Diatoms in Volcanic Soils of Mutnovsky and Gorely Volcanoes (Kamchatka Peninsula, Russia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Area
| Mutnovsky Volcano | Gorely Volcano | |
|---|---|---|
| Volcanic composition and chemical content of the rocks | Basalts and basaltic andesites with relatively low K2O and Na2O and high FeO* and Al2O3 two-peroxene dacites, composed mainly of low-potassium and calc-alkaline basalts. In terms of SiO2–K2O relations, they belong to the low- and moderate-potassium varieties of the calc-alkaline series, plotting along with the tholeiitic–calc-alkaline series; in terms of the alumina index, they are moderately aluminous rocks. The young basalts are enriched in MgO and CaO but differ in low contents of SiO2, TiO2, Al2O3, and Na2O. | Basalts and andesitodacites with low K2O, high contents of CaO, TiO2, and total iron, with a predominance of intermediate basaltic andesite rocks. All varieties of young volcanic rocks have elevated K2O contents and correspond to the high potassium calc-alkaline series, with normal alkalinity. Some lavas of the youngest eruptions have elevated alkali contents and correspond to the subalkaline series. Most lavas of the riftogenic zone are clustered around the dividing line of the calc-alkaline–tholeiitic series. |
| pH of constituent rocks | 10–15% of acid-medium rocks | Acid andesites |
| Last eruption | March 2000 | Summer 2010 |
| Concentration of SiO2 | 48–70% | 51–57% |
| Tephra | Gravel and lapilli of dense andesite with interlayers of yellowish silty sands, thin ashes with the inclusion of larger grains of sand and gravel | Black-gray volcanic sand and slag |
2.2. Sample Collection
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taxon | 1 | 2 | Habitat * | Salinity Tolerance * | pH * | Distribution * | Ecological Group * |
|---|---|---|---|---|---|---|---|
| Achnanthes linearis var. pusilla Grunow | 2 | Ep | i | al | c | Freshwater | |
| Adlafia aquaeductae (Krasske) Lange-Bertalot | 3 | B | hl | i | c | Freshwater | |
| Caloneis bacillum (Grunow) Cleve | 6 | 15 | B | i | i | c | Marine, brackish, freshwater, terrestrial |
| Caloneis dubia Krammer | 1 | B | hb | i | c | Freshwater | |
| Caloneis lancettula (Schulz) Lange-Bertalot et Witkowski | 1 | B | i | al | c | Freshwater, terrestrial | |
| Diatoma tenuis Agardh | 3 | P B | hl | al | c | Freshwater | |
| Diatoma vulgaris Bory | 1 | B Ep | i | alb | c | Freshwater | |
| Eunotia bilunaris (Ehrenberg) Schaarschmidt | 1 | L Ep | hb | ac | c | Freshwater | |
| Eunotia curtagrunowii Nörpel-Schempp et Lange-Bertalot | 15 | 1 | L Ep | hb | ac | aa | Freshwater |
| Eunotia fallax A. Cleve | 10 | Ep | hb | ac | c | Freshwater | |
| Eunotia paludosa Grunow | 2 | Ep | hb | ac | aa | Freshwater | |
| Eunotia sudetica f. minor Manguin in Bourrelly et Manguin | 3 | Ep | hb | ac | aa | Freshwater | |
| Fragilariforma virescens var. exigua (Grunow) M.Poulin | 2 | L Ep | i | i | aa | Freshwater | |
| Gomphonema parvulum (Kützing) Kützing | 1 | Ep | i | i | c | Marine, freshwater | |
| Hantzschia amphioxys (Ehrenberg) Grunow | 2 | B | i | al | c | Marine, freshwater, terrestrial | |
| Humidophila contenta (Grunow) R.L.Lowe, Kociolek, J.R.Johansen, Van de Vijver, Lange-Bertalot et Kopalová | 15 | B Ep | i | al | c | Freshwater, terrestrial | |
| Luticola mutica (Kützing) D.G.Mann | 6 | B Ep | hl | al | c | Freshwater, terrestrial | |
| Muelleria gibbula (Cleve) S.A. Spaulding et E.F. Stoermer | 4 | B | i | al | c | Freshwater | |
| Navicula cincta (Ehrenberg) Ralfs | 3 | B Ep | hl | al | c | Brackish, freshwater, terrestrial | |
| Nitzschia cf. ovalis H.J.Arnott | 1 | B | i | alb | c | Marine, brackish, freshwater, terrestrial | |
| Nitzschia palea (Kützing) W.Smith | 4 | B | i | al | c | Freshwater | |
| Pinnularia borealis Ehrenberg | 15 | 15 | B | i | ac | c | Freshwater, terrestrial |
| Pinnularia intermedia (Lagerstedt) Cleve | 2 | 1 | B | i | al | aa | Freshwater |
| Pinnularia microstauron (Ehrenberg) Cleve | 1 | 2 | B | i | i | c | Freshwater, terrestrial |
| Pinnularia cf. subcapitata W. Gregory | 7 | 12 | B | hb | ac | c | Freshwater, terrestrial |
| Pinnularia sp1. | 1 | B | i | i | c | Marine, brackish, freshwater, terrestrial | |
| Pinnularia sp.2 | 1 | B | i | i | c | Marine, brackish, freshwater, terrestrial | |
| Pinnularia sp.3 | 3 | B | i | i | c | Marine, brackish, freshwater, terrestrial | |
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 1 | Ep | i | al | c | Freshwater, terrestrial | |
| Platessa oblongella (Østrup) C.E.Wetzel, Lange-Bertalot and Ector | 2 | Ep | hb | i | c | Freshwater | |
| Psammothidium ventrale (Krasske) Bukhtiyarova and Round | 4 | B | i | i | b | Freshwater | |
| Sellaphora bacillum (Ehrenberg) D.G.Mann | 1 | B | i | al | c | Freshwater | |
| Sellaphora mutata (Krasske) Lange-Bertalot | 1 | B | hl | i | c | Freshwater | |
| Sellaphora pupula (Kützing) Mereschkovsky | 3 | B | hl | i | c | Freshwater | |
| Sellaphora submuralis (Hustedt) C.E.Wetzel, L.Ector, B.Van de Vijver, Compère et D.G.Mann | 1 | B | i | i | b | Freshwater | |
| Stauroneis anceps Ehrenberg | 1 | B | i | i | c | Freshwater | |
| Staurosirella pinnata (Ehrenberg) D.M.Williams et Round | 10 | L | hl | al | c | Marine, freshwater | |
| Tabularia fasciculata (C.Agardh) D.M.Williams et Round | 1 | Ep L | mh | al | c | Marine, brackish, freshwater | |
| 37 | 35 | 9 |
References
- Dingwell, D.B.; Lavallée, Y.; Kueppers, U. Volcanic ash: A primary agent in the Earth system. Phys. Chem. Earth 2012, 45–46, 2–4. [Google Scholar] [CrossRef]
- Crisafulli, C.M.; Swanson, F.J.; Halvorson, J.J.; Clarkson, B.D. Volcano ecology: Disturbance characteristics and assembly of biological communities. In The Encyclopedia of Volcanoes, 2nd ed.; Sigurdsson, H., Houghton, B., McNutt, S., Rymer, H., Stix, J., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 1265–1284. [Google Scholar]
- Schwabe, G.H. Blue-green algae as pioneers on post-volcanic substrate (Surtsey/Iceland). In Proceedings of the First International Symposium on Taxonomy and Biology of Blue-green Algae, Madras, India, 8–13 January 1972; pp. 419–424. [Google Scholar]
- Schwabe, G.H. Nitrogen fixing blue-green algae as pioneer plants on Surtsey 1968–1973. Surtsey Res. Progr. Rep. 1974, 7, 22–25. [Google Scholar]
- Shtina, E.A.; Andreyeva, V.M.; Kuzyakina, T.I. Algae settlement of volcanic substrates. Botanicheskiy Zhurnal 1992, 8, 33–42. [Google Scholar]
- Treub, M. Notice sur la nouvelle flore de Krakatau. Annales du Jardin Botanique de Buitenzorg 1888, 7, 213–223. [Google Scholar]
- Schwabe, G.H. On the algal settlement in craters on Surtsey during summer 1968. Surtsey Res. Progr. Rep. 1970, 5, 51–55. [Google Scholar]
- Schwabe, G.H.; Behre, K. Ökogenese der Insel Surtsey 1968–1970. Naturwiss. Rundschau. 1971, 24, 513–519. [Google Scholar]
- Henriksson, E. Algal nitrogen fixation in temperate regions. Plant Soil 1971, 35, 415–419. [Google Scholar] [CrossRef]
- Henriksson, L.E.; Enekell, P.H.; Henriksson, E. Determination of the nitrogen-fixing capacity of algae in soil. Oikos 1972, 23, 420–423. [Google Scholar] [CrossRef]
- Brock, T.D. Primary colonization of Surtsey, with special reference to the blue-green algae. Oikos 1973, 24, 239–243. [Google Scholar] [CrossRef]
- Behre, K.; Schwabe, G.H. Auf Surtsey/Island im Sommer 1968 Nachgewiesene nicht Marine Algen; Schriften Natturwiss Vereins: Schleswig, Germany, 1970; pp. 31–100, Sonderband. [Google Scholar]
- Carson, J.L.; Brown, R.M. Studies of Hawaiian freshwater and soil algae II. Algal colonization and succession on a dated volcanic substrate. J. Phycol. 1978, 14, 171–178. [Google Scholar] [CrossRef]
- Bölter, M.; Blume, H.P.; Kuhn, D. Soils and their microbiological properties from a transect from Cape Horn to the Antarctic Peninsula. Polar Biosci. 1999, 12, 54–67. [Google Scholar]
- Fermani, P.; Mataloni, G.; de Vijver, B.V. Soil microalgal communities on an Antarctic active volcano (Deception Island, South Shetlands). Polar Biol. 2007, 30, 1381–1393. [Google Scholar] [CrossRef]
- Pimenov, E.P. Function of Microorganism Complexes of in the Intensive Volcanic Ashfall Zone. Ph.D. Thesis, Institute of Mikrobiology and Virollogy of Akademy Nauk of KazSSR, Alma-Ata, Kazakhstan, 1983. [Google Scholar]
- Kuzyakina, T.I. Transformation of volcanic ash by microorganisms. In Volcanism and Associated Processes; Dalnauka: Petropavlovsk-Kamchatski, Russia, 1985; pp. 232–234. [Google Scholar]
- Nanzyo, M. Unique properties of volcanic ash soils. Global environmental research. Assoc. Int. Res. Initiat. Environ. Stud. 2002, 6, 99–112. [Google Scholar]
- Hu, C.; Gao, K.; Whitton, B.A. Semi-Arid regions and deserts. In Ecology of Cyanobacteria II: Their Diversity in Space and Time, 2nd ed.; Whitton, B.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 345–369. [Google Scholar]
- Cuadros, J.; Spiro, B.; Dubbin, W.; Jadubansa, P. Rapid microbial stabilization of unconsolidated sediment against wind erosion and dust generation. J. Soils Sediments 2010, 10, 1415–1426. [Google Scholar] [CrossRef]
- Larionov, I.A.; Marapulets, Y.V.; Shevtsov, B.M. Features of the earth surface deformations in the Kamchatka peninsula and their relation to geoacoustic emission. Solid Earth 2014, 5, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Levin, V.; Droznina, S.; Gavrilenko, M.; Carr, M.J.; Senyukov, S. Seismically active subcrustal magma source of the Klyuchevskoy volcano in Kamchatka, Russia. Geology 2014, 42, 983–986. [Google Scholar] [CrossRef] [Green Version]
- Marapulets, Y.; Rulenko, O. Joint anomalies of high-frequency geoacoustic emission and atmospheric electric field by the ground–atmosphere boundary in a seismically active region (Kamchatka). Atmosphere 2019, 10, 267. [Google Scholar] [CrossRef]
- White, J.D.L.; Houghton, B.F. Primary volcaniclastic rocks. Geology 2006, 34, 677–680. [Google Scholar] [CrossRef]
- Kalitina, E.G.; Nikulina, T.V.; Kharitonova, N.A.; Wah, E.A. Materials for the study of the diversity of microorganisms in the thermal springs of Kamchatka (Russia). In Proceedings of the All Russian Conference with International Participation “Modern Problems of Hydrogeology, Engineering Geology and Hydrogeoecology Eurasia” with Elements of a Scientific School, Tomsk, Russia, 23–27 November 2015; pp. 510–513. (In Russian). [Google Scholar]
- Nikulina, T.V.; Kalitina, E.G.; Vakh, E.A.; Kharitonova, N.A. List of diatoms from three hot springs from Kamchatka—Malkinskiye, Nachikinskiye and Verhne-Paratunskiye (Russia). In Freshwater Life; Bogatov, V.V., Ed.; Dalnauka: Vladivostok, Russia, 2016; Volume 2, pp. 108–115. (In Russian) [Google Scholar]
- Nikulina, T.V.; Grishchenko, O.V. Diatom flora of Dachnye Thermal Springs (Kamchatka Peninsula, Russia). In Vladimir Yakovlevic Levanidov’s Biennial Memorial Meetings; FNC Bioraznobrazia: Vladivostok, Russia, 2017; Volume 7, pp. 185–193. (In Russian) [Google Scholar]
- Fazlutdinova, A.I.; Gabidullin, Y.Z.; Allaguvatova, R.Z.; Gaysina, L.A. Diatoms in Kamchatka’s Hot Spring Soils. Diversity 2020, 12, 435. [Google Scholar] [CrossRef]
- Kuzyakina, T.I. Ecology and Geochemical Activity of Microorganisms on Active Volcanoes and Hydrothermal Waters (Kunashir Island, Kuril Islands Kamchatka); Dalnauka: Vladivostok, Russia, 2004; pp. 1–251. [Google Scholar]
- Abdullin, S. Cyanobacteriae and algae of lava tubes in Kamchatka, Russia. Cave Karst Sci. 2013, 40, 141–144. [Google Scholar]
- Chashchin, A.A.; Martynov, Y.A.; Perepelov, A.B.; Ekimova, N.I.; Vladimirova, T.P. Physical and chemical conditions of the formation and evolution of late pleistocene-holocene magmas of the Gorely and Mutnovsky volcanoes, southern Kamchatka. Russ. J. Pac. Geol. 2011, 5, 348–367. [Google Scholar] [CrossRef]
- Calabrese, S.; Scaglione, S.; D’Alessandro, W.; Brusca, L.; Bellomo, S.; Parello, F. A literature review and new data of trace metals fluxes from worldwide active volcanoes. In Miscelanea INGV, Proceedings of the Conferenza, A. Rittmann, Nicolosi, Catania, Italy, 12–14 December 2012; Corsaro, R.A., Ed.; Istituto Nazionale di Geofisica e Vulcanologia: Catania, Italy, 2012; pp. 41–42. [Google Scholar]
- Duggen, S.; Portnyagin, M.; Baker, J.; Ulfbeck, D.; Hoernle, K.; Garbe-Schönberg, D.; Grassineau, N. Drastic shift in lava geochemistry in the volcanic-front to rear-arc region of the Southern Kamchatkan subduction zone: Evidence for the transition from slab surface dehydration to sediment melting. Geochimica Cosmochimica Acta 2007, 71, 452–480. [Google Scholar] [CrossRef]
- Volynets, O.N.; Babanskii, A.D.; Gol’tsman, Y.V. Variations in isotopic and trace-element composition of lavas from volcanoes of the Northern Group, Kamchatka, in relation to specific features of subduction. Geochem. Int. 2000, 38, 974–989. [Google Scholar]
- Ishikawa, T.; Tera, F.; Nakazawa, T. Boron isotope and trace element systematics of the three volcanic zones in the Kamchatka arc. Geochimica Cosmochimica Acta 2001, 65, 4523–4537. [Google Scholar] [CrossRef]
- Avdeiko, G.P.; Savelyev, D.P.; Palueva, A.A.; Popruzhenko, S.V. Evolution of the Kurile-Kamchatkan volcanic arcs and dynamics of the Kamchatka-Aleutian Junction. In Volcanism and Subduction: The Kamchatka Region; Geophysical Monograph Series; Eichelberger, J., Ed.; AGU: Washington, DC, USA, 2007; Volume 172, pp. 37–55. [Google Scholar]
- Gavrilenko, M.; Ozerov, A.; Kyle, P.R.; Carr, M.J.; Nikulin, A.; Vidito, C.; Danyushevsky, L. Abrupt transition from fractional crystallization to magma mixing at Gorely volcano (Kamchatka) after caldera collapse. Bull. Volcanol. 2016, 78, 47. [Google Scholar] [CrossRef] [Green Version]
- Vereina, O.B. Natural state modeling of the Mutnovsky geothermal field, Kamchatka, Russia. In Proceedings of the Geothermal training, Reykjavik, Iceland, 14–17 September 2003; pp. 505–526. [Google Scholar]
- Sugrobov, V.M. Geothermal and Geochemical Investigations of High-Temperature Hydrothermal Systems (by the Example of Mutnovsky Geothermal Field); Nauka: Moscow, Russia, 1986; p. 305. (In Russian) [Google Scholar]
- Assaulov, S.G. A conceptual model and reservoir assessment for the Mutnovsky geothermal field, Kamchatka, Russia. In Proceedings of the Geothermal Training, Reykjavik, Iceland; 1994; pp. 1–30. [Google Scholar]
- Fedotov, S.A.; Ozerov, A.Y.; Magus’kin, M.A. The 1998–2000 Eruption of Karymskii Volcano, the Related Seismic, Geodynamic, and Postvolcanic Processes and Their Impact on the Environment. In Catastrophic Processes and their Impact on the Natural Environment: Volcanism; Laverov, N.P., Ed.; Regional’naya Obshchestvennaya Organizatsiya Uchenykh po Problemam Prikladnoi Geofiziki: Moscow, Russia, 2002; pp. 117–160. [Google Scholar]
- Kondratyuk, V.I. Climate of Kamchatka; Hydrometeoizdat: Moscow, Russia, 1974; p. 204. (In Russian) [Google Scholar]
- Neshataeva, V.Y. Vegetation of the Kamchatka Peninsula; KMK: Moscow, Russia, 2009; p. 537. (In Russian) [Google Scholar]
- Karpachevsky, L.O.; Alyabyina, I.O.; Zakharikhina, L.V.; Makeev, A.O.; Merechek, M.S.; Radyukin, A.Y.U.; Shoba, S.A. Kamchatka Soils; GEOS: Moscow, Russia, 2009; p. 224. (In Russian) [Google Scholar]
- Zharikova, E.A. Potential potassium buffer capacity of Kamchatka volcanic soils. Eurasian Soil Sci. 2011, 44, 493–499. [Google Scholar] [CrossRef]
- Zakharikhina, L.V.; Litvinenko, Y.S. Geochemical specificity of volcanic soils of Kamchatka. Eurasian Soil Sci. 2011, 43, 380–389. [Google Scholar] [CrossRef]
- Sokolov, I.A. Volcanic Activity and Soil Generation (in Kamchatka); Nauka: Moscow, Russia, 1973; p. 224. (In Russian) [Google Scholar]
- Neshataeva, V.Y. Vegetable cover of Kamchatka Peninsula and its geobotanical regionalization. Proc. Karelian Res. Cent. RAS 2011, 1, 3–22. (In Russian) [Google Scholar]
- Simon, A.; Yogodzinski, G.M.; Robertson, K.; Smith, E.; Selyangin, O.; Kiryukhin, A.; Mulcahy, S.R.; Walker, J.D. Evolution and genesis of volcanic rocks from Mutnovsky Volcano, Kamchatka. J. Volcanol. Geotherm. Res. 2014, 286, 116–137. [Google Scholar] [CrossRef]
- Semenov, V.I. the Edge of Hot Springs; Far Eastern Book Publishing House: Petropavlovsk-Kamchatsky, Russia, 1988; p. 142. (In Russian) [Google Scholar]
- Jakes, P.; Gill, J. Rare earth elements and the island arc tholeiitic series. Earth Planet. Sci. Lett. 1970, 9, 17–28. [Google Scholar] [CrossRef]
- Kuznetsov, P.Y.; Koulakov, I.; Jakovlev, A.; Abkadyrov, I.; Deev, E.; Gordeev, E.; Senyukov, S.; El Khrepy, S.; Al Arifi, N. Structure of volatile conduits beneath Gorely Volcano (Kamchatka) revealed by local earthquake tomography. Geosciences 2017, 7, 111. [Google Scholar] [CrossRef] [Green Version]
- Selyangin, O.B.; Ponomareva, V.V. Gorelovsky volcanic center, South Kamchatka: Structure and evolution. Volcanol. Seismol. 1999, 21, 163–194. [Google Scholar]
- Kirsanov, I.T.; Melekestsev, I.V. Gorely volcano. In Active Volcanoes of Kamchatka; Nauka: Moscow, Russia, 1991; Volume 1, pp. 292–315. [Google Scholar]
- Kuznetsov, A.B.; Staricova, E.V.; Maslov, A.V.; Konstantinova, G.V., Sr. Isotopic Chemostratigraphy of Precambrian Carbonate Rocksin the Amderma Rise, Pai-Khoi Ridge. Doklady Earth Sci. 2016, 246, 447–451. [Google Scholar]
- Volynets, O.N.; Flerov, G.B.; Khrenov, A.P.; Ermakov, V.A. Volcanic petrology of the Great Tolbachik fissure eruption. Akad. Nauk SSSR Doklady. Earth Sci. Sec. 1976, 238, 179–183. [Google Scholar]
- Selyangin, O.B. Structure, substance and near-surface foci of the Mutnovsky and Gorely volcanoes (Mutnovsky geothermal area, Kamchatka. Min. Inf. Anal. Bull. 2016, 31, 348–438. (In Russian) [Google Scholar]
- Khrenov, A.; Artemyev, O.; Belousov, A.; Vasiliev, V.; Girina, O.; Gordeev, E.; Dvigalo, V.; Droznin, V.; Demyanchuk, Y.; Dubrovskaya, I.; et al. Volcanoes of Kamchatka and the Kuril islands. Russ. Found. Basic Res. J. 2015, 2, 105. [Google Scholar]
- Melekestsev, I.V.; Braytseva, O.K.; Ponomareva, V.V. Dynamics of Activity of Mutnovsky and Gorely volcanoes in Holocene and potential hazard for adjacent regions. Volcanol. Seismol. 1987, 3, 3–18. (In Russian) [Google Scholar]
- Barragán, C.; Wetzel, C.E.; Ector, L. A standard method for the routine sampling of terrestrial diatom communities for soil quality assessment. J. Appl. Phycol. 2018, 30, 1095–1113. [Google Scholar] [CrossRef]
- Acker, F.; Russell, B.; Morales, E. Preparation of Diatom Slides Using Naphrax™ Mounting Medium; Protocol P-13-49; Academy of Natural Sciences of Philadelphia: Philadelphia, PA, USA; Patrick Center for Environmental Research: Philadelphia, PA, USA, 1999; pp. 13–42. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Naviculaceae. Süβwasserflora von Mitteleuropa. Band 2/1; Spectrum Academiche Verlag: Berlin, Germany, 1986; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 2. epithemiaceae, bacillariaceae, surirellaceae. In Süsswasserflora von Mitteleuropa; Pascher, A., Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; VEB Gustav Fisher Verlag: Stuttgart, Germany, 1988; p. 596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 3. centrales, fragilariaceae, eunotiaceae, achnanthaceae. In Süβwasserflora von Mitteleuropa; Pascher, A., Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; VEB Gustav Fisher Verlag: Stuttgart, Germany, 1991; p. 576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 4. achnanthaceae, kritische erganzungen zu navicula (Lineolatae) und gomphonema. In Süsswasserfloravon Mitteleuropa; Pascher, A., Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; VEB Gustav Fisher Verlag: Stuttgart, Germany, 1991; p. 436. [Google Scholar]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen im Süßwasser—Benthos von Mitteleuropa. In Bestimmungsflora Kieselalgen für die Ökologische Praxis. Über 700 der Häufigsten Arten und Ihre Ökologie; Koeltz Scientific Books: Königstein, Germany, 2013; p. 908. [Google Scholar]
- Paula, C.F.; Lowe, R.L.; Johansen, J.R. Teratology in Eunotia taxa in The Great Smoky Mountains National Park and description of Eunotia macroglossa spp. nov. Diatom Res. 2009, 24, 273–290. [Google Scholar]
- Wetzel, C.E.; Ector, L.; Vijver, B.; Compère, P.; Mann, D.G. Morphology, typification and critical analysis of some ecologically important small naviculoid species (Bacillariophyta). Fottea 2015, 15, 203–234. [Google Scholar] [CrossRef] [Green Version]
- Potapova, M.G. Diatoms of Bering Island, Kamchatka, Russia. Nova Hedwigigia 2014, 143, 63–102. [Google Scholar]
- Furey, P.C.; Manoylov, K.M.; Lowe, R.L. New and interesting aerial diatom assemblages from southwestern Iceland. Phytotaxa 2020, 428, 173–208. [Google Scholar] [CrossRef]
- Ettl, H.; Gärtner, G. Syllabus der Boden-, Luft-und Flechtenalgen; Gustav Fischer Verlag: Stuttgart, Germany, 1995; p. 721. [Google Scholar]
- Kociolek, J.P.; Spaulding, S.A. Eunotioid and asymmetrical naviculoid diatoms. In Freshwater Algae of North America: Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 655–668. [Google Scholar]
- Kociolek, J.P.; Spaulding, S.A. Symmetrical naviculoid diatoms. In Freshwater Algae of North America: Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 637–652. [Google Scholar]
- Lowe, R.L. Keeled and canalled raphid diatoms. In Freshwater Algae of North America. Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: New York, NY, USA, 2003; pp. 669–684. [Google Scholar]
- Stoermer, E.F.; Julius, M.L. Centric diatoms. In Freshwater Algae of North America: Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 559–652. [Google Scholar]
- Flechtner, V.R.; Johansen, J.R.; Belnap, J. The biological soil crusts of the San Nicolas Island: Enigmatic algae from a geographically isolated ecosystem. West. N. Am. Nat. 2008, 68, 405–436. [Google Scholar] [CrossRef] [Green Version]
- Stenina, A.S. Diatoms (Bacillariophyta) in the Lakes of the East of the Bolshezemelskaya Tundra; Institute of Biology Komi Scientific Center, Ural Branch of the Russian Academy of Sciences Publishing House: Syktyvkar, Russia, 2009; p. 179. (In Russian) [Google Scholar]
- Poradowska, A. Diatoms (Bacillariophyta) from the genus Eunotia and Pinnularia developing on soils in the open landscape of the Low Beskids. J. Ecol. Eng. 2020, 21, 257–270. [Google Scholar] [CrossRef]
- Antonelli, M.; Wetzel, C.E.; Ector, L.; Teuling, A.J.; Pfister, L. On the potential for terrestrial diatom communities and diatom indices to identify anthropogenic disturbance in soils. Ecol. Indic. 2017, 75, 73–81. [Google Scholar] [CrossRef]
- Venn, J. On the diagrammatic and mechanical representation of propositions and reasonings. Philos. Mag. J. Sci. 1880, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Hulsen, T.; de Vlieg, J.; Alkema, W. BioVenn—A web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genom. 2008, 9, 488. [Google Scholar] [CrossRef] [Green Version]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Kabirov, R.R.; Safiulina, L.M. Peculiarities of ecology and distribution of unicellular soil alga Eustigmatos magnus (J.B. Petersen) Hibberd in Southern Ural (Russia). Int. J. Algae 2008, 10, 105–116. [Google Scholar]
- Hernández, M.; Calabi, M.; Conrad, R.; Dumont, M.G. Analysis of the microbial communities in soils of different ages following volcanic eruptions. Pedosphere 2020, 30, 126–134. [Google Scholar] [CrossRef]
- Leff, J.W.; Bardgett, R.D.; Wilkinson, A.; Jackson, B.G.; Pritchard, W.; De Long, J.R.; Oakley, S.; Mason, K.E.; Ostle, M.J.; Johnson, D.; et al. Predicting the structure of soil communities from plant community taxonomy, phylogeny, and traits. Multidiscip. J Microb. Ecol. 2018, 12, 1794–1805. [Google Scholar] [CrossRef] [Green Version]
- King, G.M. Contribution of atmospheric CO and hydrogen uptake to microbial dynamics on recent Hawaiian volcanic deposits. Appl. Environ. Microbiol. 2003, 69, 4067–4075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, H.; Yagi, M.; Suzuki, J.; Fujitake, N.; Watanabe, M. Characterization of Sphingomonas species found as predominant members in the culturable bacterial community of a green pigment-containing sclerotium grain from Mt. Myoko (Japan) volcanic ash soil. Microbes Environ. 2003, 18, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Alvarez, V.; King, G.M.; Nusslein, K. Comparative bacterial diversity in recent Hawaiian volcanic deposits of different ages. FEMS Microbiol. Ecol. 2007, 60, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, M.; Matsuyama, A.; Harvard, J.P.; Bourdineaud, J.-P.; Nakamura, K. Mercury contamination in humans in Upper Maroni, French Guiana between 2004 and 2009. Bull. Envir. Contamin. Toxicol. 2012, 88, 135–139. [Google Scholar] [CrossRef]
- Guo, Z.; Wilson, M.; Zhang, L.; Zhang, M.; Cheng, Z.; Liu, J. The role of subduction channel mélanges and convergent subduction systems in the petrogenesis of post-collisional K-rich mafic magmatism in NW Tibet. Lithos 2014, 198–199, 184–201. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.; Megonigal, J.P.; Kang, H.; Seo, J.; Ding, W. Impacts of Phragmites australis invasion on soil enzyme activities and microbial abundance of tidal marshes. Microb. Ecol. 2018, 76, 782–790. [Google Scholar] [CrossRef]
- Dobrovol’skaya, T.G.; Zvyagintsev, D.G.; Chernov, I.Y.; Golovchenko, A.V.; Zenova, G.M.; Lysak, L.V.; Manucharova, A.; Marfenina, O.E.; Polyanskaya, L.M.; Stepanov, A.L.; et al. The role of microorganisms in the ecological functions of soils. Eurasian Soil Sci. 2015, 48, 959–967. [Google Scholar] [CrossRef]
- Geisen, S.; Mitchell, E.A.D.; Adl, S.M.; Bonkowski, M.; Dunthorn, M.; Ekelund, F.; Fernandez, L.D.; Jousset, A.; Krashevska, V.; Singer, D.; et al. Soil protists: A fertile frontier in soil biology research. FEMS Microbiol. Rev. 2018, 42, 293–323. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Dudenhöffer, J.H.; Widmer, F.; Heijden, M.G.A. Linking diversity, synchrony and stability in soil microbial communities. Funct. Ecol. 2018, 32, 1280–1292. [Google Scholar] [CrossRef]
- Pfister, L.; Wetzel, C.E.; Klaus, J.; Martínez-Carreras, N.; Antonelli, M.; Teuling, A.J.; McDonnell, J.J. Terrestrial diatoms as tracers in catchment hydrology: A review. Wileys Interdiscip. Rev. Water 2017, 4, e1241. [Google Scholar] [CrossRef] [Green Version]
- Foets, J.; Stanek-Tarkowska, J.; Teuling, A.J.; Van de Vijver, B.; Wetzel, C.E.; Pfister, L. Autecology of terrestrial diatoms under anthropic disturbance and across climate zones. Ecol. Indic. 2021, 122, 107248. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Śliwinska-Wilczewska, S.; Lewandowska, A.; Konik, M. The effect of abiotic factors on abundance and photosynthetic performance of airborne cyanobacteria and microalgae isolated from the Southern Baltic Sea region. Cells 2021, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Metting, B. The systematics and ecology of soil algae. Bot. Rev. 1981, 47, 195–312. [Google Scholar]
- Kilham, S.S.; Theriot, E.C.; Fritz, S.C. Linking planktonic diatoms and climabe change in the large lakes of the Yellowstone ecosystem using resource theory. Limnol. Oceanogr. 1996, 41, 1052–1062. [Google Scholar] [CrossRef]
- Pan, Y.; Rao, D.V.S.; Mann, K.H.; Li, W.K.W.; Harrison, W.G. Effects of silicate limitation on production of domoic acid, a neurotoxin, by the diatom Pseudo-nitzschia multiseries. II. Continuous culture studies. Mar. Ecol. Progr. 1996, 131, 235–243. [Google Scholar] [CrossRef]
- Annett, A.L.; Lapi, S.; Ruth, T.J.; Maldonado, M.T. The effects of Cu and Fe availability on the growth and Cu:C ratios of marine diatoms. Limnol. Oceanogr. 2008, 53, 2451–2461. [Google Scholar] [CrossRef] [Green Version]
- Kranzler, C.F.; Krause, J.W.; Brzezinski, M.A.; Edwards, B.R.; Biggs, W.P.; Maniscalco, M.; McCrow, J.P.; Van Mooy, B.A.S.; Bidle, K.D.; Allen, A.E.; et al. Silicon limitation facilitates virus infection and mortality of marine diatoms. Nat. Microbiol. 2019, 4, 1790–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Rechav, K.; Kaplan-Ashiri, I.; Assaf, G. Imaging and quantifying homeostatic levels of intracellular silicon in diatoms. Sci. Adv. 2020, 6, eaaz7554. [Google Scholar] [CrossRef]
- Lukešova, A. Soil algae in brown coal and lignite post-mining areas in Central Europe (Czech Republic and Germany). Restor. Ecol. 2001, 9, 341–350. [Google Scholar] [CrossRef]
- Fazlutdinova, A.I.; Sukhanova, N.V. Composition of soil diatoms in zones of impact from oil production complexes. Russ. J. Ecol. 2014, 45, 188–193. [Google Scholar] [CrossRef]
- Minaoui, F.; Hakkoum, Z.; Douma, M.; Mouhri, K.; Loudiki, M. Diatom communities as bioindicators of human disturbances on suburban soil quality in arid Marrakesh area (Morocco). Water Air Soil Pollut. 2021, 232, 146. [Google Scholar] [CrossRef]
- Ilchibaeva, K.V.; Kunsbaeva, D.F.; Allaguvatova, R.Z.; Fazlutdinova, A.I.; Polokhin, O.V.; Sibirina, L.A.; Gontcharov, A.A.; Singh, P.; Gaysina, L.A. Preliminary data about algae and cyanobacteria of volcanic soils on Kuril islands. Theor. Appl. Ecol. 2018, 4, 119–126. [Google Scholar] [CrossRef]
- Lowe, R.L.; Kociolek, P.; Johansen, J.R.; Van De Vijver, B.; Lange-Bertalot, H.; Kopalová, K. Humidophila gen. nov., a new genus for a group of diatoms (Bacillariophyta) formerly within the genus Diadesmis: Species from Hawai’i, including one new species. Diatom Res. 2014, 29, 351–360. [Google Scholar] [CrossRef]
- Maltsev, Y.I.; Kulikovskiy, M.S. Morphological and genetic variability of Hantzschia amphioxys (Bacillariophyceae) in terrestrial and aquatic habitats. Botanicheskiy Zhurnal 2017, 102, 1–12. [Google Scholar]
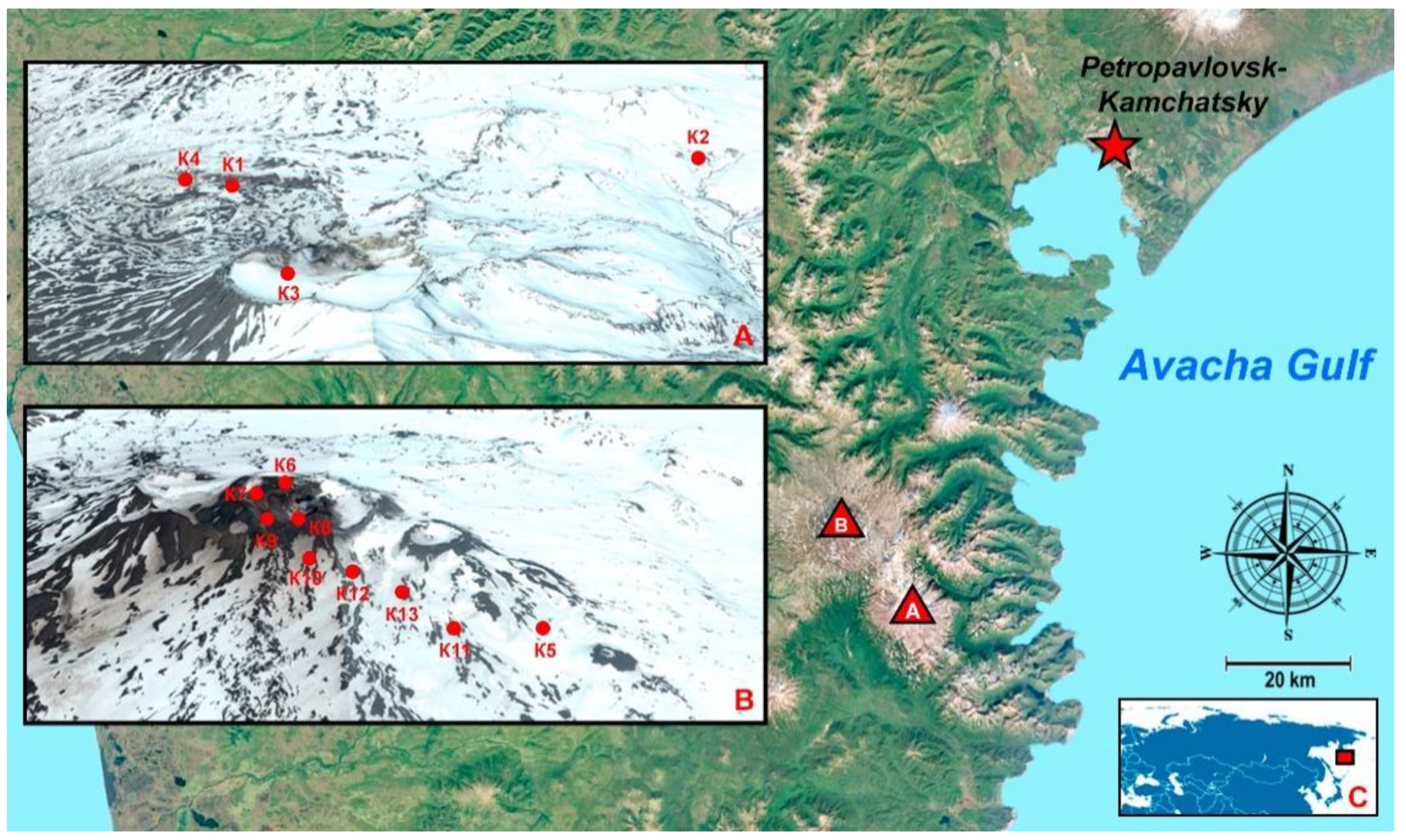


| Number | Description | Name | GPS * | Area | pH | Humidity, % | Type of Soil |
|---|---|---|---|---|---|---|---|
| 1 | Canyon of the Vulkannaya river, under the bushes | K1 | 52°28′29.4″ N 158°06′47.8″ E | M ** | 9.1 | 50–68 | Mountain–tundra illuvial–humus soils |
| 2 | At the base of the volcano, not far from Dachnye springs, alder forest | K2 | 52°31′54.6″ N 158°11′55.0″ E | M | 8.9 | 75–85 | Humus–ocher soils |
| 3 | 300 m from the top of the volcano | K3 | 52°27′26.4″ N 158°09′50.4″ E | M | 9.1 | 40–50 | Stone talus and placers, rocks |
| 4 | In the lower part of the Vulkannaya River canyon | K4 | 52°28′17.3″ N 158°06′02.4″ E | M | 9.1 | 50–58 | Rocks |
| 5 | Slope, flat area among sedges | K5 | 52°32′35.0″ N 158°03′58.2″ E | G *** | 5.8 | 60–70 | Illuvial–humus volcanic destructive soils |
| 6 | The trail along the edge of the crater, green layer on the surface of the ground | K6 | 52°33′26.4″ N 158°02′09.2″ E | G | 9.0 | 50–65 | Volcanic ash, sand |
| 7 | Down the east slope | K7 | 52°33′19.1″ N 158°01′57.4″ E | G | 9.2 | 55–65 | Tundra volcanic illuvial–humus soils |
| 8 | At the edge of a crater with a lake | K8 | 52°33′12.8″ N 158°02′20.7″ E | G | 5.0–6.0 | 40–50 | Sulfur deposits around the crater |
| 9 | Down the east slope | K9 | 52°33′10.8″ N 158°02′06.0″ E | G | 5.0–6.5 | 55–65 | Tundra volcanic illuvial–humus soils |
| 10 | Down the east slope | K10 | 52°32′53.7″ N 158°02′21.6″ E | G | 5.2–6.3 | 55–65 | Tundra volcanic illuvial–humus soils |
| 11 | 1000 m from the top of the volcano | K11 | 52°32′27.8″ N 158°03′22.0″ E | G | 5.2–6.3 | 55–65 | Tundra volcanic illuvial–humus soils |
| 12 | An active crater during the sampling, 500 m from the top of the volcano | K12 | 52°32′46.2″ N 158°02′39.9″ E | G | 8.4–9.0 | - | Volcanic ash, sand |
| 13 | 800 m from the top of the volcano, rare vegetation | K13 | 52°32′38.7″ N 158°03′03.1″ E | G | 5.2–6.3 | 65–70 | Tundra volcanic illuvial–humus soils |
| Taxa | Mutnovsky Volcano | Gorely Volcano | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | K7 | K8 | K9 | K10 | K11 | K12 | K13 | |
| Achnanthes linearis var. pusilla Grunov | 2 | ||||||||||||
| Adlafia aquaeductae (Krasske) Lange-Bertalot | 3 | ||||||||||||
| Caloneis bacillum (Grunow) Cleve | 5 | 2 | 6 | 6 | 15 | 15 | |||||||
| Caloneis lancettula (Schulz) Lange-Bertalot et Witkowski | 1 | ||||||||||||
| Caloneis dubia Krammer | 1 | ||||||||||||
| Diatoma tenuis Agardh | 3 | ||||||||||||
| Diatoma vulgaris Bory | 1 | ||||||||||||
| Eunotia bilunaris (Ehrenberg) Schaarschmidt | 1 | ||||||||||||
| Eunotia curtagrunowii Nörpel-Schempp and Lange-Bertalot | 15 | 1 | |||||||||||
| Eunotia fallax A. Cleve | 10 | 6 | |||||||||||
| Eunotia paludosa Grunow | 2 | ||||||||||||
| Eunotia sudetica f. minor Manguin in Bourrelly et Manguin | 3 | ||||||||||||
| Fragilariforma virescens var. exigua (Grunow) M.Poulin | 2 | ||||||||||||
| Gomphonema parvulum (Kützing) Kützing | 1 | ||||||||||||
| Hantzschia amphioxys (Ehrenberg) Grunow | 2 | 2 | |||||||||||
| Humidophila contenta (Grunow) R.L.Lowe, Kociolek, J.R.Johansen, Van de Vijver, Lange-Bertalot and Kopalová | 15 | 1 | |||||||||||
| Luticola mutica (Kützing) D.G.Mann | 6 | 1 | 5 | ||||||||||
| Muelleria gibbula (Cleve) S.A. Spaulding et E.F. Stoermer | 4 | ||||||||||||
| Navicula cincta (Ehrenberg) Ralfs | 3 | ||||||||||||
| Nitzschia cf. ovalis H.J. Arnott | 1 | ||||||||||||
| Nitzschia palea (Kützing) W.Smith | 4 | ||||||||||||
| Pinnularia borealis Ehrenberg | 15 | 4 | 1 | 15 | |||||||||
| Pinnularia intermedia (Lagerstedt) Cleve | 2 | 1 | 1 | ||||||||||
| Pinnularia microstauron (Ehrenberg) Cleve | 1 | 1 | 1 | ||||||||||
| Pinnularia cf.subcapitata W. Gregory | 7 | 7 | 2 | 1 | 1 | 1 | 2 | 12 | |||||
| Pinnularia sp.1 | 1 | ||||||||||||
| Pinnularia sp.2 | 1 | ||||||||||||
| Pinnularia sp.3 | 3 | ||||||||||||
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 1 | ||||||||||||
| Platessa oblongella (Østrup) C.E. Wetzel, Lange-Bertalot et Ector | 1 | 2 | |||||||||||
| Psammothidium ventrale (Krasske) Bukhtiyarova et Round | 4 | ||||||||||||
| Sellaphora bacillum (Ehrenberg) D.G.Mann | 1 | ||||||||||||
| Sellaphora mutata (Krasske) Lange-Bertalot | 1 | 1 | |||||||||||
| Sellaphora pupula (Kützing) Mereschkovsky | 1 | ||||||||||||
| Sellaphora submuralis (Hustedt) C.E.Wetzel, L.Ector, B.Van de Vijver, Compère et D.G. Mann | 1 | ||||||||||||
| Stauroneis anceps Ehrenberg | 1 | ||||||||||||
| Staurosirella pinnata (Ehrenberg) D.M.Williams et Round | 3 | 10 | |||||||||||
| Tabularia fasciculata (C. Agardh) D.M. Williams et Round | 1 | ||||||||||||
| Total number | 13 | 22 | 5 | 9 | 4 | 0 | 0 | 0 | 1 | 1 | 7 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazlutdinova, A.; Gabidullin, Y.; Allaguvatova, R.; Gaysina, L. Diatoms in Volcanic Soils of Mutnovsky and Gorely Volcanoes (Kamchatka Peninsula, Russia). Microorganisms 2021, 9, 1851. https://doi.org/10.3390/microorganisms9091851
Fazlutdinova A, Gabidullin Y, Allaguvatova R, Gaysina L. Diatoms in Volcanic Soils of Mutnovsky and Gorely Volcanoes (Kamchatka Peninsula, Russia). Microorganisms. 2021; 9(9):1851. https://doi.org/10.3390/microorganisms9091851
Chicago/Turabian StyleFazlutdinova, Alfiya, Yunir Gabidullin, Rezeda Allaguvatova, and Lira Gaysina. 2021. "Diatoms in Volcanic Soils of Mutnovsky and Gorely Volcanoes (Kamchatka Peninsula, Russia)" Microorganisms 9, no. 9: 1851. https://doi.org/10.3390/microorganisms9091851
APA StyleFazlutdinova, A., Gabidullin, Y., Allaguvatova, R., & Gaysina, L. (2021). Diatoms in Volcanic Soils of Mutnovsky and Gorely Volcanoes (Kamchatka Peninsula, Russia). Microorganisms, 9(9), 1851. https://doi.org/10.3390/microorganisms9091851








