Cholesterol-to-Coprostanol Conversion by the Gut Microbiota: What We Know, Suspect, and Ignore
Abstract
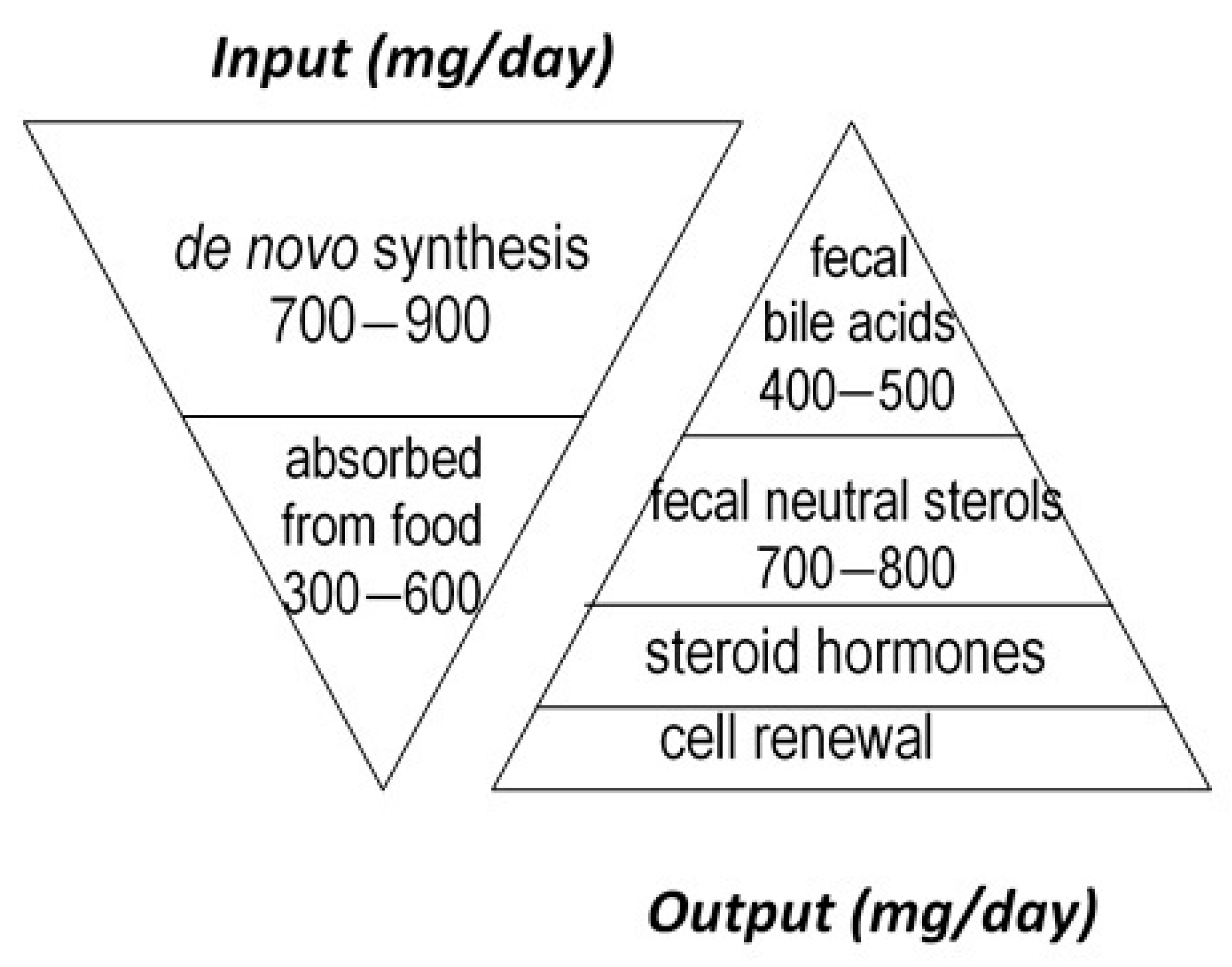
:1. Introduction
2. Historical Evidence
3. Coprostanol as a Tracer for Life on Earth and Anthropogenic Water Pollution
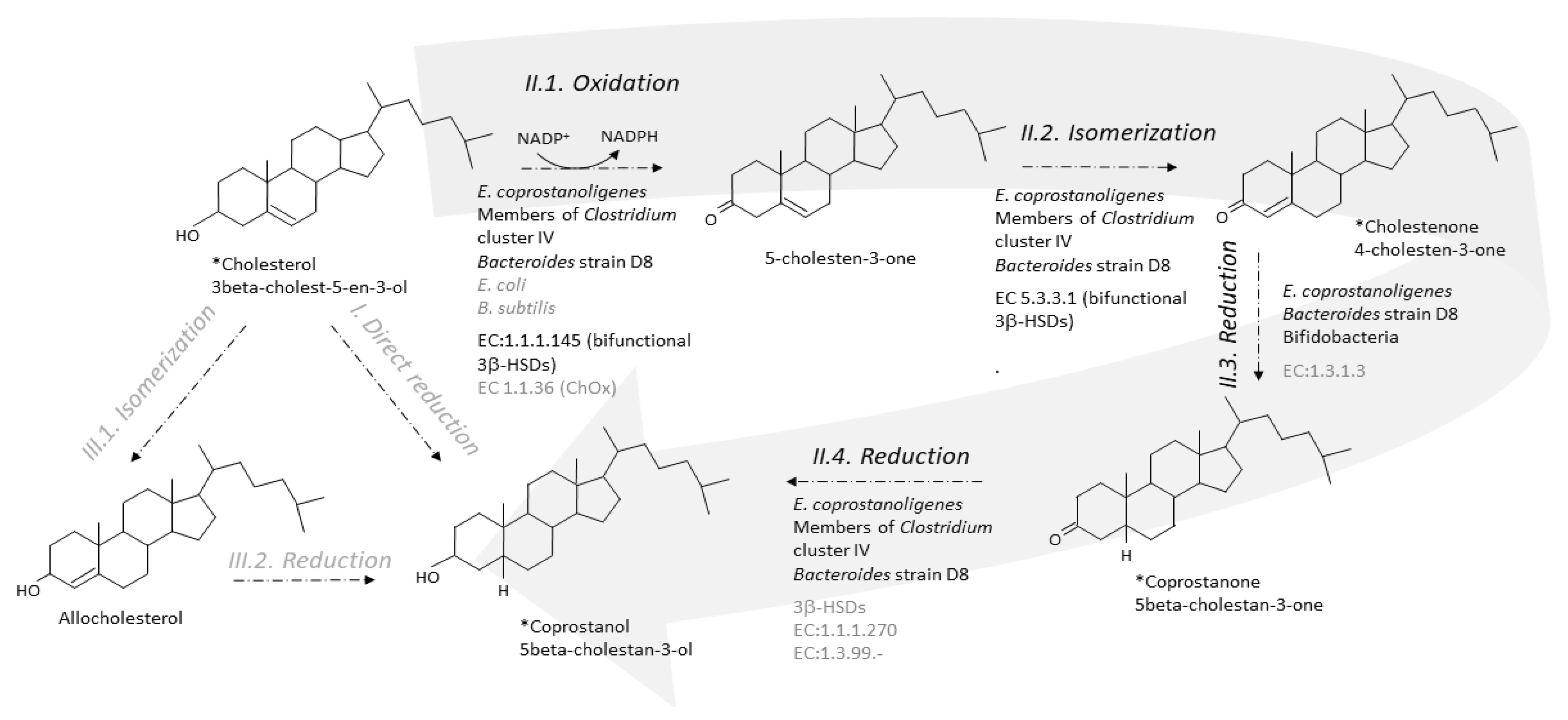
4. Gut Microbial Metabolites of Cholesterol and Suspected Pathways
5. Isolated Active Strains
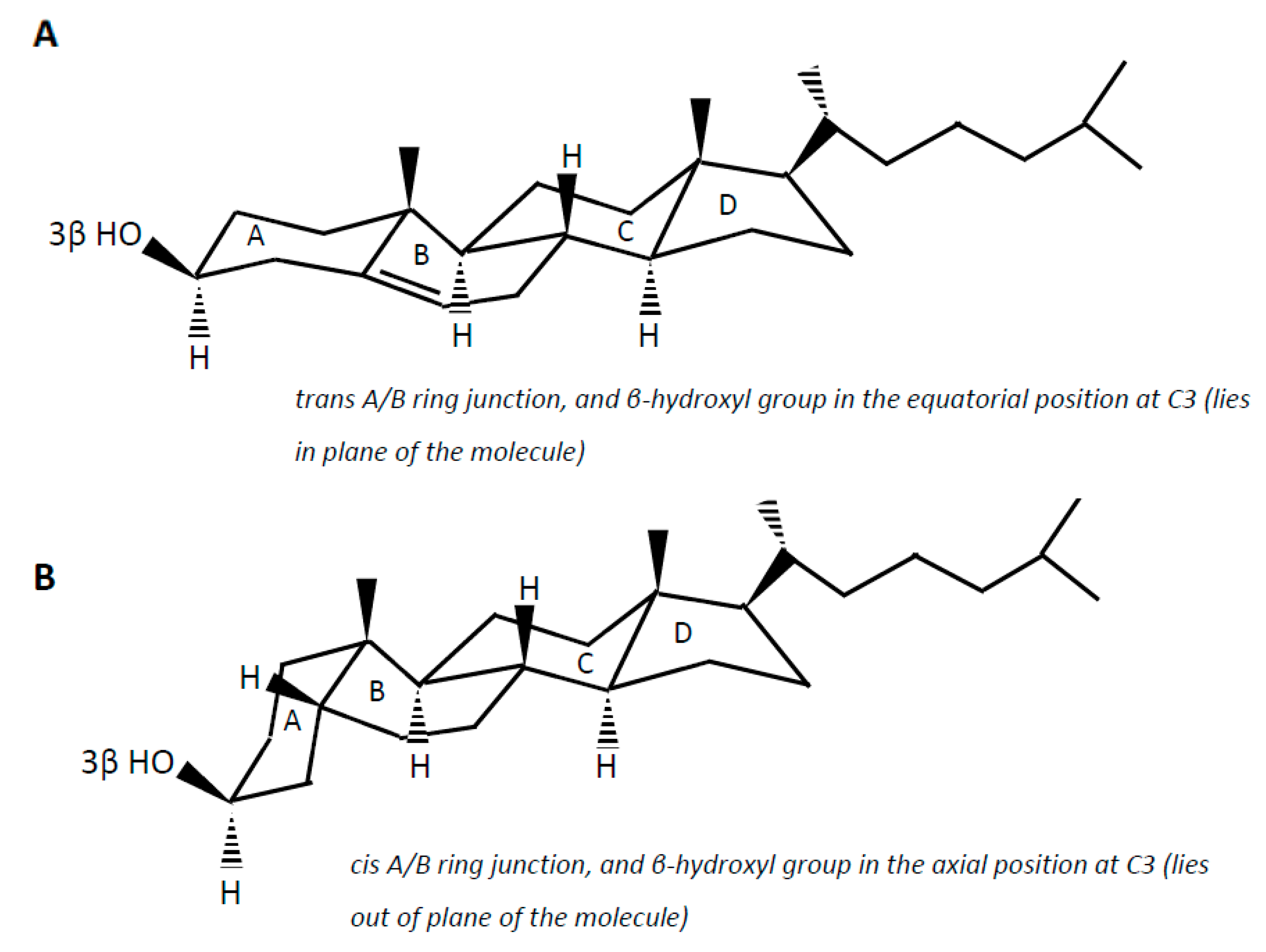
6. Step-by-Step Conversion of Cholesterol to Coprostanol
6.1. From Cholesterol to 4-Cholesten-3-One
6.2. From 4-Cholesten-3-One (Cholestenone) to 5β-Cholestan-3-One (Coprostanone)
6.3. From Coprostanone to Coprostanol
7. From Birth to Elderly
8. Dietary Regulation
8.1. Sugars
8.2. Fatty Acids
8.3. Protein Source and Amino Acids
8.4. Vegetarian Diets and Plant Sterols
8.5. Other Diets
9. Coprostanol Formation and Links to Health and Disease
10. Conclusions
Funding
Conflicts of Interest
References
- Barquera, S.; Pedroza-Tobías, A.; Medina, C.; Hernández-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef]
- Catapano, A.L.; Farnier, M.; Foody, J.M.; Toth, P.P.; Tomassini, J.E.; Brudi, P.; Tershakovec, A.M. Combination therapy in dyslipidemia: Where are we now? Atherosclerosis 2014, 237, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Beitz, D.C. Administration of Cholesterol Reductase to Humans. Patent Application No. 5,436,004, 25 July 1995. [Google Scholar]
- Li, L.; Batt, S.M.; Wannemuehler, M.; Dispirito, A.; Beitz, D.C. Effect of feeding of a cholesterol-reducing bacterium, Eubacterium coprostanoligenes, to germ-free mice. Lab. Anim. Sci. 1998, 48, 253–255. [Google Scholar]
- Beitz, D.C.; Young, J.W.; Dehal, S.S. Method of Converting Cholesterol in Food to Coprostanol. Patent Application No. 4,921,710, 1 May 1990. [Google Scholar]
- Beitz, D.C.; Young, J.W.; Li, L.; Buhman, K.K. Oral Administration of Coprostanol Producing Microorganisms to Humans to Decrease Plasma Cholesterol Concentration. Patent Application No. 5,972,685, 26 October 1999. [Google Scholar]
- Saitoh, C.; Kumazawa, H.; Aisaka, K.; Mizukami, T.; Ando, K.; Ochiai, K.; Katsumata, R.; Ouchi, K. Process for Producing a Cholesterol-Reduced Substance. U.S. Patent TVPP US20010871656, 4 June 2001. [Google Scholar]
- Li, L.; Baumann, C.A.; Meling, D.D.; Sell, J.L.; Beitz, D.C. Effect of orally administered Eubacterium coprostanoligenes ATCC 51222 on plasma cholesterol concentration in laying hens. Poult. Sci. 1996, 75, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Buhman, K.K.; Hartman, P.A.; Beitz, D.C. Hypocholesterolemic effect of Eubacterium coprostanoligenes ATCC 51222 in rabbits. Lett. Appl. Microbiol. 1995, 20, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Groh, H.; Schade, K.; Horhold-Schub, C. Steroid metabolism with intestinal microorganisms. J. Basic Microbiol. 1993, 33, 59–72. [Google Scholar] [CrossRef]
- Li, L. Characterization and Application of Novel Cholesterol-Reducing Anaerobe, Eubacterium Coprostanolignes ATCC 51222. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1995; p. 10957. [Google Scholar]
- Cuevas-Tena, M.; Alegria, A.; Lagarda, M.J. Relationship between dietary sterols and gut microbiota: A review. Eur. J. Lipid Sci. Technol. 2018, 120, 1800054. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Gérard, P. Gastrointestinal tract: Microbial metabolism of steroids. In Health Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids. Handbook of Hydrocarbon and Lipid Microbiology; Goldfine, H., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2020; pp. 389–399. [Google Scholar]
- Molinero, N.; Ruiz, L.; Sanchez, B.; Margolles, A.; Delgado, S. Intestinal bacteria interplay with bile and cholesterol metabolism: Implications on host physiology. Front. Physiol. 2019, 14, 185. [Google Scholar] [CrossRef] [Green Version]
- Kriaa, A.; Bourgin, M.; Potiron, A.; Mkaouar, H.; Jablaoui, A.; Gérard, P.; Maguin, E.; Rhimi, M. Microbial impact on cholesterol and bile acid metabolism: Current status and future prospects. J. Lipid Res. 2019, 60, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Villette, R.; Kc, P.; Beliard, S.; Salas Tapia, M.F.; Rainteau, D.; Guerin, M.; Lesnik, P. Unraveling host-gut microbiota dialogue and its impact on cholesterol levels. Front. Pharmacol. 2020, 11, 278. [Google Scholar] [CrossRef]
- Redinbo, M.R. The microbiome revolution turns to cholesterol. Cell Host Microbe 2020, 28, 154–156. [Google Scholar] [CrossRef]
- Gérard, P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens 2014, 3, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Gérard, P. The crosstalk between the gut microbiota and lipids. OCL 2020, 27, 70. [Google Scholar] [CrossRef]
- Bondzynski, S.; Hunnicki, V. Ueber das schicksal des cholesterins im thierischen organismus. Z. Physiol. Chem. 1897, 22, 396–410. [Google Scholar] [CrossRef]
- Bondzynski, S. Ueber das cholesterin der menschlichen faeces. Ber. Dtsch. Chem. Ges. 1896, 29, 476–478. [Google Scholar] [CrossRef] [Green Version]
- Flint, A. Experimental researches into a new excretory function of the liver, consisting in the removal of cholesterine from the blood and its discharge from the body in the form of stercorine. Am. J. Med Sci. 1862, 11, 305. [Google Scholar] [CrossRef]
- Flint, A. Ueber stercorin. Z. Physiol. Chem. 1897, 23, 363. [Google Scholar] [CrossRef] [Green Version]
- Schoenheimer, R. New contributions in sterol metabolism. Science 1931, 74, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, H.; Gustafsson, B. On serum-cholesterol levels and neutral fecal sterols in germ-free rats. Bile acids and steroids 59. Arch. Biochem. Biophys. 1959, 83, 482–485. [Google Scholar] [CrossRef]
- Evrard, E.; Hoet, P.P.; Eyssen, H.; Charlier, H.; Sacquet, E. Faecal lipids in germ-free and conventional rats. Br. J. Exp. Pathol. 1964, 45, 409–414. [Google Scholar] [PubMed]
- Gustafsson, B.E.; Gustafsson, J.A.; Sjövall, J. Intestinal and fecal sterols in germfree and conventional rats. Bile acids and steroids 172. Acta Chem. Scand. 1966, 20, 1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellogg, T.F.; Wostmann, B.S. Fecal neutral steroids and bile acids from germfree rats. J. Lipid Res. 1969, 10, 495–503. [Google Scholar] [CrossRef]
- Rosenheim, O.; Webster, T.A. The mechanism of coprosterol formation in vivo 2. Its inhibition by succinyl sulphathiazole and by carbarsone. Biochem. J. 1943, 37, 580–585. [Google Scholar] [CrossRef]
- Wainfan, E.; Henkin, G.; Rice, L.I.; Marx, W. Effects of antibacterial drugs on the total cholesterol balance of cholesterol-fed mice. Arch. Biochem. Biophys. 1952, 38, 187–193. [Google Scholar] [CrossRef]
- Midtvedt, T.; Lingaas, E.; Carlstedt-Duke, B.; Höverstad, T.; Midtvedt, A.C.; Saxerholt, H.; Steinbakk, M.; Norin, E. Intestinal microbial conversion of cholesterol to coprostanol in man: Influence of antibiotics. APMIS 1990, 98, 839–844. [Google Scholar] [CrossRef]
- Wilkins, T.D.; Hackman, A.S. Two patterns of neutral steroid conversion in the feces of normal North Americans. Cancer Res. 1974, 34, 2250–2254. [Google Scholar]
- Veiga, P.; Juste, C.; Lepercq, P.; Saunier, K.; Béguet, F.; Gérard, P. Correlation between faecal microbial community structure and cholesterol-to-coprostanol conversion in the human gut. FEMS Microbiol. Lett. 2005, 242, 81–86. [Google Scholar] [CrossRef]
- Rosenfeld, R.S.; Fukushima, D.K.; Hellman, L.; Gallagher, T.F. The transformation of cholesterol to coprostanol. J. Biol. Chem. 1954, 211, 301–311. [Google Scholar] [CrossRef]
- Eyssen, H.; Parmentier, G.; Compernolle, F.C.; de Pauw, G.; Piessens-Denef, M. Biohydrogenation of sterols by Eubacterium ATCC 21,408-nova species. Eur. J. Biochem. 1973, 36, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.; Connor, W.E.; Napton, L.K.; Heizer, R.F. The steroids of 2000-year-old human coprolites. J. Lipid Res. 1978, 19, 215–221. [Google Scholar] [CrossRef]
- Müller, G.; Kanazawa, A.; Teshima, S. Sedimetary record of fecal pollution in part of Lake Constance by coprostanol determination. Naturwissenschaften 1979, 66, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Barlett, P.D. Degradation of coprostanol in an experimental system. Mar. Pollut. Bull. 1987, 18, 27–29. [Google Scholar] [CrossRef]
- Bull, I.D.; Simpson, I.A.; Dockrill, J.; Evershed, R.P. Organic geochemical evidence for the origin of ancient anthropogenic soil deposits at Tofts Ness, Sanday, Orkney. Org. Geochem. 1999, 30, 535–556. [Google Scholar] [CrossRef] [Green Version]
- Evershed, R.P.; Bethell, P.H. Application of multimolecular biomarker techniques to the identification of fecal material in archaeological soils and sediments. Archaeol. Chem. 1996, 625, 157–172. [Google Scholar]
- Bull, I.D.; Lockheart, M.J.; Elhmmali, M.M.; Roberts, D.J.; Evershed, R.P. The origin of faeces by means of biomarker detection. Environ. Int. 2002, 27, 647–654. [Google Scholar] [CrossRef]
- Simpson, I.A.; Bryant, R.G.; Tveraabak, U. Relict soils and early arable land management in Lofoten, Norway. J. Archaeol. Sci. 1998, 25, 1185–1198. [Google Scholar] [CrossRef] [Green Version]
- Simpson, I.A.; van Bergen, P.F.; Perret, V.; Elhmmali, M.M.; Roberts, D.J.; Evershed, R.P. Lipid biomarkers of manuring practice in relict anthropogenicsoils. Holocene 1999, 9, 223–229. [Google Scholar] [CrossRef]
- Bull, I.D. Muck ‘n’molecules: Organic geochemical methods for detecting ancient manuring. Antiquity 1999, 73, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, L.G.S.M.; Wagener, A.L.R.; Carreira, R.S. Geochemistry of fecal sterols in a contaminated estuary in south eastern Brazil. Org. Geochem. 2008, 39, 1097–1103. [Google Scholar] [CrossRef]
- Grimalt, J.O.; Fernandez, P.; Bayona, J.M.; Albaiges, J. Assessment of fecal sterols and ketones as indicators of urban sewage inputs to coastal waters. Environ. Sci. Technol. 1990, 24, 357–363. [Google Scholar] [CrossRef]
- White, A.J.; Stevens, L.R.; Lorenzi, V.; Munoz, S.E.; Schroeder, S.; Cao, A.; Bogdanovich, T. Fecal stanols show simultaneous flooding and seasonal precipitation change correlate with Cahokia’s population decline. Proc. Natl. Acad. Sci. USA 2019, 116, 5461–5466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, A.J.; Stevens, L.R.; Lorenzi, V.; Munoz, S.E.; Lipo, C.P.; Schroeder, S. An evaluation of fecal stanols as indicators of population change at Cahokia, Illinois. J. Archaeol. Sci. 2018, 93, 129–134. [Google Scholar] [CrossRef]
- D’Anjou, R.M.; Bradley, R.S.; Balascio, N.L.; Finkelstein, D.B. Climate impacts on human settlement and agricultural activities in northern Norway revealed through sediment biogeochemistry. Proc. Natl. Acad. Sci. USA 2012, 109, 20332–20337. [Google Scholar] [CrossRef] [Green Version]
- Kirchmer, C.J. 5β-Cholestan-3β-ol: An Indicator of Faecal Pollution. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1971. [Google Scholar]
- Chan, K.H.; Lam, M.H.W.; Poon, K.F.; Yeung, H.Y.; Chiu, T.K.T. Application of sedimentary fecal stanols and sterols in tracing sewage pollution in coastal waters. Water Res. 1998, 32, 225–235. [Google Scholar] [CrossRef]
- Venkatesan, M.I.; Mirsadeghi, F.H. Coprostanol as sewage tracer in McMurdo Sound, Antarctica. Mar. Poll. Bull. 1992, 25, 328–333. [Google Scholar] [CrossRef]
- Nagasawa, M.; Bae, M.; Tamura, G.; Arima, K. Microbial transformation of sterols. Part II. Cleavage of sterol side chains by microorganisms. Agric. Biol. Chem. 1969, 33, 1644–1650. [Google Scholar] [CrossRef]
- Giorgi, V.; Menéndez, P.; García-Carnelli, C. Microbial transformation of cholesterol: Reactions and practical aspects—An update. World J. Microbiol. Biotechnol. 2019, 35, 131. [Google Scholar] [CrossRef]
- Eyssen, H.; Parmentier, G. Biohydrogenation of sterols and fatty acids by the intestinal microflora. Am. J. Clin. Nutr. 1974, 27, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, A.W.; Gottesman, A.R.; Mott, G.E. Isolation and characterization of new strains of cholesterol-reducing bacteria from baboons. Appl. Environ. Microbiol. 1982, 43, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, R.S.; Zumoff, B.; Hellman, L. Metabolism of coprostanol-C14 and cholestanol-4-C14 in man. J. Lipid Res. 1963, 4, 337–340. [Google Scholar] [CrossRef]
- Treadwell, C.R.; Vahouny, G.V. Handbook of Physiology. Alimentary Canal; American Physiological Society: Washington, DC, USA, 1977; Volume Ill, pp. 1407–1438. [Google Scholar]
- Bhattacharyya, A.K. Differences in uptake and esterification of saturated analogues of cholesterol by rat small intestine. Am. J. Physiol. 1986, 251, G495–G500. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Gustafsson, J.A. Mechanism of microbial transformation of cholesterol into coprostanol. Eur. J. Biochem. 1971, 21, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, T.F.; Hellman, L.; Rosenfeld, R.S. The transformation of cholesterol-3d to coprostanol-d. Location of deuterium in coprostanol. J. Biol. Chem. 1956, 222, 321–323. [Google Scholar] [PubMed]
- Rosenfeld, R.A.; Gallagher, T.F. Further studies of the biotransformation of cholesterol to coprostanol. Steroids 1964, 4, 515–520. [Google Scholar] [CrossRef]
- Björkhem, I.; Gustafsson, J.A.; Wrange, O. Microbial transformation of cholesterol into coprostanol. Properties of a 3-oxo-Δ4-steroid-5β-reductase. Eur. J. Biochem. 1973, 37, 143–147. [Google Scholar] [CrossRef]
- Crowther, J.S.; Drasar, B.S.; Goddard, P.; Hill, M.J.; Johnson, K. The effect of a chemically defined diet on the faecal flora and faecal steroid concentration. Gut 1973, 14, 790–793. [Google Scholar] [CrossRef] [Green Version]
- Snog-Kjaer, A.; Prange, I.; Dam, H. Conversion of cholesterol into coprosterol by bacteria in vitro. J. Gen. Microbiol. 1956, 14, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Sadzikowski, M.R.; Sperry, J.F.; Wilkins, T.D. Cholesterol-reducing bacterium from human feces. Appl. Environ. Microbiol. 1977, 34, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Eyssen, H.; Piessens-Denef, M.; Parmentier, G. Role of the cecum in maintaing 5 -steroid- and fatty acid-reducing activity of the rat intestinal microflora. J. Nutr. 1972, 102, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Mott, G.E.; Brinkley, A.W. Plasmenylethanolamine; growth factor for cholesterol-reducing Eubacterium. J. Bacteriol. 1979, 139, 755–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freier, T.A.; Beitz, D.C.; Li, L.; Hartman, P.A. Characterization of Eubacterium coprostanoligenes sp. nov., a cholesterol-reducing anaerobe. Int. J. Syst. Bacteriol. 1994, 44, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Gerard, P.; Lepercq, P.; Leclerc, M.; Gavini, F.; Raibaud, P.; Juste, C. Bacteroides sp. strain D8, the first cholesterol-reducing bacterium isolated from human feces. Appl. Environ. Microbiol. 2007, 73, 5742–5749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, M.E.; Vanay, V.V.; Midtvedt, T.; Norin, K.E. Probiotics in gnotobiotic mice conversion of cholesterol to coprostanol in vitro and in vivo and bile acid deconjugation in vitro. Microb. Ecol. Health Dis. 2000, 12, 219–224. [Google Scholar]
- Lye, H.S.; Rusul, G.; Liong, M.T. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J. Dairy Sci. 2010, 93, 1383–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, A.B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe 2020, 28, 245–257. [Google Scholar] [CrossRef]
- Kreit, J. Microbial catabolism of sterols: Focus on the enzymes that transform the sterol 3β-hydroxy-5-en into3-keto-4-en. FEMS Microbiol. Lett. 2017, 364, fnx007. [Google Scholar] [CrossRef]
- Arima, K.; Nagasawa, M.; Bae, M.; Tamura, G. Microbial transformation of sterols. Part I. Decomposition of cholesterol by microorganisms. Agric. Biol. Chem. 1969, 33, 1636–1643. [Google Scholar]
- Kieslich, K. Microbial side-chain degradation of sterols. J. Basic Microbiol. 1985, 25, 461–474. [Google Scholar] [CrossRef]
- Chiang, Y.R.; Ismail, W.; Heintz, D.; Schaeffer, C.; Van Dorsselaer, A.; Fuchs, G. Study of anoxic and oxic cholesterol metabolism by Sterolibacterium denitrificans. J. Bacteriol. 2008, 190, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Owen, R.W.; Tenneson, M.E.; Bilton, R.F.; Mason, A.N. The degradation of cholesterol by Escherichia coli isolated from human faeces. Biochem. Soc. Trans. 1978, 6, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Kumari, L.; Kanwar, S.S. Purification and characterization of an extracellular cholesterol oxidase of Bacillus subtilis isolated from Tiger excreta. Appl. Biochem. Biotechnol. 2016, 178, 353–367. [Google Scholar] [CrossRef]
- Rost, B. Enzyme function less conserved than anticipated. J. Mol. Biol. 2002, 318, 595–608. [Google Scholar] [CrossRef]
- Tian, W.; Skolnick, J. How well is enzyme function conserved as a function of pairwise sequence identity? J. Mol. Biol. 2003, 333, 863–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanotti, I.; Turroni, F.; Piemontese, A.; Mancabelli, L.; Milani, C.; Viappiani, A.; Prevedini, G.; Sanchez, B.; Margolles, A.; Elviri, L.; et al. Evidence for cholesterol-lowering activity by Bifidobacterium bifidum PRL2010 through gut microbiota modulation. Appl. Microbiol. Biotechnol. 2015, 99, 6813–6829. [Google Scholar] [CrossRef]
- Ren, D.; Li, L.; Schwabacher, A.W.; Young, J.W.; Beitz, D.C. Mechanism of cholesterol reduction to coprostanol by Eubacterium coprostanoligenes ATCC 51222. Steroids 1996, 61, 33–40. [Google Scholar] [CrossRef]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut microbiota and extreme longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [Green Version]
- Rosenheim, O.; Webster, T.A. Precursors of coprosterol and the bile acids in the animal organism. Nature 1935, 136, 474. [Google Scholar] [CrossRef]
- Bergström, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Mølgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of intestinal microbiota during early life: A longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 2014, 80, 2889–2900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Rodriguez, J.T.; Woodward, W.E.; Nichols, B.L. Comparison of patterns of fecal bile acid and neutral sterol between children and adults. Am. J. Clin. Nutr. 1976, 29, 1196–1203. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.T.; Levine, M.M.; Woodward, W.E.; Gopalakrishna, G.S.; Nalin, D.R.; Hornick, R.B.; Nichols, B.L. Biohydrogenation of cholesterol as an index of bacterial 7α-dehydroxylase activity. Lipids 1981, 16, 670–676. [Google Scholar] [CrossRef]
- Gustafsson, J.A.; Werner, B. Fecal sterols in infants. Bile acids and steroids 194. Acta Physiol. Scand. 1968, 75, 305–310. [Google Scholar]
- Ponz de Leon, M.; Roncucci, L.; di Donato, P.; Sacchetti, C.; Pezcoller, C.; Annoni, C.; Bertani, C.; Rebecchi, P.; Balli, F.; Galli, D.; et al. Fecal neutral steroids in normal conditions and in patients with polyps or cancer of the large bowel. Cancer Res. 1987, 47, 305–310. [Google Scholar]
- Midtvedt, A.C.; Midtvedt, T. Conversion of cholesterol to coprostanol by the intestinal microflora during the first two years of human life. J. Pediatr. Gastroenterol. Nutr. 1993, 17, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, T.; Stenhammar, L.; Tjellström, B.; Magnusson, K.; Midtvedt, T.; Norin, E.; Högberg, L. Evidence of disturbed gut microbial metabolic activity in pediatric Crohn’s disease. Crohn’s Colitis 2019, 360, otz010. [Google Scholar] [CrossRef]
- Korpela, J.T.; Hämäläinen, E.; Adlercreutz, H. Effect of oxytetracycline on bacterial intestinal metabolism of neutral sterols and on serum lipids. Scand. J. Gastroenterol. 1984, 19, 401–404. [Google Scholar] [CrossRef]
- Salyers, A.A.; Sperry, J.F.; Wilkins, T.D.; Walker, A.R.; Richardson, N.J. Neutral steroid concentrations in the faeces of North American white and South African black populations at different risks for cancer of the colon. S. Afr. Med. J. 1977, 51, 823–827. [Google Scholar] [PubMed]
- Midtvedt, T.; Fredrichsen, P. Influence of antibiotics on microbial intestinal transformation of cholesterol to coprostanol in man. Scand. J. Gastroenterol. 1977, 12, 669–672. [Google Scholar] [CrossRef]
- Benno, P.; Alam, M.; Henriksson, K.; Norin, E.; Uribe, A.; Midtvedt, T. Abnormal colonic microbial function in patients with rheumatoid arthritis. Scand. J. Rheumatol. 1994, 23, 311–315. [Google Scholar] [CrossRef]
- Norin, E. Intestinal cholesterol conversion in adults and elderly from four different European countries. Ann. Nutr. Metab. 2008, 52, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Gérard, P.; Béguet, F.; Lepercq, P.; Rigottier-Gois, L.; Rochet, V.; Andrieux, C.; Juste, C. Gnotobiotic rats harboring human intestinal microbiota as a model for studying cholesterol-to-coprostanol conversion. FEMS Microbiol. Ecol. 2004, 47, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Antharam, V.C.; McEwen, D.C.; Garrett, T.J.; Dossey, A.T.; Li, E.C.; Kozlov, A.N.; Mesbah, Z.; Wang, G.P. An integrated metabolomic and microbiome analysis identified specific gut microbiota associated with fecal cholesterol and coprostanol in Clostridium difficile Infection. PLoS ONE 2016, 11, e0148824. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef]
- Wells, W.W.; Cooper, S.B. Coprostanol formation. II. The opposing action of dietary lactose and calcium on cholesterol absorption. Arch. Biochem. Biophys. 1958, 75, 273–279. [Google Scholar]
- Subbiah, M.T.R.; Naylor, M.C.; Schumacher, J.; Kottke, B.A. 5β-Reduction of [14C]cholesterol by human feces in vitro: Nonspecific inhibition by sugars. Experientia 1974, 30, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D. The effect of dietary fatty acids on coprostanol excretion bv the rat. J. Lipid Res. 1961, 2, 350–356. [Google Scholar] [CrossRef]
- Froyen, E.; Burns-Whitmore, B. The effects of linoleic acid consumption on lipid risk markers for cardiovascular disease in healthy individuals: A review of human intervention trials. Nutrients 2020, 12, 2329. [Google Scholar] [CrossRef] [PubMed]
- Catala, I.; Juste, C.; Boehler, N.; Férézou, J.; André, M.; Riottot, M.; Lutton, C.; Lafont, H.; Bornet, F.; Corring, T. Cholesterol crystallization in gall-bladder bile of pigs given cholesterol–beta-cyclodextrin-enriched diets with either casein or soyabean concentrate as protein sources. Br. J. Nutr. 2000, 83, 411–420. [Google Scholar] [PubMed]
- Tanaka, C.; Nozaki, Y. Effect of partial hydrolysates of casein and soybean protein on serum lipoproteins and fecal neutral steroids. J. Nutr. Sci. Vitaminol. 1983, 29, 439–446. [Google Scholar] [CrossRef]
- Scholz, K.E.; Kinder, E.; Hagemeister, H.; Barth, C.A. Influence of dietary casein and soy protein isolate on intestinal cholesterol and bile acid concentration. Z. Ernahrungswiss. 1985, 24, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Tomotake, H.; Shimaoka, I.; Kayashita, J.; Yokoyama, F.; Nakajoh, M.; Kato, N. A buckwheat protein product suppresses gallstone formation and plasm cholesterol more strongly than soy protein isolate in hamsters. J. Nutr. 2000, 130, 1670–1674. [Google Scholar] [CrossRef]
- Oda, H.; Fukui, H.; Hitomi, Y.; Yoshida, A. Alteration of serum lipoprotein metabolism by polychlorinated biphenyls and methionine in rats fed a soybean protein diet. J. Nutr. 1991, 121, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Oh-hashi, A.; Takei, K.; Ikai, M.; Kasaoka, S.; Kiriyama, S. Cholesterol-lowering effects of soybean, potato and rice proteins depend on their low methionine contents in rats fed a cholesterol-free purified diet. J. Nutr. 1997, 127, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, K.; Kushima, Y.; Muramatsu, K. Effects of sulfur-containing amino acids and glycine on plasma cholesterol level in rats fed on a high cholesterol diet. Agric. Biol. Chem. 1985, 49, 3455–3461. [Google Scholar]
- Gudbrandsen, O.A.; Wergedahl, H.; Liaset, B.; Espe, M.; Berge, R.K. Dietary proteins with high isoflavone content or low methionine–glycine and lysine–arginine ratios are hypocholesterolaemic and lower the plasma homocysteine level in male Zucker fa/fa rats. Br. J. Nutr. 2005, 94, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Fukada, S.; Shimada, Y.; Morita, T.; Sugiyama, K. Suppression of methionine-induced hyperhomocysteinemia by glycine and serine in rats. Biosci. Biotechnol. Biochem. 2006, 70, 2403–2409. [Google Scholar] [CrossRef] [Green Version]
- Korpela, J.T.; Adlercreutz, H. Fecal neutral sterols in omnivorous and vegetarian women. Scand. J. Gastroenterol. 1985, 20, 1180–1184. [Google Scholar] [CrossRef]
- van Faassen, A.; Bol, J.; van Dokkum, W.; Pikaar, N.A.; Ockhulzen, T.; Hermus, J.J. bile acids, neutral steroids, and bacteria in feces as affected by a mied, lacto-ovovegetarian, and a vegan diet. Am. J. Clin. Nutr. 1987, 46, 962–967. [Google Scholar] [CrossRef]
- Cuevas-Tena, M.; Bermudez, J.; de los Angeles Silvestre, R.; Alegría, A.; Lagarda, M.J. Impact of colonic fermentation on sterols after the intake of a plant sterol-enriched beverage: A randomized, double-blind crossover trial. Clin. Nutr. 2019, 38, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Weststrate, J.A.; Ayesh, R.; Bauer-Plank, C.; Drewitt, P.N. Safety evaluation of phytosterol esters. Part 4. Faecal concentrations of bile acids and neutral sterols in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food Chem. Toxicol. 1999, 37, 1063–1071. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, J.; He, Z.; Hao, W.; Liu, J.; Kwek, E.; Ma, K.Y.; Bi, Y. Plasma cholesterol-lowering activity of soybean germ phytosterols. Nutrients 2019, 11, 2784. [Google Scholar] [CrossRef] [Green Version]
- Férézou, J.; Riottot, M.; Sérougne, C.; Cohen-Solal, C.; Catala, I.; Alquier, C.; Parquet, M.; Juste, C.; Lafont, H.; Mathé, D.; et al. Hypocholesterolemic action of beta-cyclodextrin and its effects on cholesterol metabolism in pigs fed a cholesterol-enriched diet. J. Lipid Res. 1997, 38, 86–100. [Google Scholar] [CrossRef]
- Sulpice, J.C.; Férézou, J.; Lutton, C.; Mathé, D.; Chevallier, F. Diet and sterol biohydrogenation in the rat: Occurrence of epicoprostanol. Lipids 1978, 13, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Wynder, E.L. Metabolic epidemiology of colon cancer: Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer 1977, 39, 2533–2539. [Google Scholar] [CrossRef]
- Cruse, J.P.; Lewin, M.R.; Ferulano, G.P.; Clark, C.G. Cocarcinogenic effects of dietary cholesterol in experimental colon cancer. Nature 1978, 276, 822–825. [Google Scholar] [CrossRef]
- Nomura, A.M.; Wilkins, T.D.; Kamiyama, S.; Heilbrun, L.K.; Shimada, A.; Stemmermann, G.N.; Mower, H.F. Fecal neutral steroids in two Japanese populations with different colon cancer risks. Cancer Res. 1983, 43, 1910–1913. [Google Scholar]
- Mastromarino, A.; Reddy, B.S.; Wynder, E.L. Metabolic epidemiology of colon cancer: Enzymic activity of fecal flora. Am. J. Clin. Nutr. 1976, 29, 1455–1460. [Google Scholar] [CrossRef] [Green Version]
- Peuchant, E.; Salles, C.; Jensen, R. Relationship between fecal neutral steroid concentrations and malignancy in colon cells. Cancer 1987, 60, 994–999. [Google Scholar] [CrossRef]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Macé, K.; Chou, C.J. Germfree C57BL/6J mice are resistant to high fat diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar]
- Le Roy, T.; Lécuyer, E.; Chassaing, B.; Rhimi, M.; Lhomme, M.; Boudebbouze, S.; Ichou, F.; Haro Barceló, J.; Huby, T.; Guerin, M.; et al. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019, 17, 94. [Google Scholar] [CrossRef] [Green Version]
- Bourgin, M.; Labarthe, S.; Kriaa, A.; Lhomme, M.; Gérard, P.; Lesnik, P.; Laroche, B.; Maguin, E.; Rhimi, M. Exploring the bacterial impact on cholesterol cycle: A numerical study. Front. Microbiol. 2020, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Sekimoto, H.; Shimada, O.; Makanishi, M.; Nakano, T.; Katayama, O. Interrelationship between serum and fecal sterols. Jpn. J. Med. 1983, 22, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vors, C.; Joumard-Cubizolles, L.; Lecomte, M.; Combe, E.; Ouchchane, L.; Drai, J.; Raynal, K.; Joffre, F.; Meiller, L.; Le Barz, M.; et al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: Towards a gut sphingomyelin-cholesterol interplay. Gut 2020, 69, 487–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Strains | Isolated from | No. Strains Tested | No. Strains Able to Reduce Cholesterol to Coprostanol | Reference | Comment |
|---|---|---|---|---|---|
| E. coli Streptococcus faecalis1 Clostridium spp. Bacteroides spp. Bifidobacterium spp. | Human feces | 20 20 20 18 12 | 0 0 9 12 9 | [65] | No strain characterization Not pursued Stains lost |
| Eubacterium 403 | Baboon feces | _ | One isolate | [69] | Confirmed (59) Strain lost |
| Eubacterium ATCC 21408 | Rat caecal contents | _ | One isolate | [36] | Confirmed (59) Strain lost |
| Similar to Eubacterium 21408 | Feces and intestinal contents of baboons | _ | Nine isolates | [57] | Not pursued Strains lost |
| Eubacterium ATCC 51222 | Hog sewage lagoon | _ | One isolate | [70] | Confirmed Available at ATCC |
| Bacteroides sp. strain D8 | Human donor | _ | One isolate | [71] | Confirmed Available upon request in our laboratory |
| Lactobacilli L. acidophilus ATCC 314 L. acidophlus FTCC 0291 L. bulgaricus FTCC 0411 L. bulgaricus FTDC 1311 L. casei ATCC 393 | Originally isolated from the human gastrointestinal tract | 15 | 5 | [73] | Not pursued Not reproduced Available at ATCC and the Culture Collection Center of Universiti Sains Malaysia (Penang, Malaysia) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juste, C.; Gérard, P. Cholesterol-to-Coprostanol Conversion by the Gut Microbiota: What We Know, Suspect, and Ignore. Microorganisms 2021, 9, 1881. https://doi.org/10.3390/microorganisms9091881
Juste C, Gérard P. Cholesterol-to-Coprostanol Conversion by the Gut Microbiota: What We Know, Suspect, and Ignore. Microorganisms. 2021; 9(9):1881. https://doi.org/10.3390/microorganisms9091881
Chicago/Turabian StyleJuste, Catherine, and Philippe Gérard. 2021. "Cholesterol-to-Coprostanol Conversion by the Gut Microbiota: What We Know, Suspect, and Ignore" Microorganisms 9, no. 9: 1881. https://doi.org/10.3390/microorganisms9091881







