Production of Enriched Sporidiobolus sp. Yeast Biomass Cultivated on Mixed Coffee Hydrolyzate and Fat/Oil Waste Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Yeast Cultivation, Hydrolysate and Media Preparation
2.2.1. Yeast Inoculation
2.2.2. Yeast Production Media
2.2.3. Spent Coffee Grounds Hydrolysate Preparation
2.2.4. Waste Lipid Materials
2.2.5. Large-Scale Bioreactor Co-Cultivation
2.3. Analytical Methods
2.3.1. SCG Hydrolysate Analysis
2.3.2. Cell Dry Weight
2.3.3. Lipid Metabolite Analysis
2.3.4. Lipids and Fatty Acids
2.4. Statistical Analysis
3. Results
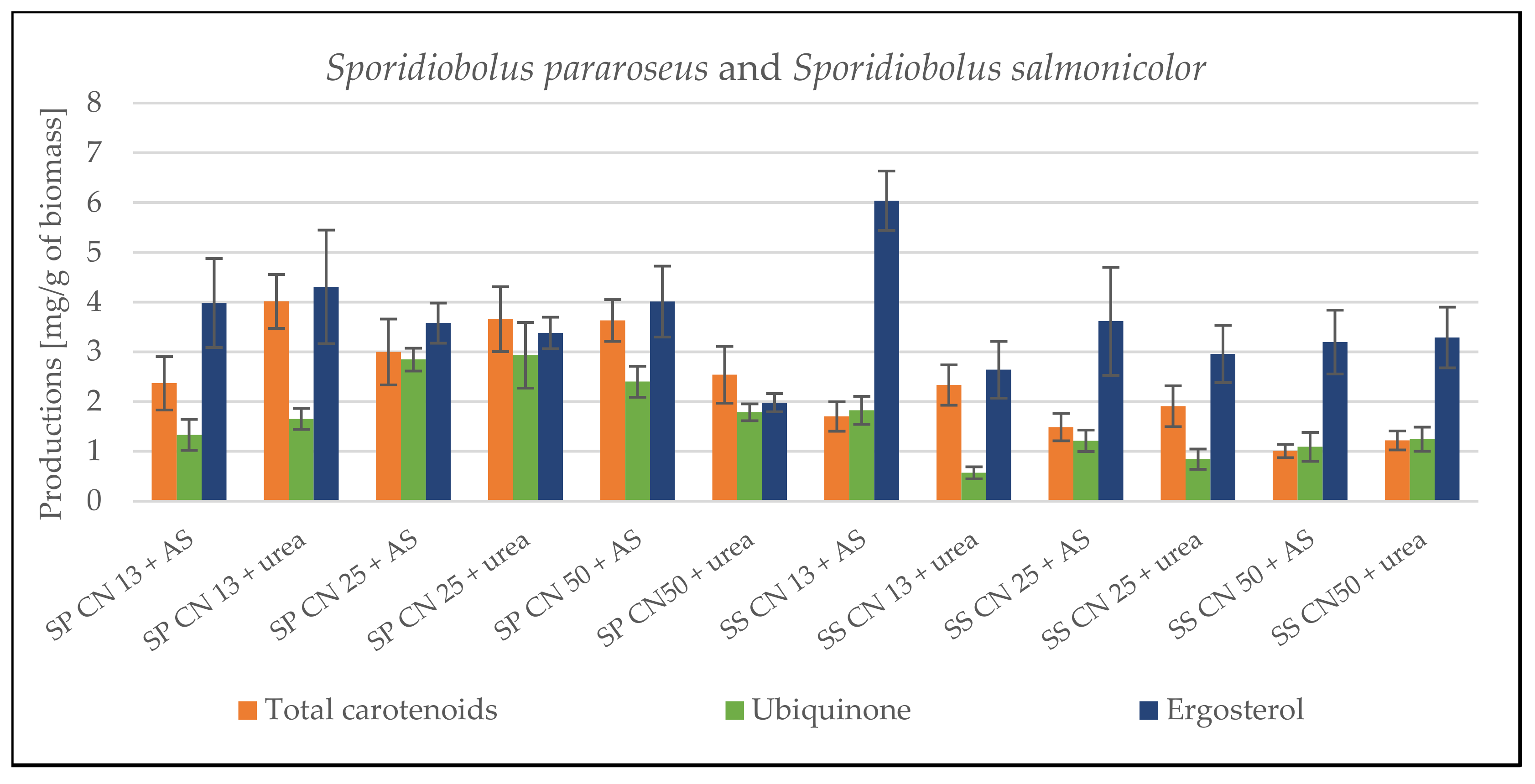
3.1. Screening Cultivation of the Yeasts of Sporidiobolus Genera on SCG Hydrolysate
3.1.1. Cultivation on Media with C/N Ratio 13
3.1.2. Cultivation on Media with C/N Ratio 25
3.1.3. Cultivation on Media with C/N Ratio 50
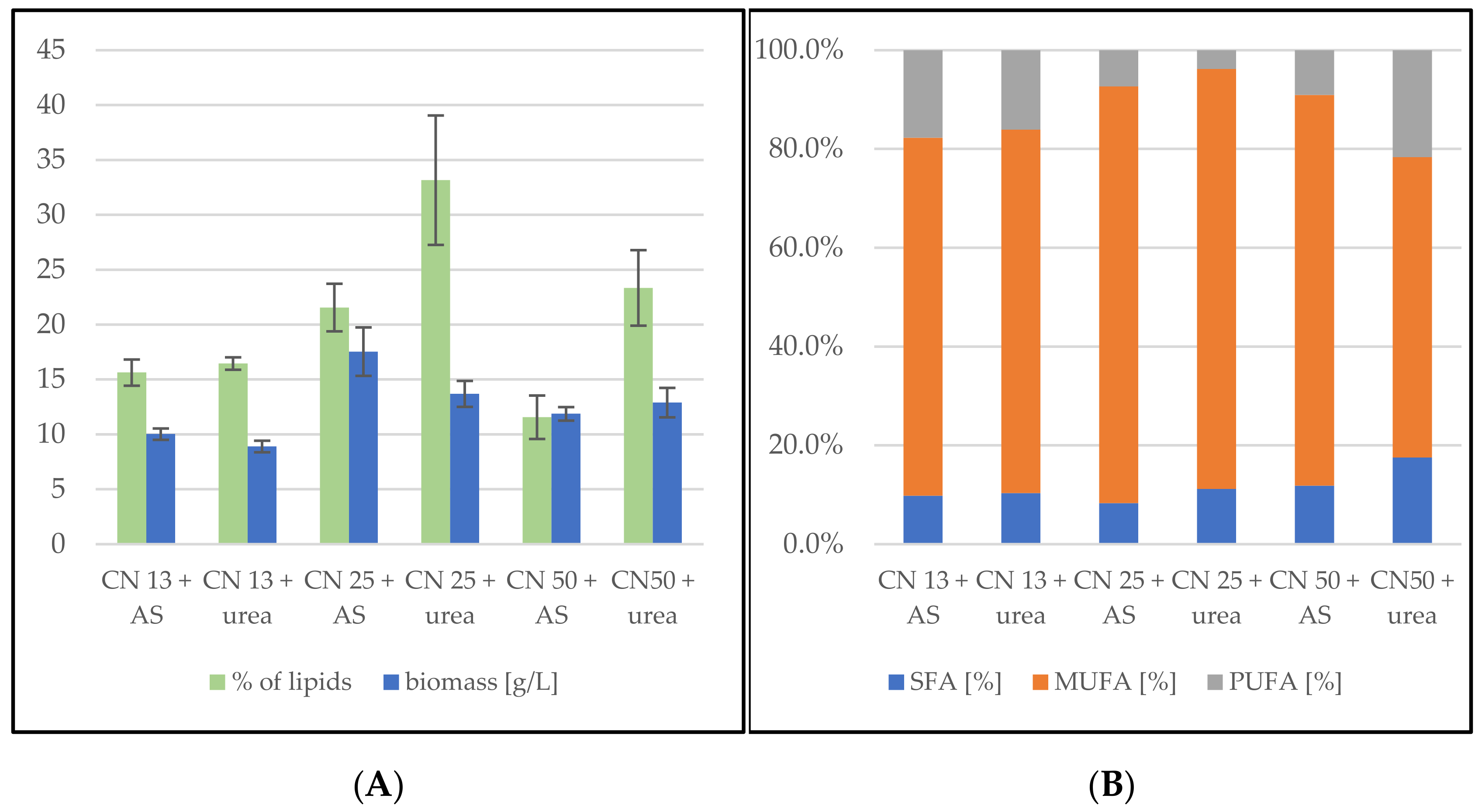
3.2. Bioreactor Cultivation SCG Hydrolysate and Waste Lipid Substrates
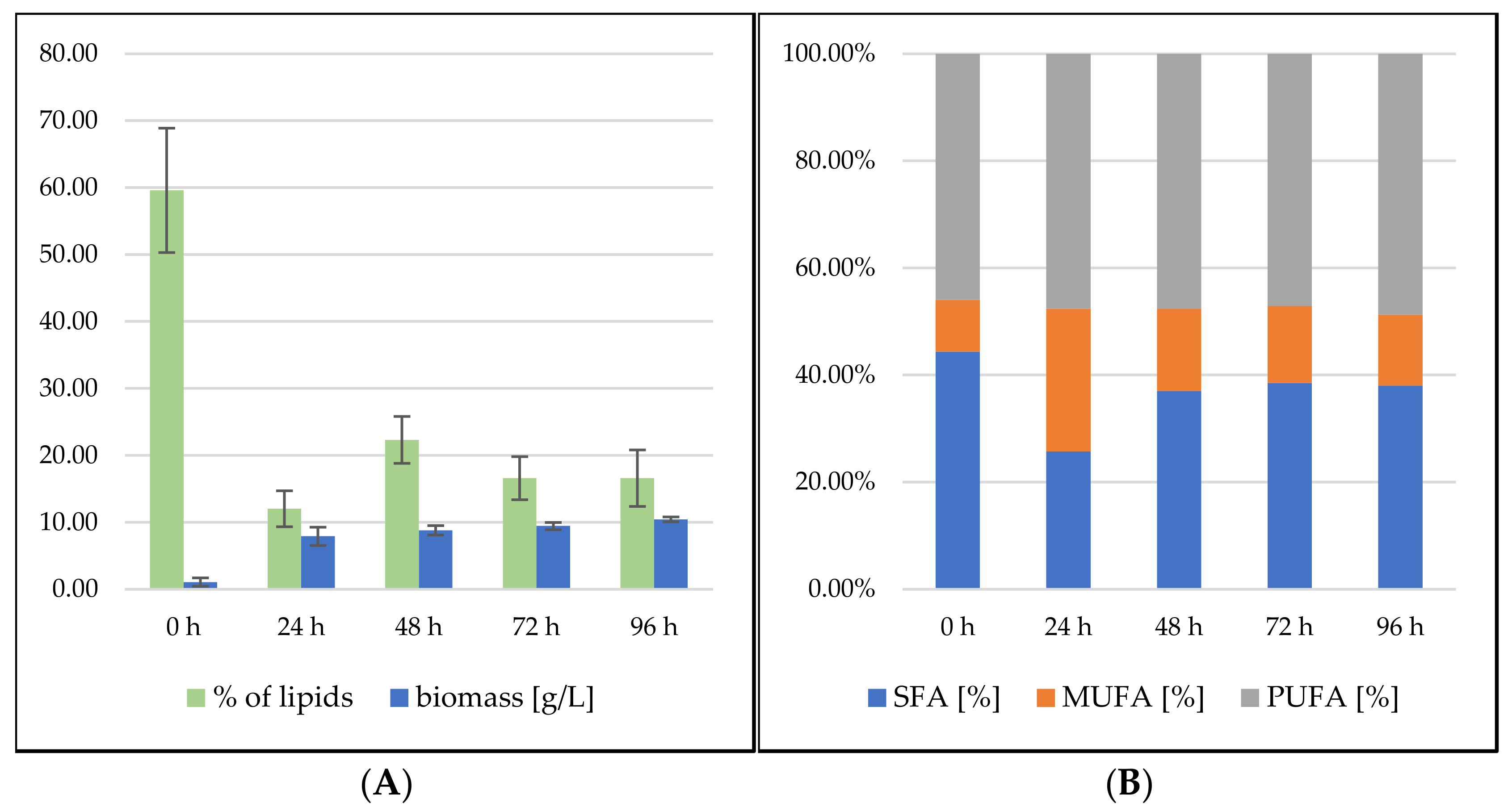
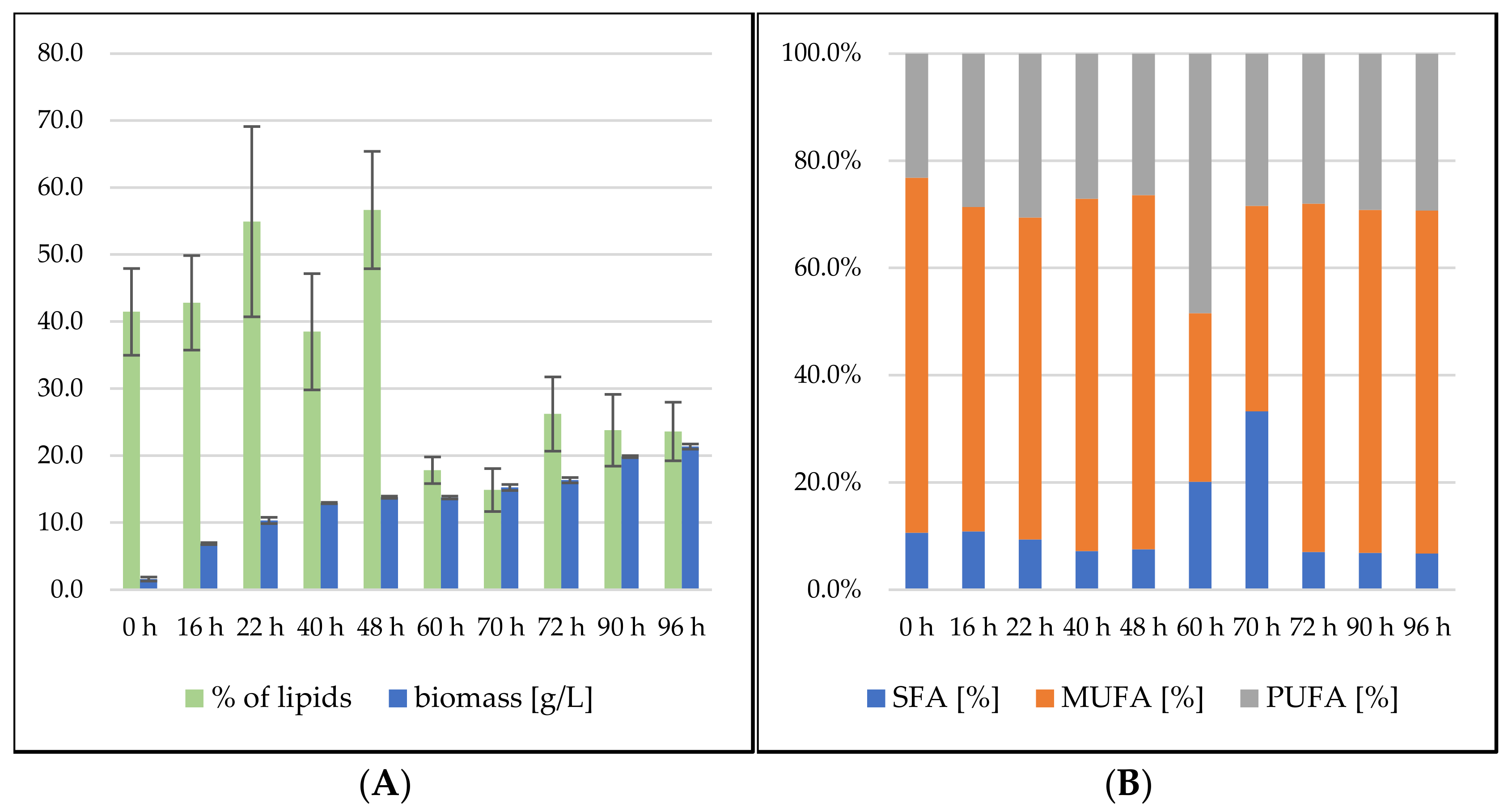
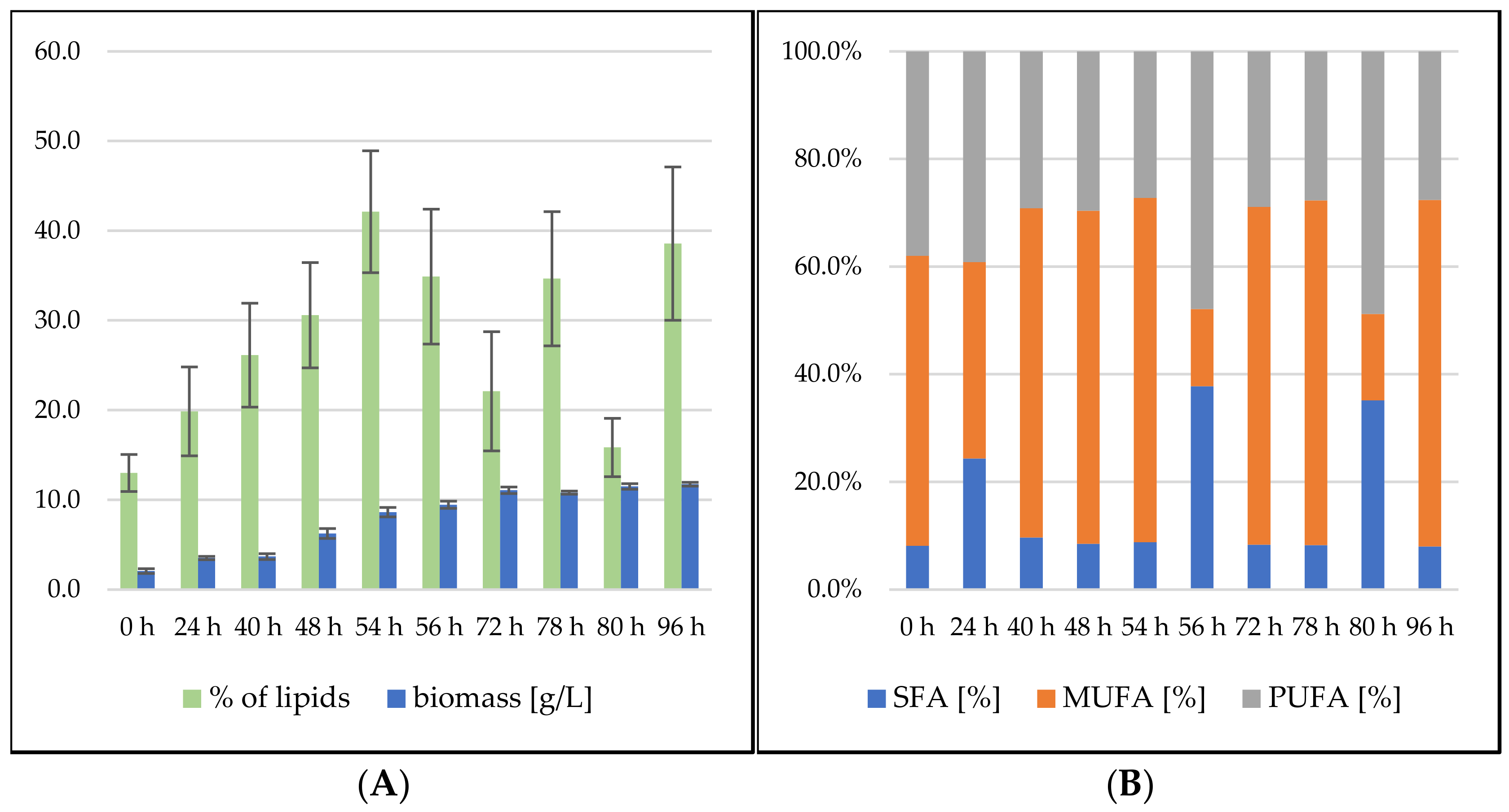
3.2.1. Bioreactor Cultivation in Medium with SCG Hydrolysate and Crude Waste Animal Fat
3.2.2. Bioreactor Cultivation in Medium with SCG Hydrolysate and Coffee Oil
Sporidiobolus pararoseus Cultivation in Medium with SCG Hydrolysate and Coffee Oil
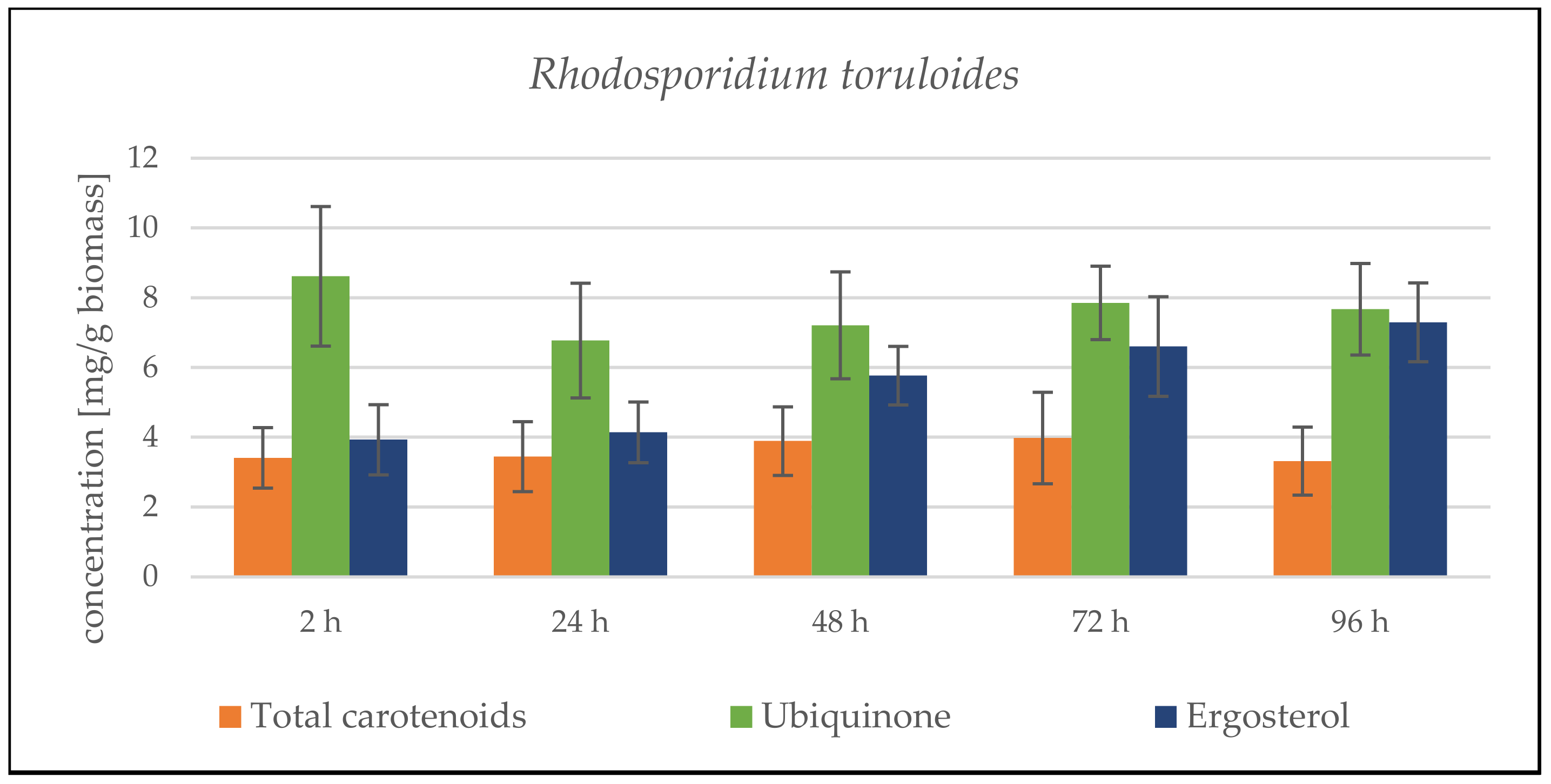
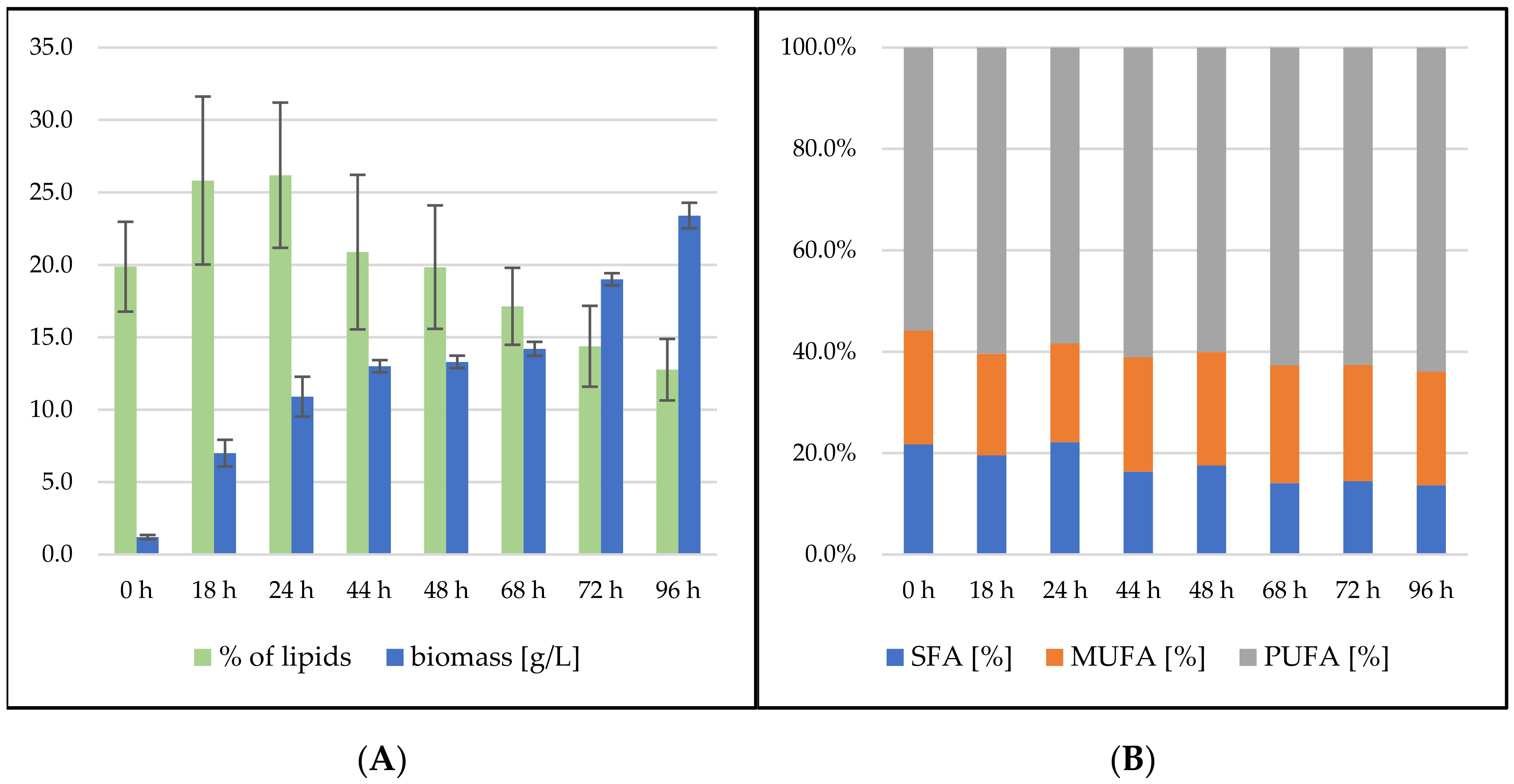
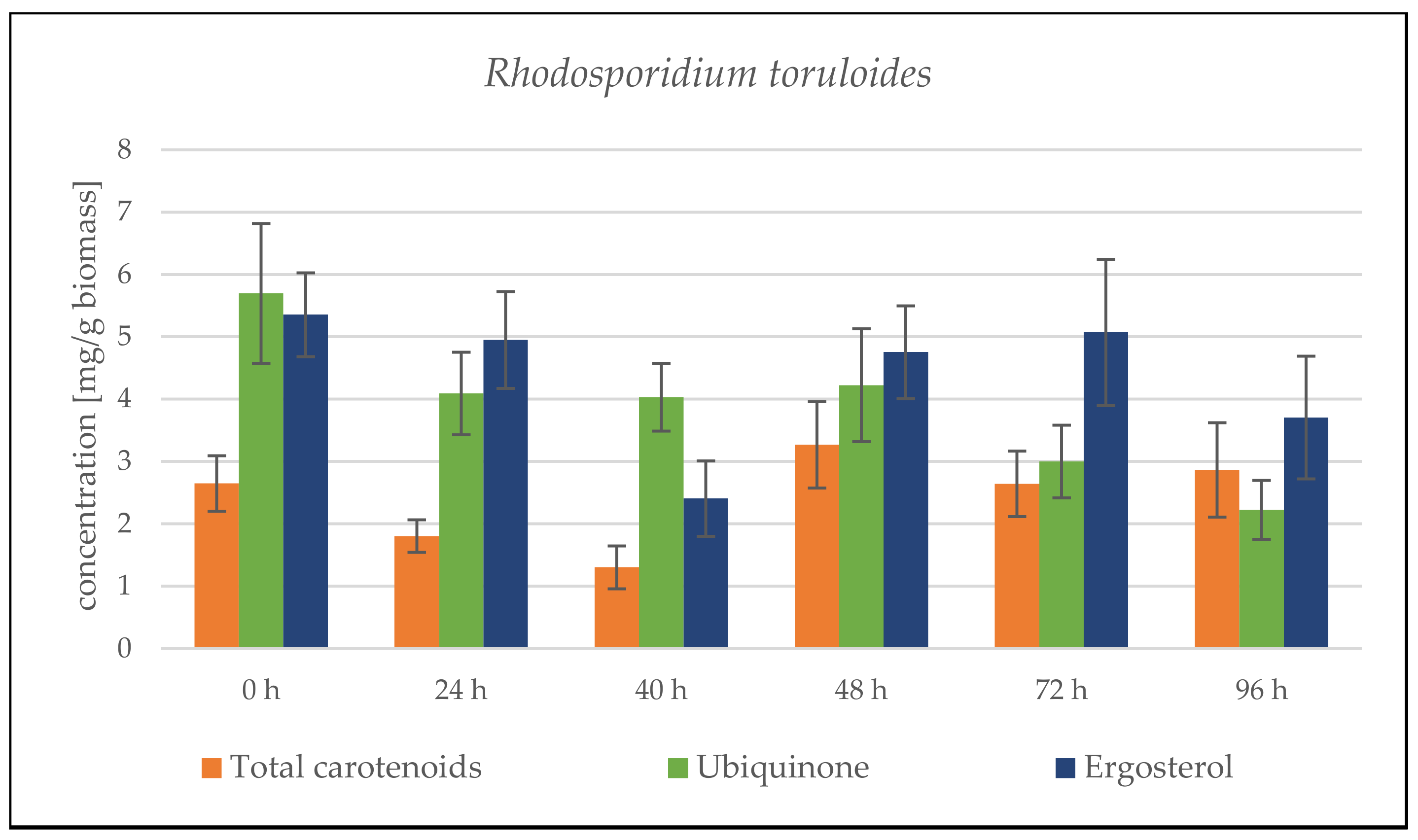
Rhodosporidium toruloides Cultivation in Medium with SCG Hydrolysate and Coffee Oil
3.2.3. Bioreactor Cultivation in Medium with SCG Hydrolysate and Waste Frying Oil
Sporidiobolus pararoseus Cultivation in Medium with SCG Hydrolysate and Waste Frying Oil
Rhodosporidium toruloides Cultivation in Medium with SCG Hydrolysate and Waste Frying Oil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schilling, C.; Weiss, S.A. Roadmap for industry to harness biotechnology for a more circular economy. New Biotech. 2021, 60, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Fang, C. Circular economy development in China-current situation, evaluation and policy implications. Environ. Impact Assess. Rev. 2020, 84, 106441. [Google Scholar] [CrossRef]
- International Coffee Organization. Coffee Development Report. Available online: http://www.ico.org (accessed on 22 July 2021).
- Jenkins, R.W.; Stageman, N.E.; Fortune, C.M.; Chuck, C.J. Effect of the type of bean, processing, and geographical location on the biodiesel produced from waste coffee grounds. Energy Fuels 2014, 28, 1166–1174. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; García, A.T.; Peréz, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Aaires, A.; Coutinho, J.; Trindade, H. Effect of different rates of spent coffee grounds (SCG) on composting process, gaseous emissions and quality of end-product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Limousy, L.; Jeguirim, M.; Dutournié, P.; Kraiem, N.; Lajili, M.; Said, R. Gaseous products and particulate matter emissions of biomass residential boiler fired with spent coffee grounds pellets. Fuel 2013, 107, 323–329. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. New Biotechnol. 2015, 32, 569–574. [Google Scholar] [CrossRef]
- Passadis, K.; Fragoulis, V.; Stoumpou, V.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. Study of valorisation routes of spent coffee grounds. Waste Biomass Valor. 2020, 11, 5295–5306. [Google Scholar] [CrossRef]
- Bautista, L.F.; Vicente, G.; Rodríguez, R.; Pacheco, M. Optimisation of FAME production from waste cooking oil for biodiesel use. Biomass Bioen. 2009, 33, 862–872. [Google Scholar] [CrossRef]
- Paul, S.; Mittal, G.S. Dynamics of fat/oil degradation during frying based on optical properties. J. Food Eng. 1996, 30, 389–403. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Dimou, A.; Fakas, S.; Diamantopoulou, P.; Philippoussis, A.; Galiotou-Panayotou, M.; Aggelis, G. Biotechnological conversion of waste cooking olive oil into lipid-rich biomass using Aspergillus and Penicillium strains. J. Appl. Microbiol. 2011, 110, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Felizardo, P.M.; Correia, J.N.; Raposo, I.; Mendes, J.F.; Berkemeier, R.; Bordaddo, J.M. Production of biodiesel from waste frying oils. Waste Manag. 2006, 26, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Mannu, A.; Ferro, M.; di Pietro, M.E.; Mele, A. Innovative applications of waste cooking oil as raw material. Sci. Progress 2019, 102, 153–160. [Google Scholar] [CrossRef]
- Kamilah, H.; Tsuge, T.; Yang, T.A.; Sudesh, K. Waste cooking oil as substrate for biosynthesis of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate): Turning waste into a value-added product. Malaysian J. Microbiol. 2013, 9, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Spalvins, K.; Geiba, Z.; Kusnere, Z.; Blumberga, D. Waste cooking oil as substrate for single cell protein production by yeast Yarrowia lipolytica. Environ. Clim. Technol. 2020, 24, 457–469. [Google Scholar] [CrossRef]
- Walker, G.M. Yeasts. Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 478–491. [Google Scholar]
- Mannazu, I.; Landolfo, S.; da Silva, T.L.; Buzzini, P. Red yeasts and carotenoid production: Outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef] [Green Version]
- Szotkowski, M.; Byrtusova, D.; Haronikova, A.; Vysoka, M.; Rapta, M.; Shapaval, V.; Marova, I. Study of metabolic adaptation of red yeast to waste animal fat substrate. Microorganisms 2019, 7, 578. [Google Scholar] [CrossRef] [Green Version]
- Byrtusova, D.; Shapaval, V.; Holub, J.; Simansky, S.; Rapta, M.; Szotkowski, M.; Kohler, A.; Marova, I. Revealing the potential of lipid and β-glucans coproduction in basidiomycetes yeast. Microorganisms 2020, 8, 1034. [Google Scholar] [CrossRef] [PubMed]
- Byrtusova, D.; Szotkowski, M.; Kurowska, K.; Shapaval, V.; Marova, I. Rhodotorula kratochvilovae CCY 20-2-26—The source of multifunctional metabolites. Microorganisms 2021, 9, 1280. [Google Scholar] [CrossRef]
- Jiru, T.M.; Groenewald, M.; Pohl, C.; Steyn, L.; Kiggundu, N.; Abate, D. Optimization of cultivation conditions for biotechnological production of lipid by Rhodotorula kratochvilovae (syn, Rhodosporidium kratochvilovae) SY89 for biodiesel preparation. 3 Biotech 2017, 7, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, M.; Gomes, A.S.; Silva, C.M.; Belo, I. Microbial lipids and added value metabolites production by Yarrowia lipolytica from pork lard. J. Biotechnol. 2018, 265, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, S.; Aggelis, G. Yarrowia lipolytica: A model microorganism used for the production of tailor-made lipids. Eur. J. Lipid Sci. Technol. 2010, 112, 639–654. [Google Scholar] [CrossRef]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S. Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 6581–6588. [Google Scholar] [CrossRef]
- Gründemann, C.; Garcia-Käufer, M.; Sauer, B.; Scheer, R.; Merdivan, S.; Bettin, P.; Huber, R.; Lindequist, U. Comparative chemical and biological investigations of β-glucan-containing products from shiitake mushrooms. J. Funct. Foods 2015, 18, 692–702. [Google Scholar] [CrossRef]
- Szotkowski, M.; Holub, J.; Simansky, S.; Hubacova, K.; Sikorova, P.; Marinicova, V.; Nemcova, A.; Marova, I. Bioreactor co-cultivation of high lipid and carotenoid producing yeast Rhodotorula kratochvilovae and several microalgae under stress. Microorganisms. 2021, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, A.M.; Vustin, M.M.; Tyaglov, B.V.; Maksimova, I.A.; Sinekoiy, S.P. Pigmented Basidiomycetous yeasts are a promising source of carotenoids and ubiquinone Q10. Microbiology 2008, 77, 1–6. [Google Scholar] [CrossRef]
- Petrik, S.; Hároniková, A.; Márová, I.; Kostovová, I.; Breierová, E. Production of biomass, carotenoids and other lipid metabolites by several red yeast strains cultivated on waste glycerol from biofuel production-a comparative screening study. Ann. Microbiol. 2013, 63, 1537–1551. [Google Scholar] [CrossRef]
- Linder, T. Making the case for edible microorganisms as an integral part of a more sustainable and resilient food production system. Food Secur. 2019, 11, 265–278, ISSN 1876-4517. [Google Scholar] [CrossRef] [Green Version]




| Media volume | 2.0 L |
| Stirring | 300–800 rpm, regulated by oxygen consumption |
| pH | 6.5 |
| pO2 | 30% |
| Temperature | 25 °C |
| Aeration | 3 L per minute |
| Illumination | 300 μmol·m2·s−1 of photons |
| Retention Time (min) | Mobile Phase A (%) | Mobile Phase B (%) | |
|---|---|---|---|
| 1 | 0.0 | 90% | 10% |
| 2 | 1.0 | 90% | 10% |
| 3 | 5.0 | 85% | 15% |
| 4 | 10.0 | 80% | 20% |
| 5 | 21.0 | 25% | 75% |
| 6 | 26.0 | 55% | 45% |
| 7 | 30.0 | 90% | 10% |
| Retention Time (min) | Mobile Phase A (%) | Mobile Phase B (%) | |
|---|---|---|---|
| 1 | 0.0 | 100% | 0% |
| 2 | 13.0 | 0% | 100% |
| 3 | 19.0 | 0% | 100% |
| 4 | 20.0 | 100% | 0% |
| 5 | 25.0 | 100% | 0% |
| Retention Time (min) | Gradient (°C·min−1) | Temperature (°C) | Retention (min) | |
|---|---|---|---|---|
| 1 | 0.000 | start | - | - |
| 2 | 1.000 | 0.000 | 80.000 | 1.000 |
| 3 | 5.000 | 15.000 | 140.000 | 0.000 |
| 4 | 21.667 | 3.000 | 190.000 | 0.000 |
| 5 | 24.467 | 25.000 | 260.000 | 1.000 |
| 6 | 24.467 | stop | - | - |
| Media Type | S. pararoseus | S. metaroseus | S. salmonicolor | S. roseus |
|---|---|---|---|---|
| CN 13 + AS | 10.03 ± 0.52 | 3.41 ± 0.79 | 10.40 ± 0.62 | 7.74 ± 0.82 |
| CN 13 + urea | 8.91 ± 0.53 | 5.87 ± 0.37 | 9.44 ± 0.26 | 6.48 ± 0.64 |
| CN 25 + AS | 17.54 ± 2.21 | 3.58 ± 0.47 | 14.13 ± 0.49 | 12.09 ± 0.65 |
| CN 25 + urea | 13.69 ± 1.19 | 1.78 ± 0.74 | 12.27 ± 1.06 | 10.37 ± 0.69 |
| CN 50 + AS | 11.88 ± 0.62 | 2.65 ± 0.80 | 4.49 ± 0.83 | 11.73 ± 3.32 |
| CN50 + urea | 12.89 ± 1.34 | 3.021 ± 0.90 | 11.01 ± 1.43 | 14.96 ± 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szotkowski, M.; Holub, J.; Šimanský, S.; Hubačová, K.; Hladká, D.; Němcová, A.; Marová, I. Production of Enriched Sporidiobolus sp. Yeast Biomass Cultivated on Mixed Coffee Hydrolyzate and Fat/Oil Waste Materials. Microorganisms 2021, 9, 1848. https://doi.org/10.3390/microorganisms9091848
Szotkowski M, Holub J, Šimanský S, Hubačová K, Hladká D, Němcová A, Marová I. Production of Enriched Sporidiobolus sp. Yeast Biomass Cultivated on Mixed Coffee Hydrolyzate and Fat/Oil Waste Materials. Microorganisms. 2021; 9(9):1848. https://doi.org/10.3390/microorganisms9091848
Chicago/Turabian StyleSzotkowski, Martin, Jiří Holub, Samuel Šimanský, Klára Hubačová, Dagmar Hladká, Andrea Němcová, and Ivana Marová. 2021. "Production of Enriched Sporidiobolus sp. Yeast Biomass Cultivated on Mixed Coffee Hydrolyzate and Fat/Oil Waste Materials" Microorganisms 9, no. 9: 1848. https://doi.org/10.3390/microorganisms9091848
APA StyleSzotkowski, M., Holub, J., Šimanský, S., Hubačová, K., Hladká, D., Němcová, A., & Marová, I. (2021). Production of Enriched Sporidiobolus sp. Yeast Biomass Cultivated on Mixed Coffee Hydrolyzate and Fat/Oil Waste Materials. Microorganisms, 9(9), 1848. https://doi.org/10.3390/microorganisms9091848







