Sensitivity of Rapid Antigen Testing and RT-PCR Performed on Nasopharyngeal Swabs versus Saliva Samples in COVID-19 Hospitalized Patients: Results of a Prospective Comparative Trial (RESTART)
Abstract
:1. Introduction
- (1)
- Compare the diagnostic performance of NP RT-PCR and salivary RT-PCR;
- (2)
- Evaluate the reliability of RATs in hospitalized patients;
- (3)
- Compare the sensitivity of RATs performed on NP swab versus saliva in this specific population;
- (4)
- Evaluate the impact of VTM on the diagnostic performance of RATs.
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Diagnostic Tests
2.3. Quantitative SARS-CoV-2 PCR and RAT
2.4. In Vitro Testing of Dilution Effect
- (i)
- One Step Immunoassay Exdia® COVID-19 Ag (Precision Biosensor, Daejeon, Korea) and Standard Q® COVID-19 Rapid Antigen Test (Roche-Switzerland, Basel, Switzerland) were performed after mixing 350 µL of VTM with the buffer solution provided in the RAT kit;
- (ii)
- A clean swab was first inoculated in the VTM, then mixed with the buffer solution (according to manufacturer instructions) and tested with the previously mentioned commercial kits; and
- (iii)
- An amount of 300 µL of VTM was used for RT-PCR analysis.
2.5. Statistical Analysis
2.6. Ethics
3. Results
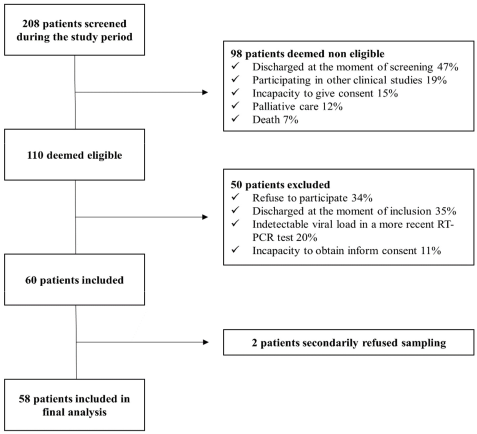
3.1. Patients’ Characteristics
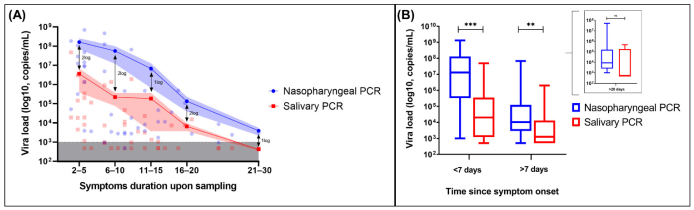
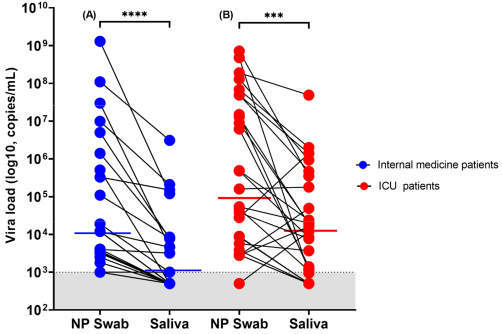
3.2. Diagnostic Performance of RT-PCR (NP versus Saliva) and Rapid Antigen Testing
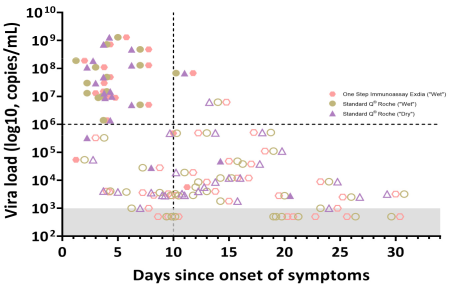
3.3. In Vitro Dilution Effect
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Diagnostic Testing for SARS-CoV-2. Available online: https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2 (accessed on 7 April 2021).
- Butler-Laporte, G.; Lawandi, A.; Schiller, I.; Yao, M.; Dendukuri, N.; McDonald, E.G.; Lee, T.C. Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2021, 181, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.J.; Mozafarihashjin, M.; Coomes, E.; Powis, J.; Li, A.X.; Paterson, A.; Anceva-Sami, S.; Barati, S.; Crowl, G.; Faheem, A.; et al. Sensitivity of nasopharyngeal swabs and saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 72, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Pasomsub, E.; Watcharananan, S.P.; Boonyawat, K.; Janchompoo, P.; Wongtabtim, G.; Suksuwan, W.; Sungkanuparph, S.; Phuphuakrat, A. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: A cross-sectional study. Clin. Microbiol. Infect. 2021, 27, 285.e1–285.e4. [Google Scholar] [CrossRef] [PubMed]
- Schwob, J.-M.; Miauton, A.; Petrovic, D.; Perdrix, J.; Senn, N.; Jaton, K.; Opota, O.; Maillard, A.; Minghelli, G.; Cornuz, J.; et al. Antigen rapid tests, nasopharyngeal PCR and saliva PCR to detect SARS-CoV-2: A prospective comparative clinical trial. medXriv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.11.23.20237057v1 (accessed on 7 April 2021).
- Huber, M.; Schreiber, P.W.; Scheier, T.; Audigé, A.; Buonomano, R.; Rudiger, A.; Braun, D.L.; Eich, G.; Keller, D.; Hasse, B.; et al. Large parallel screen of saliva and nasopharyngeal swabs in a test center setting proofs utility of saliva as alternate specimen for SARS-CoV-2 detection by RT-PCR. MedRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.12.01.20241778v1.full (accessed on 7 April 2021).
- FIND. SARS-CoV-2 Diagnostic Pipeline. Available online: https://www.finddx.org/covid-19/pipeline/?avance=all&type=Rapid+diagnostic+tests&test_target=Antigen&status=all§ion=show-all&action=default (accessed on 25 January 2021).
- Caruana, G.; Croxatto, A.; Coste, A.T.; Opota, O.; Lamoth, F.; Jaton, K.; Greub, G. Diagnostic strategies for SARS-CoV-2 infection and interpretation of microbiological results. Clin. Microbiol. Infect. 2020, 26, 1178–1182. [Google Scholar] [CrossRef]
- Teo, A.K.J.; Choudhury, Y.; Tan, I.B.; Cher, C.Y.; Chew, S.H.; Wan, Z.Y.; Cheng, L.T.E.; Oon, L.L.E.; Tan, M.H.; Chan, K.S.; et al. Saliva is more sensitive than nasopharyngeal or nasal swabs for diagnosis of asymptomatic and mild COVID-19 infection. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Skolimowska, K.; Rayment, M.; Jones, R.; Madona, P.; Moore, L.S.P.; Randell, P. Non-invasive saliva specimens for the diagnosis of COVID-19: Caution in mild outpatient cohorts with low prevalence. Clin. Microbiol. Infect. 2020, 12, 1711–1713. [Google Scholar] [CrossRef]
- Berenger, B.M.; Conly, J.M.; Fonseca, K.; Hu, J.; Louie, T.; Schneider, A.R.; Singh, T.; Stokes, W.; Ward, L.; Zelyas, W. Saliva collected in universal transport media is an effective, simple and high-volume amenable method to detect SARS-CoV-2. Clin. Microbiol. Infect. 2020, 27, 656–657. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Carcano, G.; Gianfagna, F.; Grossi, P.; Gasperina, D.D.; Genoni, A.; Fasano, M.; Sessa, F.; Tettamanti, L.; Carinci, F.; et al. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020, 81, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Caruana, G.; Croxatto, A.; Kampouri, E.; Kritikos, A.; Opota, O.; Foerster, M.; Brouillet, R.; Senn, L.; Lienhard, R.; Egli, A.; et al. ImplemeNting SARS-CoV-2 Rapid antigen testing in the Emergency wArd of a Swiss univErsity hospital: The INCREASE study. Microorganisms 2021, 9, 798. [Google Scholar] [CrossRef]
- Kirkland, P.D.; Frost, M.J. The impact of viral transport media on PCR assay results for the detection of nucleic acid from SARS-CoV-2. Pathology 2020, 52, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.A.; Baumann, R.E.; Borillo, G.A.; Kagan, R.M.; Batterman, H.J.; Galdzicka, M.M.; Marlowe, E.M. Evaluation of Transport Media and Specimen Transport Conditions for the Detection of SARS-CoV-2 by Use of Real-Time Reverse Transcription-PCR. J. Clin. Microbiol. 2020, 58, e00708-20. [Google Scholar] [CrossRef]
- Walsh, P.; Overmyer, C.L.; Pham, K.; Michaelson, S.; Gofman, L.; DeSalvia, L.; Tran, T.; Gonzalez, D.; Pusavat, J.; Feola, M.; et al. Comparison of respiratory virus detection rates for infants and toddlers by use of flocked swabs, saline aspirates, and saline aspirates mixed in universal transport medium for room temperature storage and shipping. J. Clin. Microbiol. 2008, 46, 2374–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opota, O.; Brouillet, R.; Greub, G.; Jaton, K. Comparison of SARS-CoV-2 RT-PCR on a high-throughput molecular diagnostic platform and the cobas SARS-CoV-2 test for the diagnostic of COVID-19 on various clinical samples. Pathog. Dis. 2020, 78, ftaa061. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Nsoga, M.T.N.; Perez-Rodriguez, F.J.; Aad, Y.A.; Sattonnet-Roche, P.; Gayet-Ageron, A.; Jaksic, C.; Torriani, G.; Boehm, E.; Kronig, L.; et al. Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers. PLoS ONE 2021, 16, e0248921. [Google Scholar] [CrossRef]
- Prevention CfDCa. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html#additional-resources (accessed on 7 April 2021).
- Marty, F.M.; Chen, K.; Verrill, K.A. How to Obtain a Nasopharyngeal Swab Specimen. N. Engl. J. Med. 2020, 382, e76. [Google Scholar] [CrossRef] [PubMed]
- Jacot, D.; Greub, G.; Jaton, K.; Opota, O. Viral load of SARS-CoV-2 across patients and compared to other respiratory viruses. Microbes. Infect. 2020, 22, 617–621. [Google Scholar] [CrossRef]
- Desmet, T.; Paepe, P.; Boelens, J.; Coorevits, L.; Padalko, E.; Vandendriessche, S.; Leroux-Roels, I.; Aerssens, A.; Callens, A.; Braeckel, E.V.; et al. Combined oropharyngeal/nasal swab is equivalent to nasopharyngeal sampling for SARS-CoV-2 diagnostic PCR. BMC Microbiol. 2021, 21, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vlek, A.L.M.; Wesselius, T.S.; Achterberg, R.; Thijsen, S.F.T. Combined throat/nasal swab sampling for SARS-CoV-2 is equivalent to nasopharyngeal sampling. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.E.; Caliendo, A.M.; Arias, C.A.; Hayden, M.K.; Englund, J.A.; Lee, M.J.; Loeb, M.; Patel, R.; El Alayli, A.; Altayar, O.; et al. The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing. Clin Infect. Dis. 2021. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/ (accessed on 7 April 2021).
- Adrian Egli, R.L.; Jaton, K.; Gilbert Greub for the Swiss Society of Microbiology. Recommendation of the Swiss Society of Microbiology for usage of SARS-CoV-2 specific antigen tests. PIPETTE—SWISS LABORATORY MEDICINE NR. 6 December 2020. Available online: https://issuu.com/sulm-pipette/docs/pipette_6-2020-web (accessed on 7 April 2021).
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claer, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Gorochov, G.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13. Available online: https://www.science.org/doi/10.1126/scitranslmed.abd2223 (accessed on 7 April 2021). [CrossRef] [PubMed]
- Weitzel, T.; Legarraga, P.; Iruretagoyena, M.; Pizarro, G.; Vollrath, V.; Araos, R.; Munita, J.M.; Porte, L. Comparative evaluation of four rapid SARS-CoV-2 antigen detection tests using universal transport medium. Travel Med. Infect. Dis. 2021, 39, 101942. [Google Scholar] [CrossRef] [PubMed]
- Furness, S.; Worthington, H.V.; Bryan, G.; Birchenough, S.; McMillan, R. Interventions for the management of dry mouth: Topical therapies. Cochrane Database Syst. Rev. 2011, 12. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008934.pub2/full (accessed on 7 April 2021). [CrossRef] [PubMed]
| Characteristics | Internal Medicine Patients (n = 26) | ICU Patients (n = 32) | Overall (n = 58) |
|---|---|---|---|
| Age (y), median (IQR) | 70 (61–77) | 71 (62–78) | 70 (61–77) |
| Male, n (%) | 20 (77) | 25 (78) | 45 (77) |
| Patients with symptoms upon sampling, n (%) | 21 (81) | 28 (87) | 49 (84) |
| Duration of symptoms, median (IQR) | 11 (5–19) | 11 (6–19) | 11 (5–19) |
| Type of symptoms, n (%) | |||
| Fever | 5 (19) | 5 (16) | 10 (17) |
| Cough | 8 (31) | 11 (34) | 19 (33) |
| Dyspnea | 7 (27) | 20 (62) | 27 (46) |
| Fatigue | 4 (15) | 3 (9) | 7 (12) |
| Anosmia/Dysgeusia | 1 (4) | 2 (6) | 3 (5) |
| Other | 6 (23) | 8 (25) | 14 (24) |
| Viral load, median (IQR) | 43,000 (3700–9,100,000) | 82,000 (4500–12,000,000) | 48,000 (3800–9,900,000) |
| Diagnostic Test | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) | |
|---|---|---|---|---|---|
| Nasopharyngeal RT-PCR | 98 (89–100) | 100 (66–100) | 100 (93–100) | 90 (56–98) | |
| Salivary RT-PCR PCR | 69 (55–82) | 100 (66–100) | 100 (90–100) | 37.5 (28–48) | |
| Nasopharyngeal rapid antigen test | Exdia®(“Wet”) | 41 (27–56) | 100 (63–100) | 100 (83–100) | 22 (18–26) |
| Roche® (“Wet”) | 35 (22–50) | 100 (66–100) | 100 (82–100) | 22 (19–26) | |
| Roche® (“Dry”) | 47 (35–62) | 100 (66–100) | 100 (86–100) | 26 (21–31) | |
| Salivary rapid antigen test | Exdia®(“Wet”) | 8 (2–20) | 100 (66–100) | 100 (51–100) | 17 (15–18) |
| Roche®(“Wet”) | 4 (0.5–14) | 100 (66–100) | 100 (18–100) | 16 (15–17) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kritikos, A.; Caruana, G.; Brouillet, R.; Miroz, J.-P.; Abed-Maillard, S.; Stieger, G.; Opota, O.; Croxatto, A.; Vollenweider, P.; Bart, P.-A.; et al. Sensitivity of Rapid Antigen Testing and RT-PCR Performed on Nasopharyngeal Swabs versus Saliva Samples in COVID-19 Hospitalized Patients: Results of a Prospective Comparative Trial (RESTART). Microorganisms 2021, 9, 1910. https://doi.org/10.3390/microorganisms9091910
Kritikos A, Caruana G, Brouillet R, Miroz J-P, Abed-Maillard S, Stieger G, Opota O, Croxatto A, Vollenweider P, Bart P-A, et al. Sensitivity of Rapid Antigen Testing and RT-PCR Performed on Nasopharyngeal Swabs versus Saliva Samples in COVID-19 Hospitalized Patients: Results of a Prospective Comparative Trial (RESTART). Microorganisms. 2021; 9(9):1910. https://doi.org/10.3390/microorganisms9091910
Chicago/Turabian StyleKritikos, Antonios, Giorgia Caruana, René Brouillet, John-Paul Miroz, Samia Abed-Maillard, Geraldine Stieger, Onya Opota, Antony Croxatto, Peter Vollenweider, Pierre-Alexandre Bart, and et al. 2021. "Sensitivity of Rapid Antigen Testing and RT-PCR Performed on Nasopharyngeal Swabs versus Saliva Samples in COVID-19 Hospitalized Patients: Results of a Prospective Comparative Trial (RESTART)" Microorganisms 9, no. 9: 1910. https://doi.org/10.3390/microorganisms9091910
APA StyleKritikos, A., Caruana, G., Brouillet, R., Miroz, J.-P., Abed-Maillard, S., Stieger, G., Opota, O., Croxatto, A., Vollenweider, P., Bart, P.-A., Chiche, J.-D., & Greub, G. (2021). Sensitivity of Rapid Antigen Testing and RT-PCR Performed on Nasopharyngeal Swabs versus Saliva Samples in COVID-19 Hospitalized Patients: Results of a Prospective Comparative Trial (RESTART). Microorganisms, 9(9), 1910. https://doi.org/10.3390/microorganisms9091910







