
| AT7 | 4.80 ± 0.46 n | 25.54 ± 1.22 l1 | 49.14 ± 0.94 k2 | 26.49 j3 | 34.47 j4 |
| BT1 | 5.30 ± 0.29 k | 26.10 ± 1.3 i1 | 51.21 ± 0.92 g2 | 27.54 h3 | 31.88 l4 |
| BT2 | 4.10 ± 0.14 r | 24.47 ± 1.75 m1 | 46.62 ± 1.26 n2 | 25.06 n3 | 38.01 d4 |
| BT3 | 6.10 ± 0.38 e | 26.51 ± 1.25 e1 | 52.10 ± 0.76 e2 | 28.24 f3 | 30.15 n4 |
| BT4 | 3.50 ± 0.57 j | 19.65 ± 1.22 r1 | 39.84 ± 0.62 r2 | 21.00 r3 | 48.05 a4 |
| BT5 | 5.70 ± 0.36 h | 25.84 ± 1.50 j1 | 53.45 ± 1.31 c2 | 28.33 e3 | 29.92 o4 |
| BT6 | 4.25 ± 0.37 p | 23.41 ± 1.40 o1 | 50.12 ± 1.16 i2 | 25.92 l3 | 35.88 i4 |
| BT7 | 6.30 ± 0.37 d | 29.31 ± 0.79 c1 | 58.57 ± 0.6 b2 | 31.39 b3 | 22.35 r4 |
| BT8 | 6.00 ± 0.38 f | 27.46 ± 1.30 d1 | 51.60 ± 1.21 f2 | 28.35 d3 | 29.87 p4 |
| BT9 | 5.51 ± 0.63 i | 21.23 ± 1.07 q1 | 49.60 ± 1.24 j2 | 25.44 o3 | 37.07 e4 |
| BT10 | 7.41 ± 0.33 b | 31.35 ± 1.67 b1 | 44.52 ± 0.77 o2 | 27.76 g3 | 31.33 m4 |
| Control | 9.84 ± 0.89a | 41.25 ± 1.14 a1 | 70.20 ± 1.31 a2 | 40.43 a3 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohiddin, F.A.; Padder, S.A.; Bhat, A.H.; Ahanger, M.A.; Shikari, A.B.; Wani, S.H.; Bhat, F.A.; Nabi, S.U.; Hamid, A.; Bhat, N.A.; et al. Phylogeny and Optimization of Trichoderma harzianum for Chitinase Production: Evaluation of Their Antifungal Behaviour against the Prominent Soil Borne Phyto-Pathogens of Temperate India. Microorganisms 2021, 9, 1962. https://doi.org/10.3390/microorganisms9091962
Mohiddin FA, Padder SA, Bhat AH, Ahanger MA, Shikari AB, Wani SH, Bhat FA, Nabi SU, Hamid A, Bhat NA, et al. Phylogeny and Optimization of Trichoderma harzianum for Chitinase Production: Evaluation of Their Antifungal Behaviour against the Prominent Soil Borne Phyto-Pathogens of Temperate India. Microorganisms. 2021; 9(9):1962. https://doi.org/10.3390/microorganisms9091962
Chicago/Turabian StyleMohiddin, Fayaz A., Shahid A. Padder, Arif H. Bhat, Mohammad A. Ahanger, Asif B. Shikari, Shabir H. Wani, Farooq A. Bhat, Sajad Un Nabi, Aflaq Hamid, Nazir A. Bhat, and et al. 2021. "Phylogeny and Optimization of Trichoderma harzianum for Chitinase Production: Evaluation of Their Antifungal Behaviour against the Prominent Soil Borne Phyto-Pathogens of Temperate India" Microorganisms 9, no. 9: 1962. https://doi.org/10.3390/microorganisms9091962
APA StyleMohiddin, F. A., Padder, S. A., Bhat, A. H., Ahanger, M. A., Shikari, A. B., Wani, S. H., Bhat, F. A., Nabi, S. U., Hamid, A., Bhat, N. A., Sofi, N. R., Waza, S. A., Hamid, B., Parveen, S., Hussain, A., Bhat, A. N., Ali, O. M., Dar, M. S., & Abdel Latef, A. A. H. (2021). Phylogeny and Optimization of Trichoderma harzianum for Chitinase Production: Evaluation of Their Antifungal Behaviour against the Prominent Soil Borne Phyto-Pathogens of Temperate India. Microorganisms, 9(9), 1962. https://doi.org/10.3390/microorganisms9091962










