Titanium Wear Particles Exacerbate S. epidermidis-Induced Implant-Related Osteolysis and Decrease Efficacy of Antibiotic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
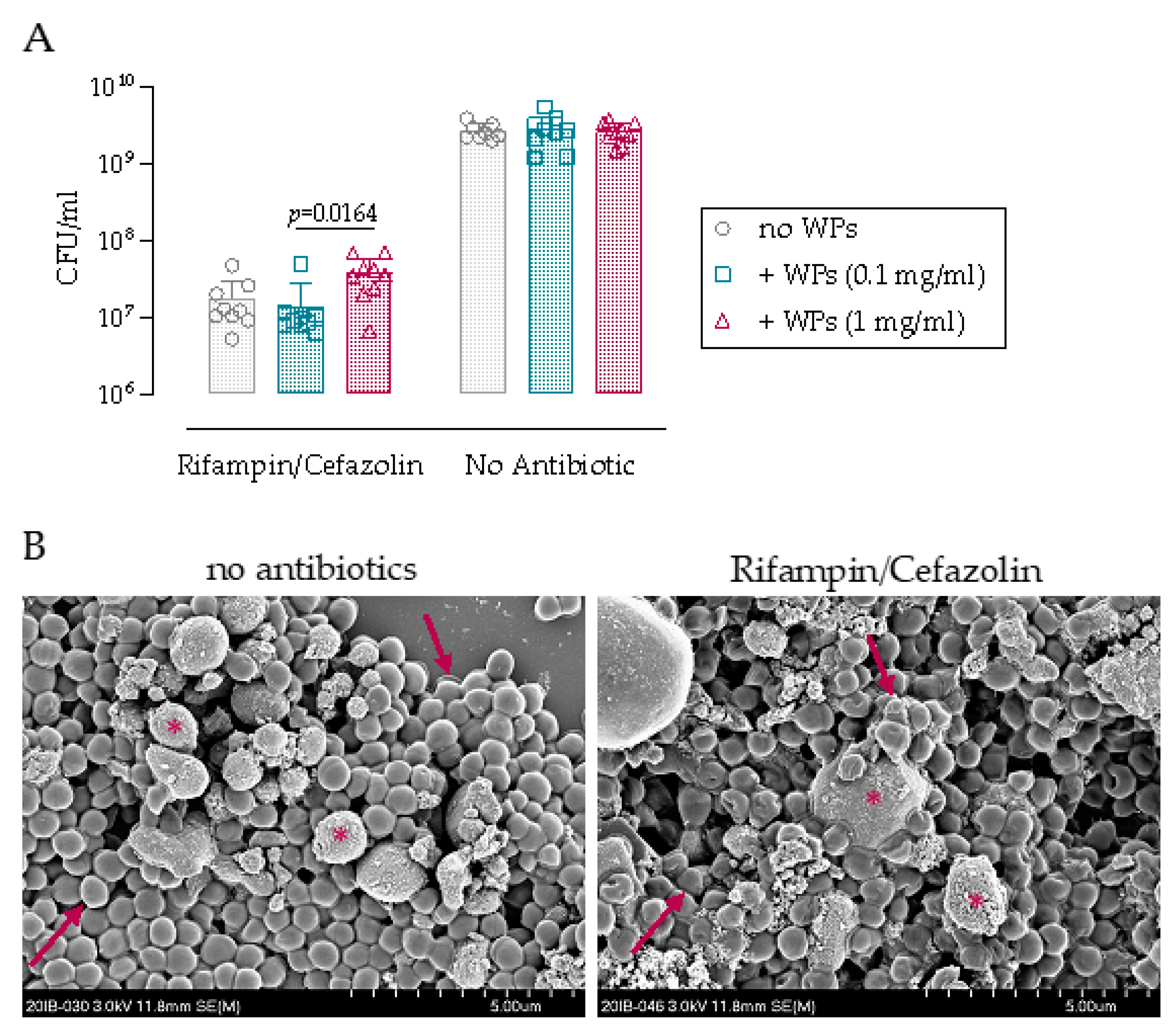
2.2. Antibiotic Challenge of S. epidermidis Grown on Titanium WPs
2.3. Confirmation of Biofilm Formation by Scanning Electron Microscopy
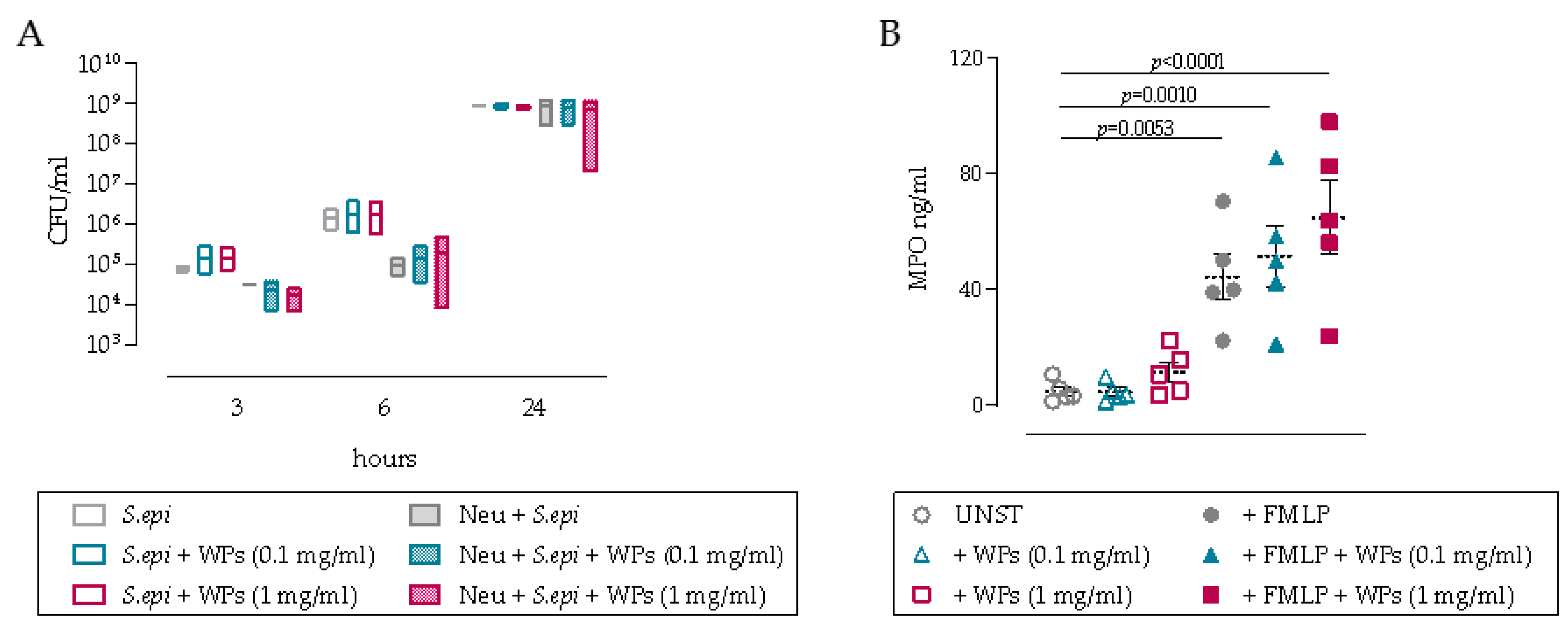
2.4. Assessment of Antibacterial Efficacy of Neutrophils
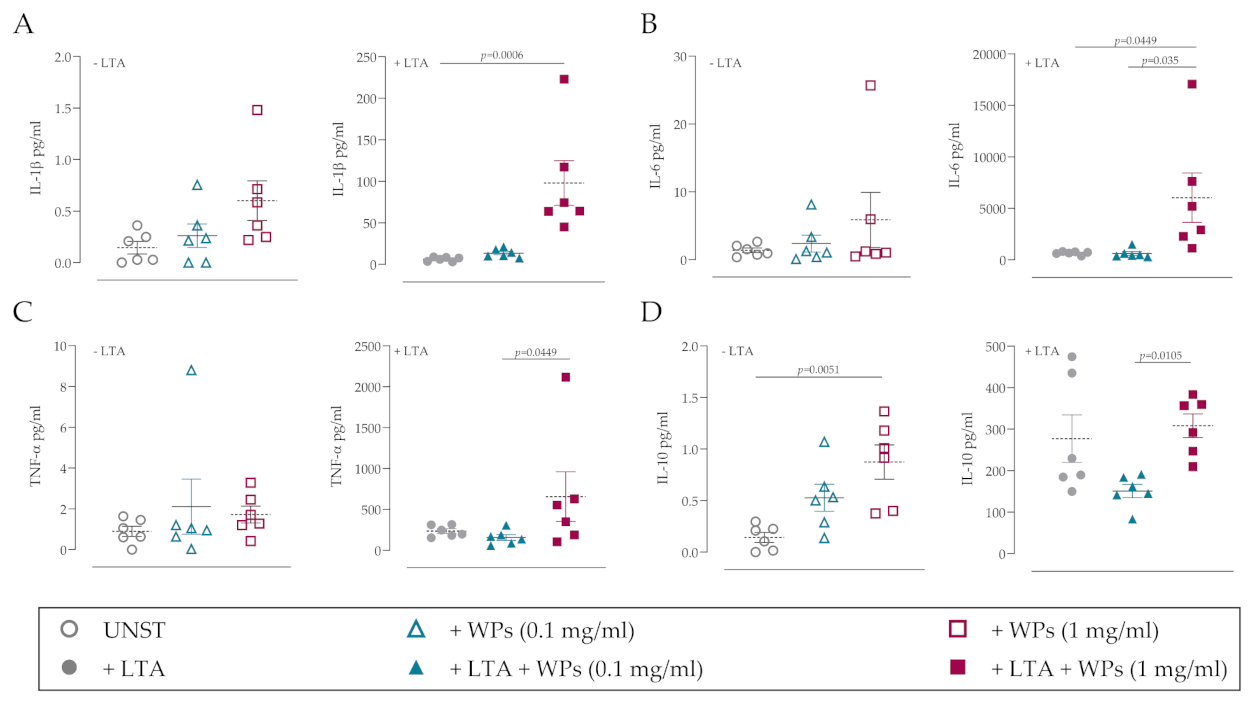
2.5. WP-Induced Soluble Mediator Production by Neutrophils and PBMCs
2.6. In Vivo Study Outline
2.7. Implant Design and Manufacturing
2.8. Bacterial Inoculum Preparation for In Vivo Study
2.9. Animal Welfare, Observation and Euthanasia
2.10. Anesthesia, Surgery and Medication Administration
2.11. In Vivo MicroCT and Image Processing
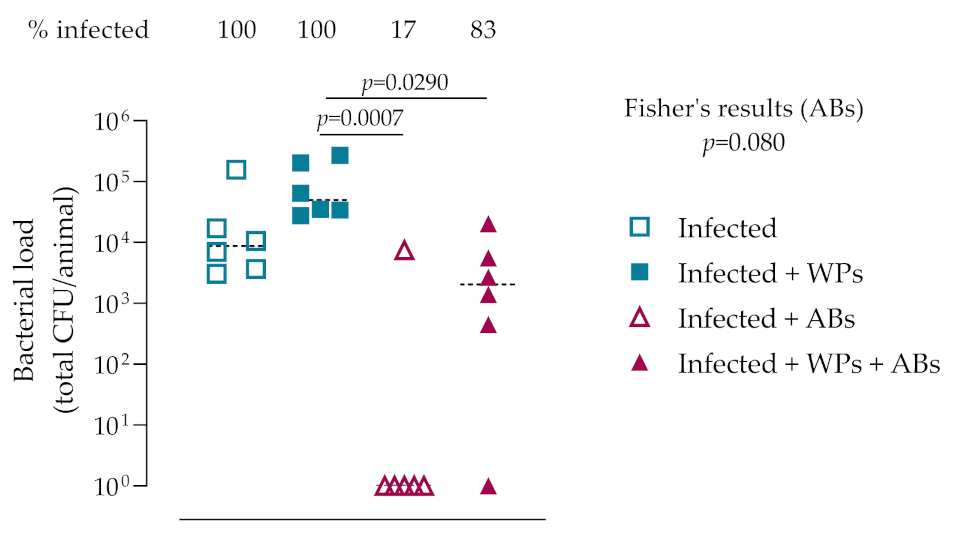
2.12. Bacteriology
2.13. Statistical Analysis
3. Results
3.1. Antibiotic Challenge of Biofilm on Wear Particles
3.2. Impact of WPs on Neutrophil and PBMC Function
3.3. In Vivo Bacteriology
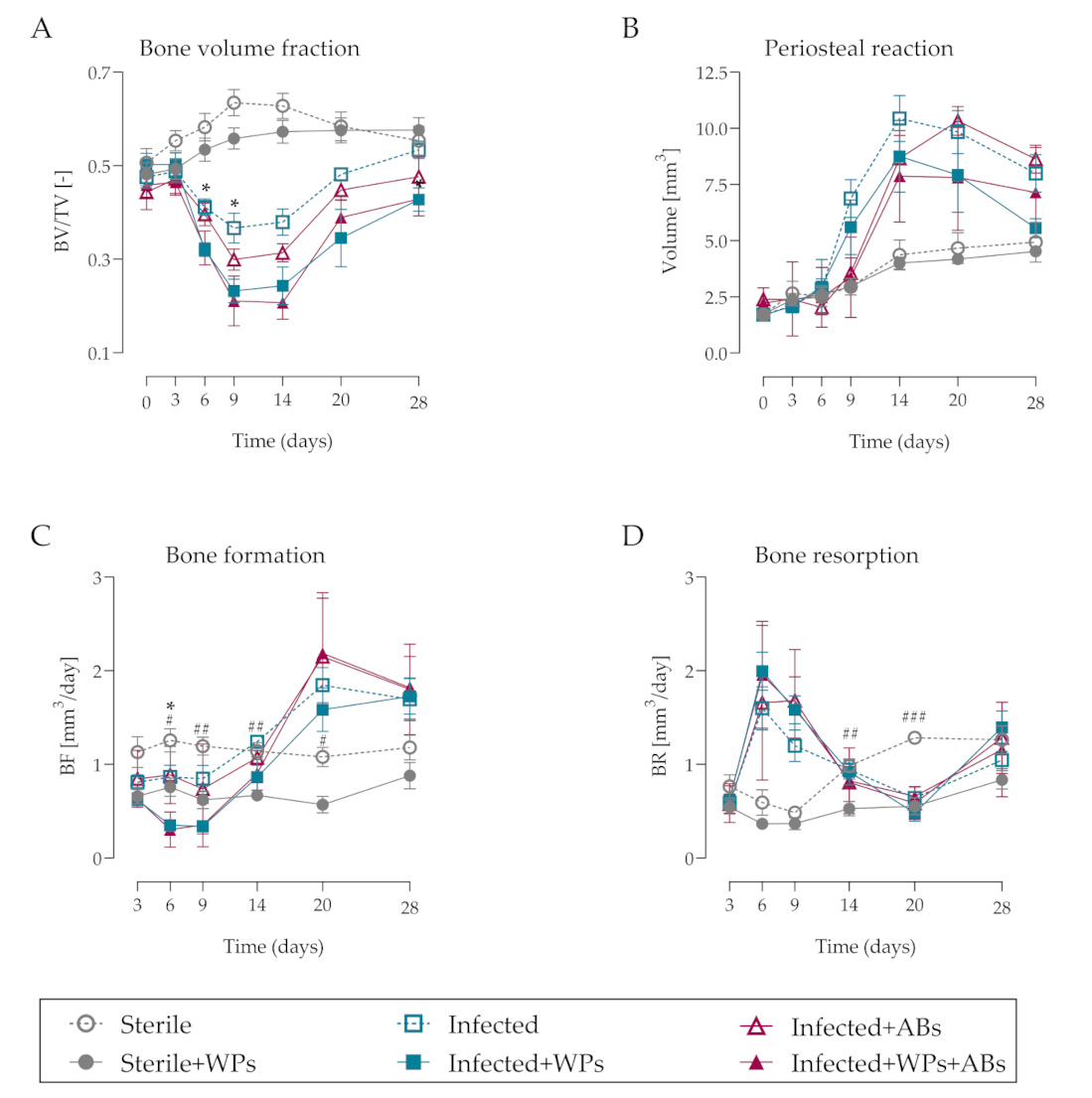
3.4. MicroCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otto-Lambertz, C.; Yagdiran, A.; Wallscheid, F.; Eysel, P.; Jung, N. Periprosthetic Infection in Joint Replacement. Dtsch. Aerzteblatt Int. 2017, 114, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Etkin, C.D.; Springer, B.D. The American Joint Replacement Registry—The first 5 years. Arthroplast. Today 2017, 3, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, Y.; An, B.; Yang, Y.; Shi, L.; Han, X.; Gao, S. Risk factors for dislocation after revision total hip arthroplasty: A systematic review and meta-analysis. Int. J. Surg. 2017, 38, 123–129. [Google Scholar] [CrossRef]
- Gwam, C.U.; Mistry, J.B.; Mohamed, N.S.; Thomas, M.; Bigart, K.C.; Mont, M.A.; Delanois, R.E. Current Epidemiology of Revision Total Hip Arthroplasty in the United States: National Inpatient Sample 2009 to 2013. J. Arthroplast. 2017, 32, 2088–2092. [Google Scholar] [CrossRef]
- Weber, M.; Renkawitz, T.; Voellner, F.; Craiovan, B.; Greimel, F.; Worlicek, M.; Grifka, J.; Benditz, A. Revision Surgery in Total Joint Replacement Is Cost-Intensive. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip Pelvis 2018, 30, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Puetzler, J.; Zalavras, C.; Moriarty, T.F.; Verhofstad, M.H.; Kates, S.L.; Raschke, M.-J.; Rosslenbroich, S.; Metsemakers, W.-J. Clinical practice in prevention of fracture-related infection: An international survey among 1197 orthopaedic trauma surgeons. Injury 2019, 50, 1208–1215. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.-J. Pathogenesis and management of fracture-related infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis Pathogenesis. Methods Mol. Biol. 2014, 1106, 17–31. [Google Scholar]
- Rogers, K.L.; Fey, P.D.; Rupp, M.E. Coagulase-Negative Staphylococcal Infections. Infect. Dis. Clin. North Am. 2009, 23, 73–98. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Davidson, D.J.; Spratt, D.; Liddle, A.D. Implant materials and prosthetic joint infection: The battle with the biofilm. EFORT Open Rev. 2019, 4, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Jacobs, J.J. Biologic effects of implant debris. Bull. NYU Hosp. Jt. Dis. 2009, 67, 182–188. [Google Scholar]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The biological response to orthopaedic implants for joint replacement: Part I: Metals. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Gibon, E.; Córdova, L.A.; Lu, L.; Lin, T.-H.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The biological response to orthopedic implants for joint replacement. II: Polyethylene, ceramics, PMMA, and the foreign body reaction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1685–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingham, E.; Fisher, J. Biological reactions to wear debris in total joint replacement. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2000, 214, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Catelas, I.; Wimmer, M.A.; Utzschneider, S. Polyethylene and metal wear particles: Characteristics and biological effects. Semin. Immunopathol. 2011, 33, 257–271. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Wang, J.; Sheng, C.; Li, Z. Wear Mechanism of Artificial Joint Failure Using Wear Debris Analysis. J. Nanosci. Nanotechnol. 2018, 18, 6805–6814. [Google Scholar] [CrossRef]
- Wooley, P.H.; Morren, R.; Andary, J.; Sud, S.; Yang, S.-Y.; Mayton, L.; Markel, D.; Sieving, A.; Nasser, S. Inflammatory responses to orthopaedic biomaterials in the murine air pouch. Biomaterials 2002, 23, 517–526. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gallo, J.; Gibon, E.F.; Takagi, M. Diagnosis and management of implant debris-associated inflammation. Expert Rev. Med. Devices 2019, 17, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Koulouvaris, P.; Ly, K.; Ivashkiv, L.B.; Bostrom, M.P.; Nestor, B.J.; Sculco, T.P.; Purdue, P.E. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J. Orthop. Res. 2007, 26, 106–116. [Google Scholar] [CrossRef]
- Anwar, H.A.; Aldam, C.H.; Visuvanathan, S.; Hart, A.J. The effect of metal ions in solution on bacterial growth compared with wear particles from hip replacements. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 1655–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadelmann, V.A.; Thompson, K.; Zeiter, S.; Camenisch, K.; Styger, U.; Patrick, S.; McDowell, A.; Nehrbass, D.; Richards, R.G.; Moriarty, T.F. Longitudinal time-lapse in vivo micro-CT reveals differential patterns of peri-implant bone changes after subclinical bacterial infection in a rat model. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Freitag, L.; Styger, U.; Camenisch, K.; Zeiter, S.; Arens, D.; Richards, R.G.; Moriarty, T.F.; Stadelmann, V.A. Impact of low bone mass and antiresorptive therapy on antibiotic efficacy in a rat model of orthopedic device-related infection. J. Orthop. Res. 2021, 39, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Kyluik-Price, D.; Kumaran, D.; Scott, M.D.; Toyofuku, W.; Ramirez-Arcos, S. Bacterial survival in whole blood depends on plasma sensitivity and resistance to neutrophil killing. Transfusion 2019, 59, 3674–3682. [Google Scholar] [CrossRef]
- Freitag, L.; Günther, C.; Eberli, U.; Fürst, A.; Zeiter, S.; Stadelmann, V.A. Relative effects of age on implant integration in a rat model: A longitudinal in vivo microct study. J. Orthop. Res. 2019, 37, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, V.A.; Potapova, I.; Camenisch, K.; Nehrbass, D.; Richards, R.G.; Moriarty, T.F. In Vivo MicroCT Monitoring of Osteomyelitis in a Rat Model. Biomed. Res. Int. 2015, 2015, 587857. [Google Scholar]
- Burch, M.-A.; Keshishian, A.; Wittmann, C.; Nehrbass, D.; Styger, U.; Muthukrishnan, G.; Arens, D.; Stadelmann, V.; Richards, R.; Moriarty, T.; et al. The non-steroidal anti-inflammatory drug carprofen negatively impacts new bone formation and antibiotic efficacy in a rat model of orthopaedic-device-related infection. Eur. Cells Mater. 2021, 41, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Versalovic, J.; Koeuth, T.; Lupski, R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic. Acids. Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Vaudaux, P.; Huggler, E.; Stern, R.; Fréhel, C.; Francois, P.; Lew, D.; Hoffmeyer, P. Inactivation of a subpopulation of human neutrophils by exposure to ultrahigh-molecular-weight polyethylene wear debris. FEMS Immunol. Med Microbiol. 2007, 49, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Bernard, L.; Vaudaux, P.; Merle, C.; Stern, R.; Huggler, E.; Lew, D.P.; Hoffmeyer, P. The inhibition of neutrophil antibacterial activity by ultra-high molecular weight polyethylene particles. Biomaterials 2005, 26, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Hosman, A.H.; Bulstra, S.K.; Sjollema, J.; Van Der Mei, H.C.; Busscher, H.J.; Neut, D. The influence of Co-Cr and UHMWPE particles on infection persistence: An in vivo study in mice. J. Orthop. Res. 2011, 30, 341–347. [Google Scholar] [CrossRef]
- Schwarz, E.M.; Lu, A.P.; Goater, J.J.; Benz, E.B.; Kollias, G.; Rosier, R.N.; Puzas, Z.E.; O’Keefe, R.J. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J. Orthop. Res. 2000, 18, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, R.; Hock, C.; Bechtold, C.D.; Proulx, S.; Bukata, S.V.; Ito, H.; Awad, H.; Nakamura, T.; O’Keefe, R.J.; Schwarz, E.M. Differential effects of biologic versus bisphosphonate inhibition of wear debris-induced osteolysis assessed by longitudinal micro-CT. J. Orthop. Res. 2008, 26, 1340–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, G.; Li, S.; Ouyang, N.; Wu, J.; Li, C.; Liu, W.; Qiu, J.; Peng, P.; Qin, L.; Ding, Y. Antiresorptive Agents are More Effective in Preventing Titanium Particle-Induced Calvarial Osteolysis in Ovariectomized Mice Than Anabolic Agents in Short-Term Administration. Artif. Organs 2018, 42, E259–E271. [Google Scholar] [CrossRef]
- Tatro, J.M.; Taki, N.; Islam, A.S.; Goldberg, V.M.; Rimnac, C.M.; Doerschuk, C.M.; Stewart, M.C.; Greenfield, E.M. The balance between endotoxin accumulation and clearance during particle-induced osteolysis in murine calvaria. J. Orthop. Res. 2007, 25, 361–369. [Google Scholar] [CrossRef]
- Greenfield, E.M.; Bi, Y.; Ragab, A.A.; Goldberg, V.M.; Nalepka, J.L.; Seabold, J.M. Does endotoxin contribute to aseptic loosening of orthopedic implants? J. Biomed. Mater. Res. 2004, 72, 179–185. [Google Scholar] [CrossRef]
- Nalepka, J.L.; Lee, M.J.; Kraay, M.J.; Marcus, R.E.; Goldberg, V.M.; Chen, X.; Greenfield, E.M. Lipopolysaccharide Found in Aseptic Loosening of Patients with Inflammatory Arthritis. Clin. Orthop. Relat. Res. 2006, 451, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Akisue, T.; Bauer, T.W.; Farver, C.F.; Mochida, Y. The effect of particle wear debris on NFkappaB activation and pro-inflammatory cytokine release in differentiated THP-1 cells. J. Biomed. Mater. Res. 2002, 59, 507–515. [Google Scholar] [CrossRef]
- Dapunt, U.; Prior, B.; Kretzer, J.P.; Giese, T.; Zhao, Y. Bacterial Biofilm Components Induce an Enhanced Inflammatory Response Against Metal Wear Particles. Ther. Clin. Risk Manag. 2020, 16, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- St. Pierre, C.A.; Chan, M.; Iwakura, Y.; Ayers, D.C.; Kurt-Jones, E.A.; Finberg, R.W. Periprosthetic osteolysis: Characterizing the innate immune response to titanium wear-particles. J. Orthop. Res. 2010, 28, 1418–1424. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gallo, J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, R.J.; Münch, H.J.; Bonefeld, C.M.; Thyssen, J.P.; Sloth, J.J.; Geisler, C.; Søballe, K.; Jellesen, M.S.; Jakobsen, S.S. Cytokine Profile in Patients with Aseptic Loosening of Total Hip Replacements and Its Relation to Metal Release and Metal Allergy. J. Clin. Med. 2019, 8, 1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenfield, E.M.; Beidelschies, M.A.; Tatro, J.M.; Goldberg, V.M.; Hise, A. Bacterial Pathogen-associated Molecular Patterns Stimulate Biological Activity of Orthopaedic Wear Particles by Activating Cognate Toll-like Receptors. J. Biol. Chem. 2010, 285, 32378–32384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearl, J.I.; Ma, T.; Irani, A.R.; Huang, Z.; Robinson, W.H.; Smith, R.L.; Goodman, S.B. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials 2011, 32, 5535–5542. [Google Scholar] [CrossRef] [Green Version]
- Utsunomiya, T.; Zhang, N.; Lin, T.; Kohno, Y.; Ueno, M.; Maruyama, M.; Huang, E.; Rhee, C.; Yao, Z.; Goodman, S.B. Suppression of NF-κB-induced chronic inflammation mitigates inflammatory osteolysis in the murine continuous polyethylene particle infusion model. J. Biomed. Mater. Res. 2021, 109, 1828–1839. [Google Scholar]
- Dapunt, U.; Spranger, O.; Gantz, S.; Burckhardt, I.; Zimermann, S.; Schmidmaier, G.; Moghaddam, A. Are atrophic long-bone nonunions associated with low-grade infections? Ther. Clin. Risk Manag. 2015, 11, 1843–1852. [Google Scholar] [CrossRef] [Green Version]
- Obst, U.; Marten, S.-M.; Niessner, C.; Hartwig, E. Bacterial DNA from Orthopedic Implants after Routine Removal. Int. J. Artif. Organs 2011, 34, 856–862. [Google Scholar] [CrossRef]
- Liu, X.; Qu, X.; Wu, C.; Zhai, Z.; Tian, B.; Li, H.; Ouyang, Z.; Xu, X.; Wang, W.; Fan, Q.; et al. The effect of enoxacin on osteoclastogenesis and reduction of titanium particle-induced osteolysis via suppression of JNK signaling pathway. Biomaterials 2014, 35, 5721–5730. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kang, H.; Guo, C.-A.; Fan, W.-S.; Wang, Y.-M.; Deng, L.-F.; Yan, Z.-Q. Rifampin suppresses osteoclastogenesis and titanium particle-induced osteolysis via modulating RANKL signaling pathways. Biochem. Biophys. Res. Commun. 2017, 484, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Group | Inoculum | Group Size | |
|---|---|---|---|
| 1 | Control | Sterile | 6 |
| S. epidermidis | 6 | ||
| S. epidermidis + antibiotics | 6 | ||
| 2 | Wear particles | Sterile | 6 |
| S. epidermidis | 6 | ||
| S. epidermidis + antibiotics | 6 | ||
| TOTAL | 36 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siverino, C.; Freitag, L.; Arens, D.; Styger, U.; Richards, R.G.; Moriarty, T.F.; Stadelmann, V.A.; Thompson, K. Titanium Wear Particles Exacerbate S. epidermidis-Induced Implant-Related Osteolysis and Decrease Efficacy of Antibiotic Therapy. Microorganisms 2021, 9, 1945. https://doi.org/10.3390/microorganisms9091945
Siverino C, Freitag L, Arens D, Styger U, Richards RG, Moriarty TF, Stadelmann VA, Thompson K. Titanium Wear Particles Exacerbate S. epidermidis-Induced Implant-Related Osteolysis and Decrease Efficacy of Antibiotic Therapy. Microorganisms. 2021; 9(9):1945. https://doi.org/10.3390/microorganisms9091945
Chicago/Turabian StyleSiverino, Claudia, Linda Freitag, Daniel Arens, Ursula Styger, R. Geoff Richards, T. Fintan Moriarty, Vincent A. Stadelmann, and Keith Thompson. 2021. "Titanium Wear Particles Exacerbate S. epidermidis-Induced Implant-Related Osteolysis and Decrease Efficacy of Antibiotic Therapy" Microorganisms 9, no. 9: 1945. https://doi.org/10.3390/microorganisms9091945
APA StyleSiverino, C., Freitag, L., Arens, D., Styger, U., Richards, R. G., Moriarty, T. F., Stadelmann, V. A., & Thompson, K. (2021). Titanium Wear Particles Exacerbate S. epidermidis-Induced Implant-Related Osteolysis and Decrease Efficacy of Antibiotic Therapy. Microorganisms, 9(9), 1945. https://doi.org/10.3390/microorganisms9091945








