Electrochemical Determination of Antioxidant Capacity of Traditional Homemade Fruit Vinegars Produced with Double Spontaneous Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Harvesting and Selection of Plant Feedstocks
2.3. Sampling Procedures
2.4. Production of Homemade Fruit Vinegars (HMV)
2.5. Physicochemical Characterization
2.6. Analytical Methods for Determination of the Antioxidant Capacity of Vinegars
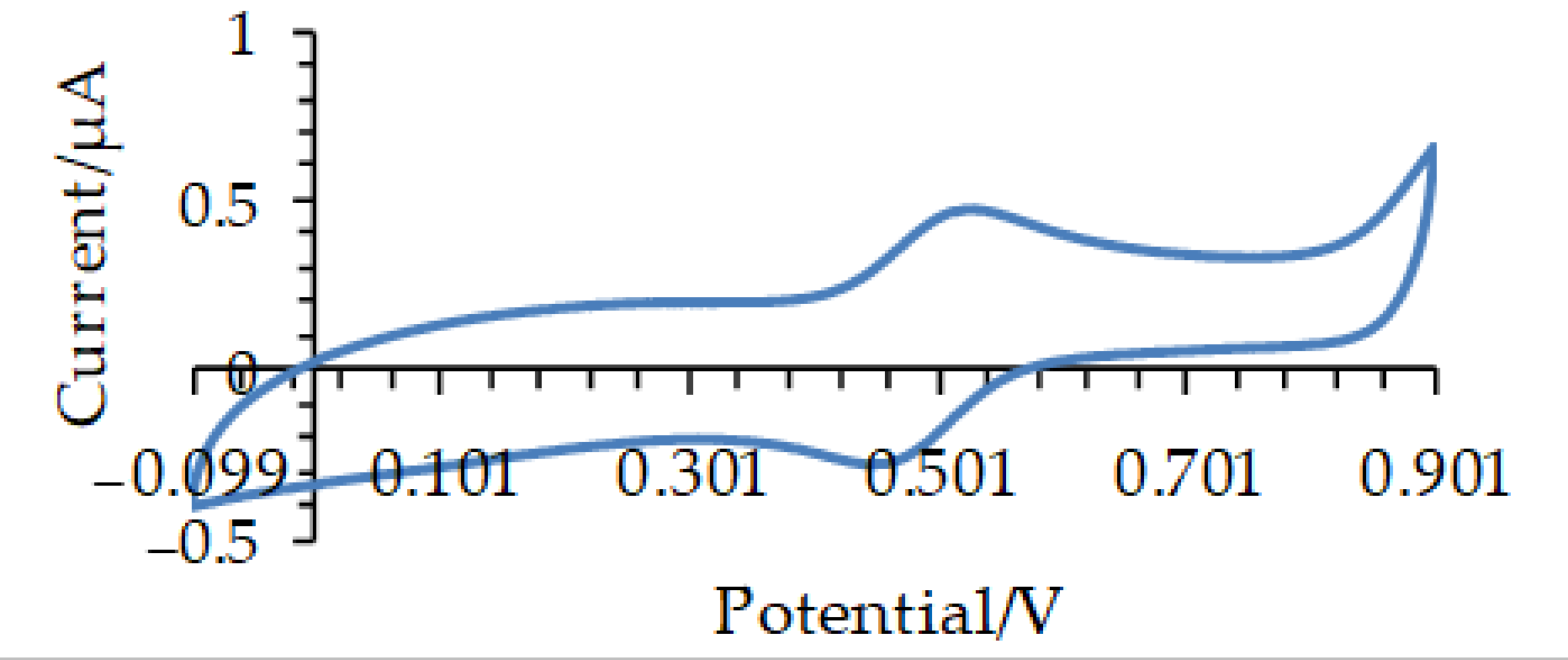
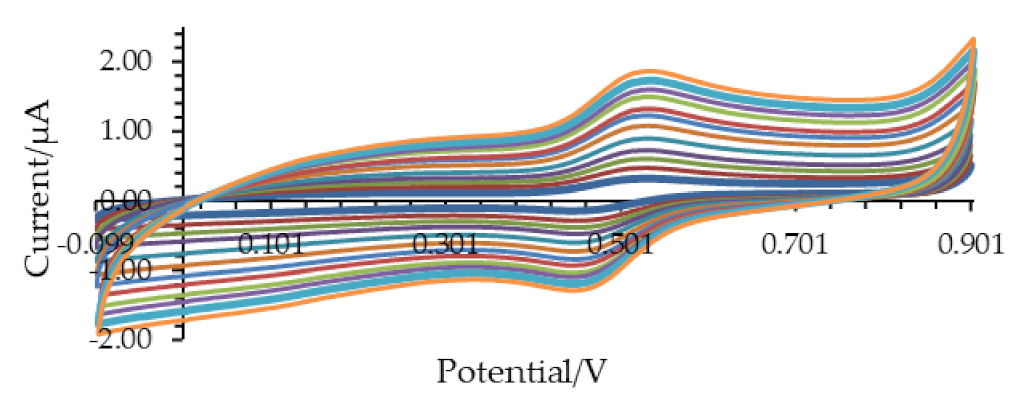
2.6.1. Cyclic Voltammetry
2.6.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.6.3. Electrochemical Characterization of 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS)
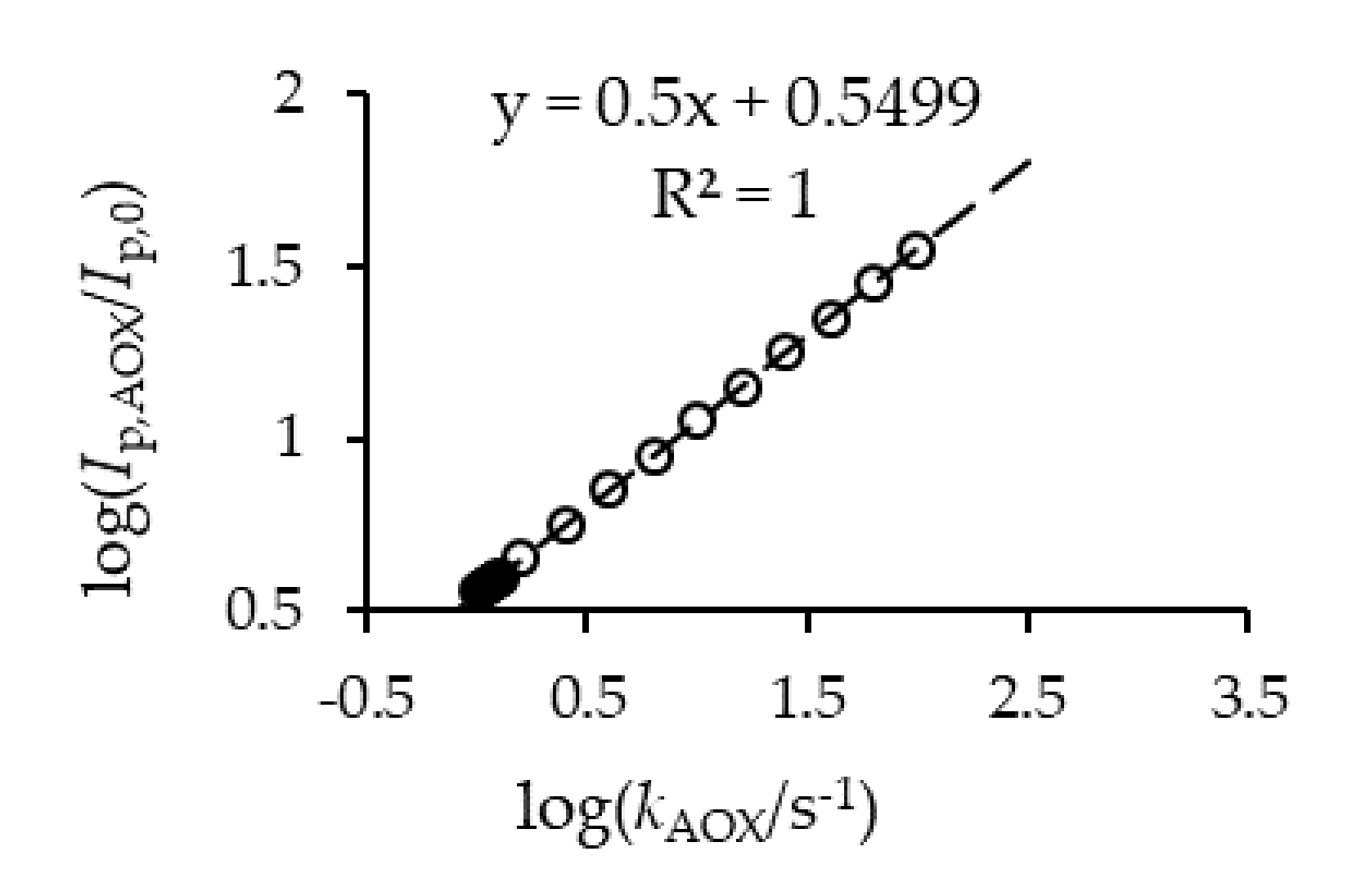
2.6.4. Determination of Antioxidant Capacity by Cyclic Voltammetry
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of Homemade Fruit Vinegars (HMV) Produced by Double Consecutive Spontaneous Fermentations
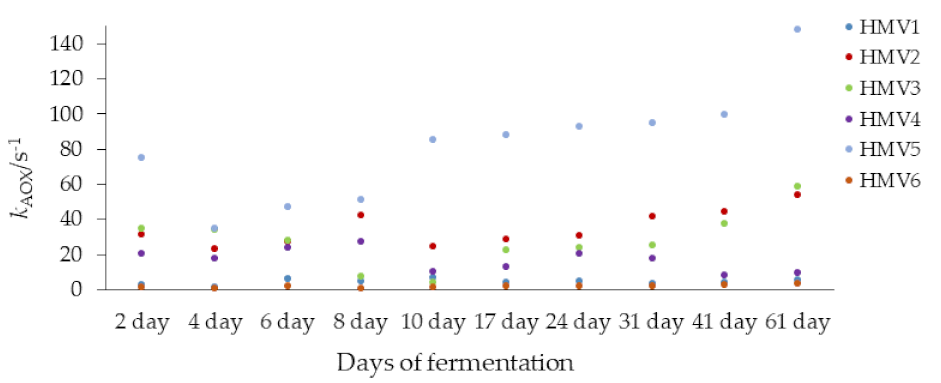
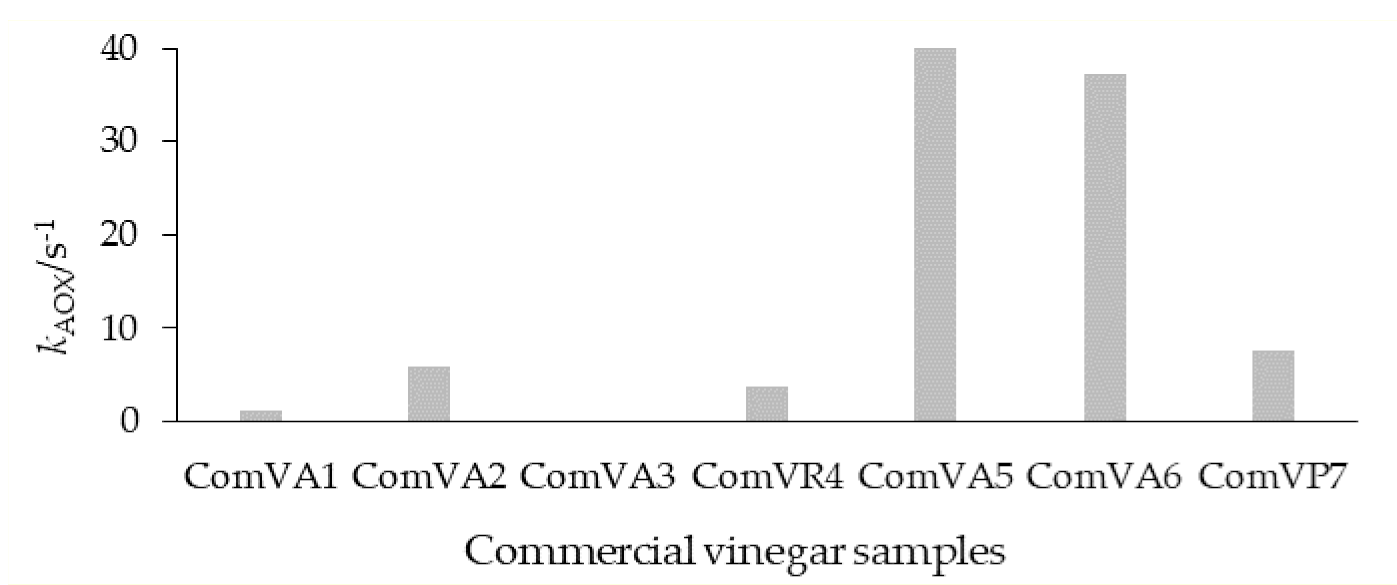
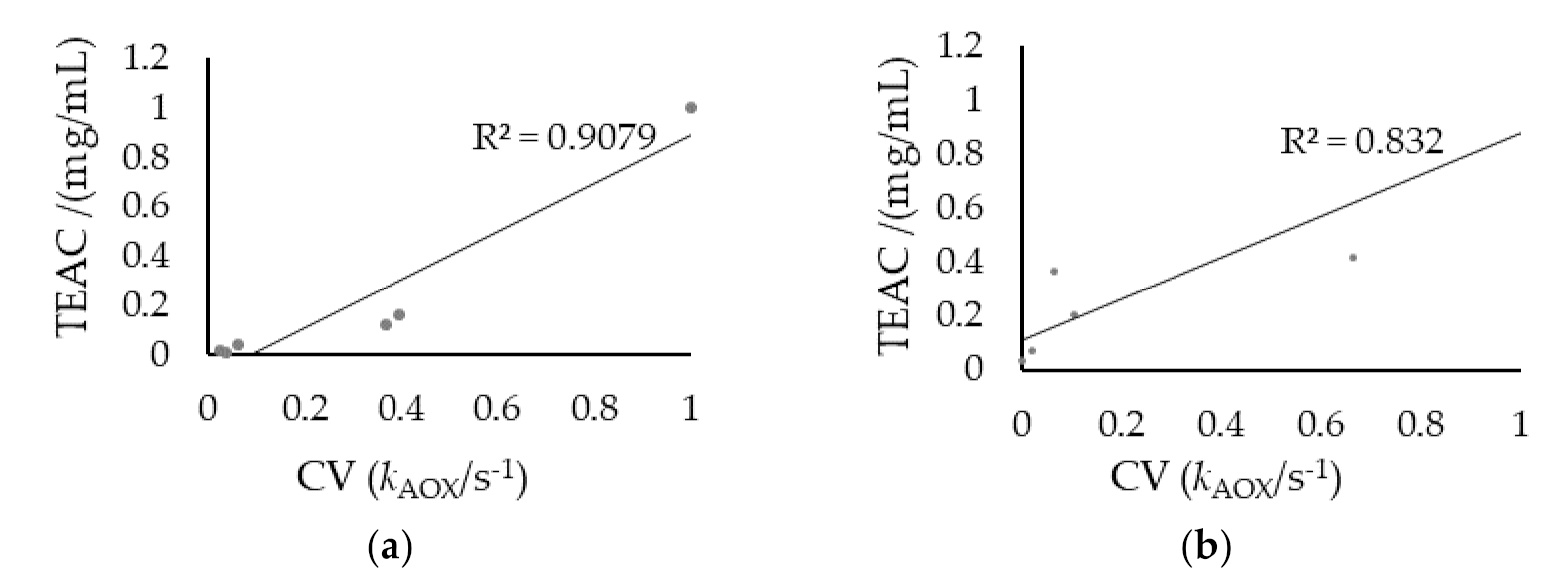
3.2. Determination of the Antioxidant Capacity of Homemade Fruit (HMV) and Commercial (ComV) Vinegars by Cyclic Voltammetry and TEAC Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) |
| ComV | commercial vinegars |
| CV | cyclic voltammetry |
| HMV | homemade fruit vinegars |
| TEAC | Trolox equivalent antioxidant capacity |
References
- Tesfaye, W.; Morales, M.L.; Garcia-Parrilla, M.C.; Troncoso, M.A. Evolution of phenolic compounds during an experimental aging in wood of Sherry vinegar. J. Agric. Food Chem. 2002, 50, 7053–7061. [Google Scholar] [CrossRef]
- Aguiar, A.; Rodrigo, A.A.N.; Ferretti, L.P.; Adílson, R.G. Determination of organic acids and ethanol in commercial vinegars. Braz. J. Food Technol. 2005, 5, 51–56. [Google Scholar]
- Dogaru, D.V.; Hădărugă, N.; Traşcă, T.; Jianu, C.; Jianu, I. Researches regarding the antioxidant capacity of some fruits vinegar. J. Agroaliment. Process. Technol. 2009, 15, 506–510. [Google Scholar]
- Vikas Bhat, S.; Akhtar, R.; Amin, T. An overview on the biological production of vinegar. Int. J. Fermented Foods 2014, 3, 139–155. [Google Scholar] [CrossRef]
- Garcia-Parrilla, M.C.; Torija, M.J.; Mas, A.; Cerezo, A.B.; Troncoso, A.M. Vinegars and other fermented condiments. Fermented Foods Health Dis. Prev. 2017, 25, 577–591. [Google Scholar]
- Cerezo, A.B.; Tesfaye, W.; Soria-Dias, M.E.; Torija, M.J.; Estibaliz, M.; Garcia-Parrilla, M.K.; Troncoso, A.M. Effect of wood on the phenolic profile and sensory properties of wine vinegars during ageing. J. Food Comp. Anal. 2010, 23, 175–186. [Google Scholar] [CrossRef]
- Hidalgo, C.; Estibaliz, M.; Cerezo, A.B.; Jesús, M. Technological process for persimmon and strawberry vinegars. Int. J. Wine Res. 2010, 2, 55–61. [Google Scholar] [CrossRef]
- Budak, H.N.; Güzel-Seydim, Z. Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. J. Sci. Food Agric. 2010, 90, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Plessi, M. Vinegar. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 5996–6004. [Google Scholar]
- Collins, B. Making & Using Vinegar: Recipes That Celebrate Vinegar’s Versatility; Storey Publishing, LLC: North Adams, MA, USA, 2014. [Google Scholar]
- Adams, M.R. Vinegar. Encyclopedia of Food Microbiology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 717–721. [Google Scholar]
- Dabija, A.; Hatnean, C.A. Study concerning the quality of apple vinegar obtained through classical method. J. Agroaliment. Process. Technol. 2014, 20, 304–310. [Google Scholar]
- Galvez, M.C.; Barroso, C.G.; Perez-Bustamante, J.A. Analysis of polyphenolic compounds of different vinegar samples. Z. Lebensm. Unters. Forsch. 1994, 199, 29–31. [Google Scholar] [CrossRef]
- Davalos, A.; Bartolome, B.; Gomez-Cordove, C. Antioxidant properties of commercial grape juices and vinegars. Food Chem. 2005, 93, 325–330. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. Antioxidant properties of traditional balsamic vinegar and boiled must model systems. Eur. Food Res. Technol. 2008, 227, 835–843. [Google Scholar] [CrossRef]
- Pinsirodom, P.; Rungcharoen, J.; Liumminful, A. Quality of commercial wine vinegars evaluated on the basis of total polyphenol content and antioxidant properties. Asian J. Food Agro-Ind. 2010, 3, 389–397. [Google Scholar]
- Heikefelt, C. Chemical and sensory analyses of juice, cider and vinegar produced from different apple cultivars. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2011. [Google Scholar]
- Ubeda, C.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Evaluation of antioxidant activity and total phenols index in persimmon vinegars produced by different process. LWT Food Sci. Technol. 2011, 44, 1591–1596. [Google Scholar] [CrossRef]
- Skenderi, P.K.; Haligiannis, I.; Sitaras, M.N. Total Antioxidant capacity and phenolic compounds of selected vinegars in the greek market. J. Food Nutr. Disor. 2013, 2, 2–7. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Rad. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, H. A cyclic voltammetry method suitable to characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Yakovleva, K.E.; Kurzeev, S.A.; Stepanova, E.V.; Fedorova, T.V.; Kuznetsov, B.A.; Koroleva, O.V. Characterization of plant phenolic compounds by cyclic voltammetry. Appl. Biochem. Microbiol. 2007, 43, 661–668. [Google Scholar] [CrossRef]
- Chaisuksant, R.; Damwan, K.; Poolkasem, A. Simple electrochemical methods for antioxidant capacity test. Acta Hortic. 2012, 943, 297–302. [Google Scholar] [CrossRef]
- Arteaga, J.F.; Ruiz-Montoya, M.; Palma, A.; Alonso-Garrido, G.; Pintado, S.; Rodríguez-Mellado, J.M. Comparison of the simple cyclic voltammetry and DPPH assays for the determination of antioxidant capacity of active principles. Molecules 2012, 17, 5126–5138. [Google Scholar] [CrossRef]
- Davčeva, M.; Mirčeski, V.; Komorsky-Lovrić, S. Evaluation of the antioxidative activity by measuring the rate of the homogeneous oxidation reaction with ferroceniumdimethanol cation. Comparative analysis of glutathione and ascorbic acid. Int. J. Electrochem. Sci. 2011, 6, 2718–2729. [Google Scholar]
- Yun, J.; Zhao, F.; Zhang, W.; Yan, H.; Zhao, F.; Ai, D. Monitoring the microbial community succession and diversity of Liangzhou fumigated vinegar during solid-state fermentation with next-generation sequencing. Ann. Mirobiol. 2019, 69, 279–289. [Google Scholar] [CrossRef]
- AOAC Official Methods Program Manual. 942.06-1942, Alcohol by Volume in Distilled Liquors. Available online: www.aoac.org/vmeth/omamanual/omamanual.htm (accessed on 21 October 2020).
- AOAC Official Methods Program Manual. 30.071, Total acid content. Available online: www.aoac.org/vmeth/omamanual/omamanual.htm (accessed on 31 May 2019).
- AOAC. Official Methods Program Manual. Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Winesand. Available online: www.aoac.org/vmeth/omamanual/omamanual.htm (accessed on 5 May 2020).
- Ilieva, F.; Petrov, K.; Kostadinović Veličkovska, S.; Gunova, N.; Dimovska, V.; João Rocha, J.M.F.; Esatbeyoglu, T. Influence of autochthonous and commercial yeast strains on fermentation and quality of wines produced from Vranec and Cabernet Sauvignon grape varieties from Tikveš wine-growing region, Republic of North Macedonia. Appl. Sci. 2021, 11, 6135. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, F.; Menezes, V.; Rogez, H. Spontaneous postharvest fermentation of acai (Euterpe oleracea) fruit. Postharv. Biol. Technol. 2013, 86, 294–299. [Google Scholar] [CrossRef]
- Coelho, E.; Genisheva, Z.; Oliveira, J.M.; Teixeira, J.A.; Domingues, L. Vinegar production from fruit concentrates: Effect on volatile composition and antioxidant activity. J. Food Sci. Technol. 2017, 54, 4112–4122. [Google Scholar] [CrossRef] [Green Version]
- Bakir, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Capanoglu, E. Fruit antioxidants during vinegar processing: Changes in content and in vitro bio-accessibility. Int. J. Mol. Sci. 2016, 17, 1658. [Google Scholar] [CrossRef]
- Küley, E.; Özyurt, G.; Özogul, I.; Boga, M.; Akyol, I.; Rocha, J.M.; Özogul, F. The role of selected lactic acid bacteria on organic acid accumulation during wet and spray-dried fish-based silages. Contributions to the winning combination of microbial food safety and environmental sustainability. Microorganisms 2020, 8, 172. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.M.; Malcata, F.X. Microbiological profile of maize and rye flours, and sourdough used for the manufacture of traditional Portuguese bread. Food Microbiol. 2012, 31, 72–88. [Google Scholar] [CrossRef]
- Rocha, J.M.; Malcata, F.X. Behavior of the complex micro-ecology in maize and rye flour and mother-dough for broa throughout storage. J. Food Qual. 2016, 39, 218–233. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.M.; Malcata, F.X. Microbial ecology dynamics in Portuguese broad sourdough. J. Food Qual. 2016, 39, 634–648. [Google Scholar] [CrossRef]
- Ozturk, I.; Caliskan, O.; Tornuk, F.; Ozcan, N.T.; Yalcin, H.; Baslar, M.; Sagdic, O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT Food Sci. Technol. 2015, 63, 144–151. [Google Scholar] [CrossRef]
- Du Toit, W.J.; Pretorius, I.S. The occurrence and esoteric effect of acetic acid bacteria in wine making. Ann. Microbiol. 2002, 52, 155–179. [Google Scholar]
- Budak, H.N.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional properties of vinegar, Institute of Food Technologists. J. Food Sci. 2014, 79, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; Giudici, P. Acetic acid bacteria in traditional balsamic vinegar: Phenotypic traits relevant for starter cultures selection. Int. J. Food Microbiol. 2008, 125, 46–53. [Google Scholar] [CrossRef]
- Kim, K.O.; Kim, S.M.; Kim, S.M.; Kim, D.Y.; Jo, D.; Yeo, S.H. Physicochemical properties of commercial fruit vinegars with different fermentation methods. J. Korean Soc. Food Sci. Nutr. 2013, 42, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Sengun, I.Y.; Kilic, G.; Ozturk, B. Screening physicochemical, microbiological and bioactive properties of fruit vinegars produced from various raw materials. Food Sci. Biotechnol. 2020, 29, 401–408. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. CL 2000/18-EURO. Available online: http://www.fao.org/3/X8537e/X8537e (accessed on 2 July 2001).
- Hailu, S.; Shimelis Admassu, S.; Jha, Y.K. Vinegar production technology—An overview. Beverage Food World 2012, 2, 29–32. [Google Scholar]
- Mirceski, V.; Komorsky-Lovric, S.; Lovric, M. Square-Wave Voltammetry Theory and Application; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Gulaboski, R.; Pereira, C.M. Electroanalytical techniques and instrumentation in food analysis. In Handbook of Food Analysis Instruments; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Kalemba-Drożdż, M.; Kwiecień, I.; Szewczyk, A.; Agnieszka Cierniak, A.; Agata Grzywacz-Kisielewska, A. Fermented vinegars from apple peels, raspberries, rosehips, lavender, mint, and rose petals: The composition, antioxidant power, and genoprotective abilities in comparison to acetic macerates, decoctions, and tinctures. Antioxidants 2020, 9, 1121. [Google Scholar] [CrossRef]
- Sakanaka, S.; Ishihara, Y. Comparison of antioxidant properties of persimmon vinegar and some other commercial vinegars in radical-scavenging assays and on lipid oxidation in tuna homogenates. Food Chem. 2008, 107, 739–744. [Google Scholar] [CrossRef]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem. 2007, 105, 564–571. [Google Scholar] [CrossRef]
- Su, M.S.; Po-Jung, C. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) fluid products as affected by fermentation. Food Chem. 2007, 104, 182–187. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, E.; Ombódi, A. Total polyphenol content and antioxidant capacity of rosehips of some Rosa species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Masino, F.; Chinnici, F.; Bendini, A.; Montevecchi, G.; Antonelli, A. A study on relationships among chemical, physical, and qualitative assessment in traditional balsamic vinegar. Food Chem. 2008, 106, 90–95. [Google Scholar] [CrossRef]
- Jang, Y.K.; Lee, M.Y.; Kim, H.Y.; Lee, S.; Yeo, S.H.; Baek, S.Y.; Lee, H.C. Comparison of traditional and commercial vinegars based on metabolite profiling and antioxidant activity. J. Microbiol. Biotechnol. 2015, 25, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.J.; Ma, Y.K.; Zhang, F.F.; Chen, F.S. Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of “Shanxi aged vinegar”, a traditional Chinese vinegar. Food Microbiol. 2012, 30, 289–297. [Google Scholar] [CrossRef]
- Cappozi, V.; Fragasso, M.G.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous food fermentations and potential risks for human health. Fermentation 2017, 3, 49. [Google Scholar] [CrossRef]
- Matloob, M.H. Zahadi date vinegar: Production and characterization. Am. J. Food Technol. 2014, 9, 231–245. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Cho, H.K.; Shin, H.S. Physicochemical properties and antioxidant activities of commercial vinegar drinks in Korea. Food Sci. Biotechnol. 2012, 21, 1729–1734. [Google Scholar] [CrossRef]
- Hafzan, Y.; Saw, J.W.; Fadzilah, I. Physicochemical properties, total phenolic content, and antioxidant capacity of homemade and commercial date (Phoenix dactylifera L.) vinegar. Int. Food Res. J. 2017, 24, 2557–2562. [Google Scholar]
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Kelebek, H.; Kadiroğlu, P.; Demircan, N.B.; Selli, S. Screening of bioactive components in grape and apple vinegars: Antioxidant and antimicrobial potential. J. Inst. Brew. 2017, 123, 407–416. [Google Scholar] [CrossRef] [Green Version]
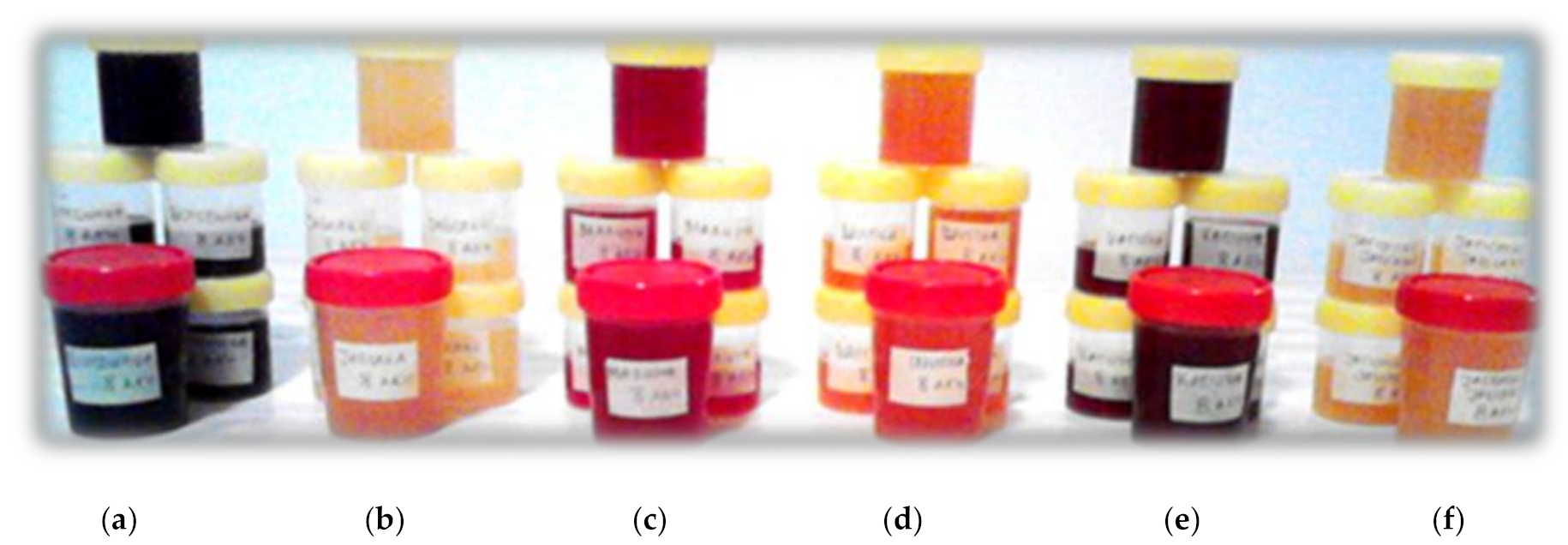
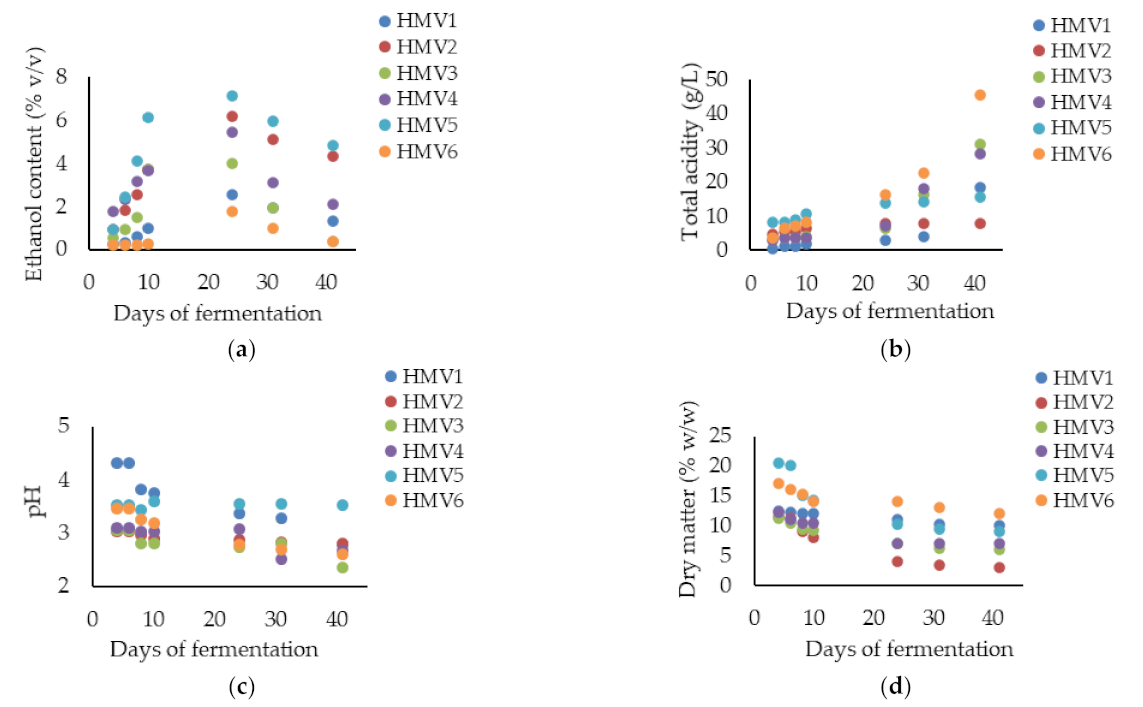

| Final HMV | Fruit | Ethanol (%, v/v) | Total Acid (g/L) | pH | Dry Matter (%, w/w) | Total Phenolic Content (mg of Gallic Acid/mL) |
|---|---|---|---|---|---|---|
| HMV1 | Apple | 0.13 ± 0.01 d | 20.4 ± 2.1 b | 2.7 ± 0.0 b | 11.1 ± 1.4 a | 0.29± 0.1 d |
| HMV2 | Raspberry | 4.24 ± 0.08 a | 9.0 ± 0.8 c | 2.9 ± 0.1 b | 3.0 ± 0.7 d | 0.99 ± 0.0 b,c |
| HMV3 | Blueberry | 0.47 ± 0.00 c | 43.6 ± 4.7 a | 2.5 ± 0.0 b | 6.2 ± 0.9 c | 1.24 ± 0.5 b |
| HMV4 | Blackberry | 0.54 ± 0.01 c | 42.6 ± 5.1 a | 2.3 ± 0.2 b | 8.1 ± 1.1 b | 1.70 ± 0.8 b |
| HMV5 | Rose hip | 3.7 ± 0.07 b | 17.4 ± 1.1 b | 3.4 ± 0.1 a | 9.3 ± 2.3 b | 20.2 ± 2.4 a |
| HMV6 | Persimmon | 0.34 ± 0.01 c | 46.8 ± 6.0 a | 2.4 ± 0.0 b | 12.1 ± 2.2 a | 0.44 ± 0.0 d |
| Figure | Fruit | TEAC (mg/mL) | ComV Sample | Fruit | TEAC (mg/mL) |
|---|---|---|---|---|---|
| HMV1 | Apple | 0.103 ± 0.05 c | ComVA1 | Apple | 0.049 ± 0.11 d |
| HMV2 | Raspberry | 1.286 ± 0.06 b | ComVA2 | Apple | 0.100 ± 0.15 c |
| HMV3 | Blueberry | 1.625 ± 0.07 b | ComVA3 | Apple | 0.024 ± 0.03 d |
| HMV4 | Blackberry | 0.431 ± 0.01 c | ComVR4 | Raspberry | 0.248 ± 0.02 b |
| HMV5 | Rose hip | 10.312 ± 0.05 a | ComVA5 | Apple | 0.683 ± 0.08 a |
| HMV6 | Persimmon | 0.171 ± 0.03 c | ComVA6 | Apple | 0.286 ± 0.04 b |
| Acetic acid (100 g/L) | --- | 0.008 ± 0.0006 d | ComVP7 | Plum | 0.695 ± 0.06 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chochevska, M.; Jančovska Seniceva, E.; Veličkovska, S.K.; Naumova-Leţia, G.; Mirčeski, V.; Rocha, J.M.F.; Esatbeyoglu, T. Electrochemical Determination of Antioxidant Capacity of Traditional Homemade Fruit Vinegars Produced with Double Spontaneous Fermentation. Microorganisms 2021, 9, 1946. https://doi.org/10.3390/microorganisms9091946
Chochevska M, Jančovska Seniceva E, Veličkovska SK, Naumova-Leţia G, Mirčeski V, Rocha JMF, Esatbeyoglu T. Electrochemical Determination of Antioxidant Capacity of Traditional Homemade Fruit Vinegars Produced with Double Spontaneous Fermentation. Microorganisms. 2021; 9(9):1946. https://doi.org/10.3390/microorganisms9091946
Chicago/Turabian StyleChochevska, Maja, Elizabeta Jančovska Seniceva, Sanja Kostadinović Veličkovska, Galaba Naumova-Leţia, Valentin Mirčeski, João Miguel F. Rocha, and Tuba Esatbeyoglu. 2021. "Electrochemical Determination of Antioxidant Capacity of Traditional Homemade Fruit Vinegars Produced with Double Spontaneous Fermentation" Microorganisms 9, no. 9: 1946. https://doi.org/10.3390/microorganisms9091946
APA StyleChochevska, M., Jančovska Seniceva, E., Veličkovska, S. K., Naumova-Leţia, G., Mirčeski, V., Rocha, J. M. F., & Esatbeyoglu, T. (2021). Electrochemical Determination of Antioxidant Capacity of Traditional Homemade Fruit Vinegars Produced with Double Spontaneous Fermentation. Microorganisms, 9(9), 1946. https://doi.org/10.3390/microorganisms9091946









