The Effectiveness of Biostimulation, Bioaugmentation and Sorption-Biological Treatment of Soil Contaminated with Petroleum Products in the Russian Subarctic
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Territory of the Study
2.2. Climate
2.3. Soils
2.4. Sorbents
2.5. Associations of Hydrocarbon Oxidizing Microorganisms
2.6. Experiment Design
2.7. Determination of the Total Petroleum Hydrocarbons Content in Soil
2.8. Determination of Physical-Chemical Properties of Soil
2.9. Biological Parameters
2.10. Statistical Processing
3. Results
3.1. Degradation Activity of Microfungi in Laboratory Experiments
- (a)
- Species with high hydrocarbon oxidizing activity, reducing the petroleum hydrocarbon content by 80–98%.
- (b)
- Species with medium hydrocarbon oxidizing activity, reducing the petroleum hydrocarbon content by 50–79%.
- (c)
- Species with low hydrocarbon oxidizing activity, reducing the petroleum hydrocarbon content by 49% or less.
3.2. Analysis of the Bioremediation Effectiveness in a Field Experiment
3.2.1. Content of Total Petroleum Hydrocarbons
3.2.2. Soil Humidity and Temperature
3.2.3. Actual Acidity
3.2.4. The Number of Hydrocarbon-Oxidizing Microorganisms
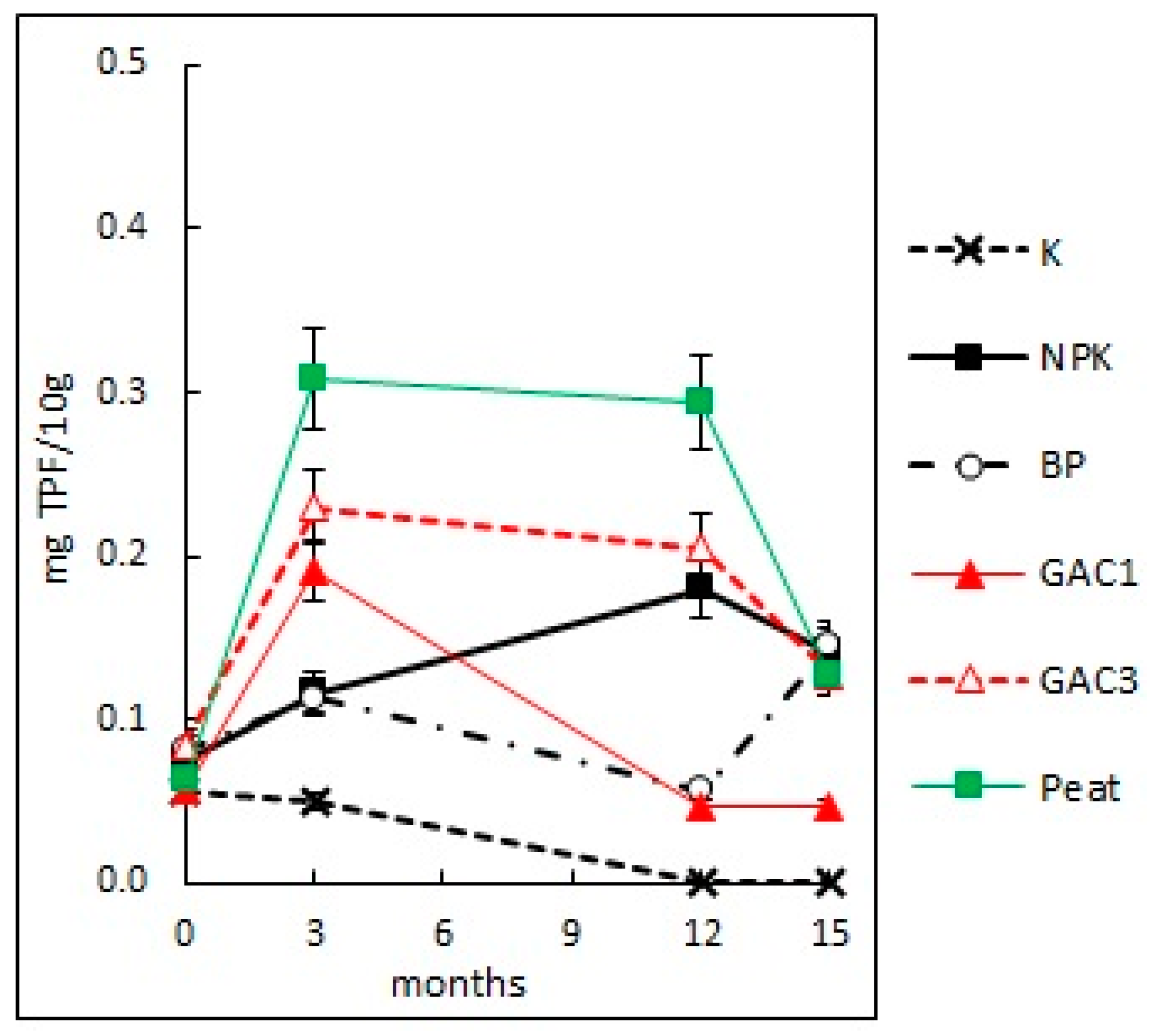
3.2.5. Soil Dehydrogenase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Pikovsky, Y.I.; Gennadiev, A.G.; Chernyansky, S.S.; Sakharov, G.N. The problem of diagnostics and regulation of soil pollution by oil and oil products. Pochvovedeniye 2003, 9, 1132–1140. [Google Scholar]
- Stroud, J.L.; Paton, G.I.; Semple, K.T. Microbe-aliphatic hydrocarbon interactions in soil: Implications for biodegradation and bioremediation. J. Appl. Microbiol. 2007, 102, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Nechaeva, I.A.; Filonov, A.E.; Akhmetov, L.I.; Puntus, I.F.; Boronin, A.M. Stimulation of microbial destruction of oil in the soil by introducing bacterial associations and mineral fertilizers in laboratory and field conditions. Biotechnology 2009, 1, 64–70. [Google Scholar]
- Sokolov, Y.I. Arctic: On the problem of accumulated environmental damage. Arctic: Ecology Econ. 2013, 2, 18–27. [Google Scholar]
- State (National) Report “On the State and Use of Land in the Russian Federation in 2018”; Rosreestr: Moscow, Russia, 2019; p. 198.
- Minaeva, T. (Ed.) Ecological Restoration in Arctic: Review of the International and Russian Practices; Triada: Petersburg, Russia, 2016; p. 288. [Google Scholar]
- Mishra, S.; Jyot, J.; Kuhad, R.C.; Lai, B. Evalution of inoculum addition to stimulate in situ bioremediation of oily-sludge-contaminated soil. Appl. Environ. Microbiol. 2001, 67, 1675–1681. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.; Nicklin, S.; Faull, J.L. Biodegradation of fuel oils and lubricants: Soil and water bioremediation options. Biotransform. Bioremediat. Technol. Health Environ. Prot. 2002, 36, 69–100. [Google Scholar] [CrossRef]
- Ouyang, W.; Liu, H.; Murygina, V.; Yongyong, Y.; Zengde, X.; Kalyuzhnyi, S. Comparison of bio-augmentation and composting for remediation of oily sludge: A field-scale study in China. Process Biochem. 2005, 40, 3763–3768. [Google Scholar] [CrossRef]
- Molla, A.H.; Fakhru’l-Razi, A. Mycoremediation—A prospective environmental friendly technique of bioseparation and dewatering of domestic wastewater sludge. Environ. Sci. Pollut. Res. 2012, 19, 1612–1619. [Google Scholar] [CrossRef]
- Aranda, E. Promising approaches towards biotransformation of polycyclic aromatic hydrocarbons with Ascomycota fungi. Curr. Opin. Biotechnol. 2016, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Treu, R.; Falandysz, J. Mycoremediation of hydrocarbons with basidiomycetes—A review. J. Environ. Sci. Health 2017, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Schluter, R.; Dallinger, A.; Kabisch, J.; Duldhardt, I.; Schauer, F. Fungal biotransformation of short-chain nalkylcycloalkanes. Appl. Microbiol. Biotechnol. 2019, 103, 4137–4151. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, V.D.; Vrvic, M.M. Potential of pure and mixed cultures of Cladosporium cladosporioides and Geotrichum candidum for application in bioremediation and detergent industry. Saudi J. Biol. Sci. 2018, 25, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obruca, S.; Marova, I.; Matouskova, P.; Haronikova, A.; Lichnova, A. Production of lignocellulose-degrading enzymes employing Fusarium solani F-552. Folia Microbiol. 2012, 57, 221–227. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Brar, S.K.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Environ. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, N.N.; Varese, G.C.; Prigione, V.; Dubrovskaya, E.V.; Balandina, S.A.; Turkovskaya, O.V. Degradative properties of two newly isolated strains of the Ascomycetes Fusarium oxysporum and Lecanicillium aphanocladii. Int. Microbiol. 2019, 22, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Chukhareva, N.V.; Shishmina, L.V. Comparison of the sorption properties of high-moor and lowland peat in relation to commercial oil and stable gas condensate. Khimiya Rastit. Syr’ya 2012, 4, 193–200. [Google Scholar]
- Mahtab, A.M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Bandura, L.; Woszuk, A.; Kolodynska, D.; Franus, W. Application of mineral sorbents for removal of petroleum substances: A review. Minerals 2017, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Nazarko, M.D.; Romanova, K.N.; Ksandopulo, S.Y.; Shcherbakov, V.G.; Alexandrova, A.V. Sorbent for cleaning soil from oil pollution. Fundam. Issled. 2006, 11, 96–98. [Google Scholar]
- Vasilyeva, G.K.; Bakhaeva, L.P.; Strijakova, E.R.; Shea, P.J. Biodegradation of 3,4-dichloroaniline and 2,4,6-trinitritiluene in soil in the presence of natural adsorbents. Environ. Chem Lett. 2003, 1, 176–183. [Google Scholar] [CrossRef]
- Vasilyeva, G.K.; Strijakova, E.R.; Shea, P.J. Use of activated carbon for soil bioremediation. In Viable Methods of Soil and Water Pollution Monitoring, Protection and Remediation; NATO Collection; Springer: Dordrecht, The Netherlands, 2006; pp. 309–322. [Google Scholar] [CrossRef]
- Yatsenko, V.S.; Strizhakova, E.R.; Vasilyeva, G.K.; Zinnatshina, L.V. A method for reducing environmental risks when carrying out an in-situ bioremediation of oil-contaminated soils. Probl. Anal. Riska. 2014, 11, 6–17. [Google Scholar]
- Vasilyeva, G.K.; Strizhakova, E.R. The use of activated carbon in bioremediation of contaminated soils and sediments (review). Vestn. RFFI 2008, 4, 32–43. [Google Scholar]
- Vasilyeva, G.K.; Strizhakova, E.R.; Baryshnikova, E.R. The use of sorbents to improve the efficiency of bioremediation of contaminated soils. Sorbenty kak faktor kachestva zhizni i zdorov’ya. In Mater. IV-y Mezhd. Konf.; BGU: Belgorod, Russia, 2012; pp. 194–200. [Google Scholar]
- Vasilyeva, G.K.; Strizhakova, E.R.; Bocharnikova, E.A.; Semenyuk, N.N.; Yatsenko, V.S.; Slyusarevskij, A.V.; Baryshnikova, E.A. Petroleum and petroleum products as soil pollutants. Technology of combined physical and biological treatment of contaminated soils. Ross. Khimicheskiy Zhurnal 2013, 57, 79–104. [Google Scholar]
- Semenyuk, N.N.; Yatsenko, V.S.; Strijakova, E.R.; Filonov, A.E.; Petrikov, K.V.; Zavgorodnyaya, Y.A.; Vasilyeva, G.K. Effect of Activated Charcoal on Bioremediation of Diesel Fuel Contaminated Soil. Microbiology 2014, 83, 589–598. [Google Scholar] [CrossRef]
- Myazin, V.A.; Isakova, E.A.; Vasilyeva, G.K. Influence of granular activated carbon on the rate of bioremediation of soils in the Murmansk region, historically contaminated with oil products. Probl. Reg. Ekol. 2020, 2, 20–26. [Google Scholar] [CrossRef]
- Polikarpova, N.V. Chronicle of the Nature Reserve “Pasvik”; Polikarpova, N.V., Ed.; KSC RAN. Publishing House: Apatity, Russia, 2011; Book 15.314. [Google Scholar]
- Archive of Weather in Nikel. Weather Statistics. Electron. Data: Weather Schedule, Cop. 2016–2021. Available online: http://rp5.ru/Weather_archive_in_Nickel (accessed on 21 June 2021).
- Zenkova, I.V. Summer temperature dynamics in mountain soils of the Pasvik reserve. Vestn. MGTU 2013, 16, 715–724. [Google Scholar]
- Myazin, V.A. The dynamic of some biogenic elements content in the soil during remediation of oil contaminated sites. Vestnik MGTU 2019, 22, 90–100. [Google Scholar] [CrossRef]
- Mukhin, V.M.; Tarasov, A.V.; Klushin, V.N. Active coals of Russia; Metallurgiya: Moscow, Russia, 2000; p. 352. [Google Scholar]
- Evdokimova, G.A.; Masloboev, V.A.; Mozgova, N.P.; Myazin, V.A.; Fokina, N.V. Bioremediation of oil-polluted cultivated soils in the Euro-Arctic Region. Environ. Sci. Eng. 2012, 9, 1130–1136. [Google Scholar]
- Korneykova, M.V.; Chaporgina, A.A.; Redkina, V.V. Oil destructive activity of fungi isolated from the soils of the Kola Peninsula Urbanization. In Challenge and Opportunity for Soil Functions and Ecosystem Services: Proceedings of the 9th Suitma Congress; Vasenev, V., Dovletyarova, E., Cheng, Z., Prokofieva, T., Morel, J., Ananyeva, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 123–134. [Google Scholar] [CrossRef]
- Quantitative Chemical Analysis of Soil. Methods for Measuring the Mass Fraction of Petroleum Products in Mineral, Organogenic, Organic-Mineral Soils and Bottom Sediments Using IR Spectrometry (Environmental Save Regulation Documents PND F 16.1:2.2.22-98); RussianGost|Official Regulatory Library: Moscow, Russia, 1998; p. 17.
- Soils. Preparation of Salt Extract and Determination of Its рН by CINAO Method (Environmental Save Regulation Documents GOST 26483-85); RussianGost|Official Regulatory Library: Moscow, Russia, 1985; p. 6. [Google Scholar]
- Galstyan, A.S. Enzymatic Activity of Soils in Armenia; Hayastan: Yerevan, Armenia, 1974; p. 276. [Google Scholar]
- Mineev, V.G. Practical Work on Agrochemistry; Izd-vo MGU: Moscow, Russia, 2001; pp. 330–332. [Google Scholar]
- Evdokimova, G.A.; Zenkova, I.V.; Mozgova, N.P.; Pereverzev, V.N. Soil and Soil Biota in the Environments of Fluorine Pollution; Academy of Sciences: Apatity, Russia, 2005; p. 155. [Google Scholar]
- Marfenina, O.E. Anthropogenic Ecology of Soil Fungi; RussianGost|Official Regulatory Library: Moscow, Russia, 2005; p. 195. [Google Scholar]
- Bashkin, V.N.; Bashkin, V.N. Geoecological Risk Management in Polar Areas; Bashkin, V.N., Galiulin, R.V., Eds.; Springer: Basel, Switzerland, 2019; p. 156. [Google Scholar]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Vidali, M. Bioremediation. An overview. Pure Appl. Chem. 2001, 73, 1163–1172. [Google Scholar] [CrossRef]
- Vasilyeva, G.; Kondrashina, V.; Striyakova, E.; Ortega-Calvo, J.-J. Adsorptive bioremediation of soil highly contaminated with crude oil. Sci. Total Environ. 2019, 706, 135739. [Google Scholar] [CrossRef] [PubMed]
- Pleshakova, E.V.; Kabantseva, E.G.; Chernovol, B.C. Dehydrogenase activity in oil-contaminated soils as a tool for monitoring bioremediation technologies. Izv. Saratov. 2010, 10, 40–46. [Google Scholar]
- Bashkin, V.N.; Galiulin, R.V.; Galiulina, R.A. Diagnostics of reagent soil contamination in the gas industry by analyzing cellulase activity. Zashchita Okruz. Sredy Neftegazov. Kompleks. 2009, 8, 4–7. [Google Scholar]
- Galiulin, R.V.; Galiulina, R.A.; Bashkin, V.N. Diagnostics of the remediation of hydrocarbon-contaminated soil. Territ. Neftegaz 2011, 10, 64–67. [Google Scholar]
- Ivanova, T.I.; Kuzmina, N.P.; Chevychelov, A.P. The number of microorganisms and the levels of microbiological activity of permafrost anthropogenically transformed pale-colored soils of Yakutia. Eurasian Soil Sci. 2008, 11, 1371–1380. [Google Scholar]
- Boopathy, R. Factors limiting bioremediation technologies. Bioresour. Technol. 2000, 74, 63–67. [Google Scholar] [CrossRef]
- Dias, R.L.; Ruberto, L.; Calabró, A.; Lo Balbo, A.; Del Panno, M.T.; Mac Cormack, W.P. Hydrocarbon removal and bacterial community structure in on-site biostimulated biopile systems designed for bioremediation of diesel-contaminated Antarctic soil. Polar Biol. 2015, 38, 677–687. [Google Scholar] [CrossRef]
- Atlas, R.M. Microbial Degradation of Petroleum Hydrocarbons: An Environmental Perspective. Microbiol. Rev. 1981, 45, 180–209. [Google Scholar] [CrossRef]
- Supriya, J.; Sameer, S.; Sibi, G. Microbial Degradation of Petroleum Hydrocarbons and Factors Influencing the Degradation Process. Bioprocess Eng. 2019, 3, 6–11. [Google Scholar] [CrossRef]
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial Degradation of Hydrocarbons—Basic Principles for Bioremediation: A Review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, S.E.; Elmquist, M.; Brändli, R.; Hartnik, T.; Jakob, L.; Henriksen, T.; Werner, D.; Cornelissen, G. Activated carbon amendment to sequester PAHs in contaminated soil: A lysimeter field trial. Chemosphere 2012, 87, 177–184. [Google Scholar] [CrossRef]
- Freidman, B.L.; Speirs, L.B.M.; Churchill, J.; Gras, S.L.; Tucci, J.; Snape, I.; Stevens, G.W.; Mumford, K.A. Biofilm communities and biodegradation within permeable reactive barriers at fuel spill sites in Antarctica. Int. Biodeterrior. Biodegrad. 2017, 125, 45–53. [Google Scholar] [CrossRef]
- Galiulin, R.V.; Bashkin, V.N.; Galiulina, R.A. Degradation of petroleum hydrocarbons in soil under the action of peat compost. Solid Fuel Chem. 2012, 46, 328–329. [Google Scholar] [CrossRef]
- Tarabukin, D.V. Assessment of the Lowland Bog Biomass for Ex Situ Remediation of Petroleum-Contaminated Soils. Environments 2020, 7, 86. [Google Scholar] [CrossRef]
- Novosolova, L.; Sirotkina, E. Peat based sorbents for treatment of polluted environments (review). Chem. Solid Fuel 2008, 4, 64–77. [Google Scholar]
- Porshnov, D.; Klavins, M. Sorption of Hydrocarbons on Peat, and Possibilities for Using Peat-Based Oil Sorbent for Treatment of Polluted Areas. In Proceedings of the Linnaeus ECO-TECH ‘10, Kalmar, Sweden, 22–24 November 2010; pp. 1032–1045. [Google Scholar] [CrossRef]


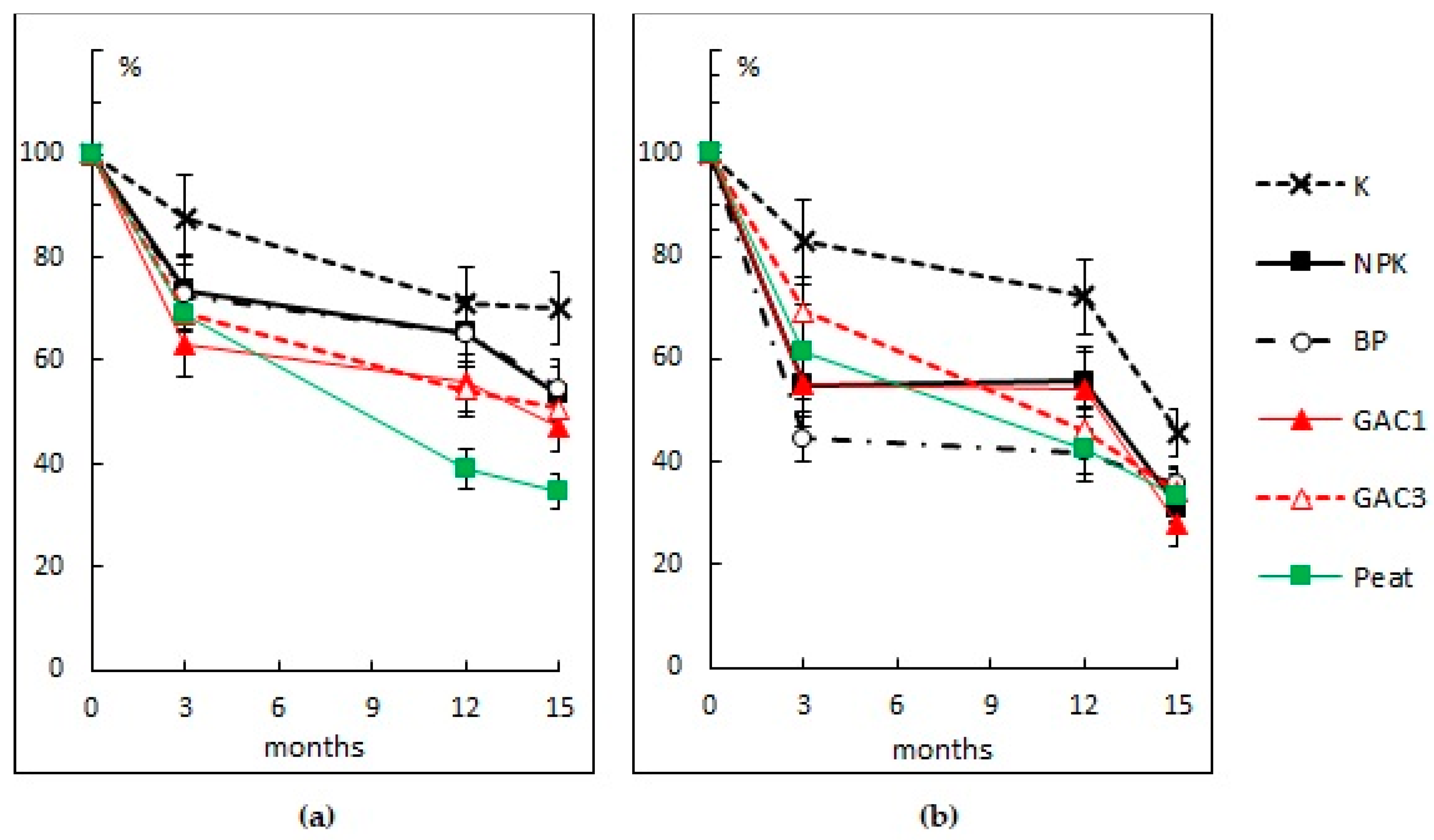
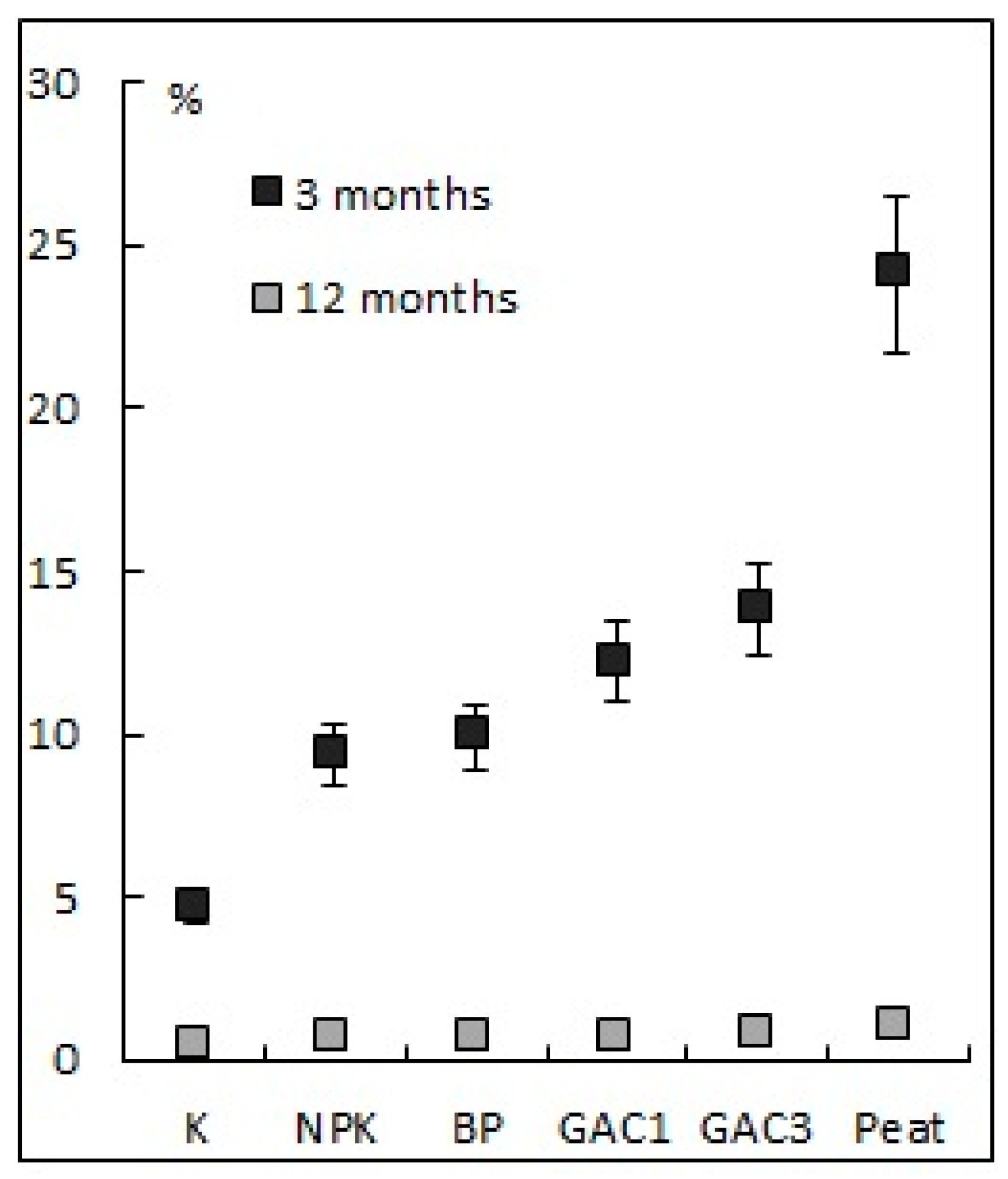
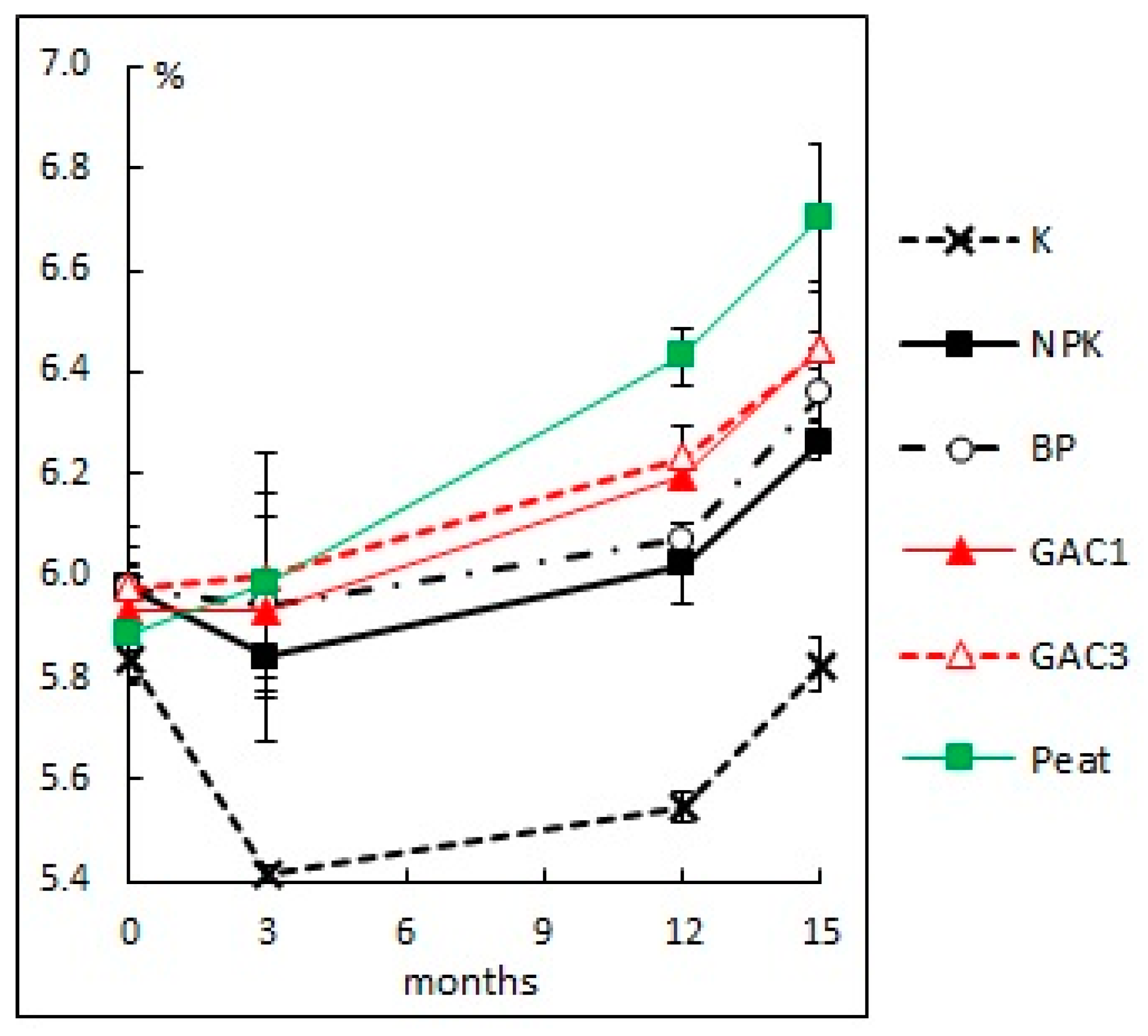
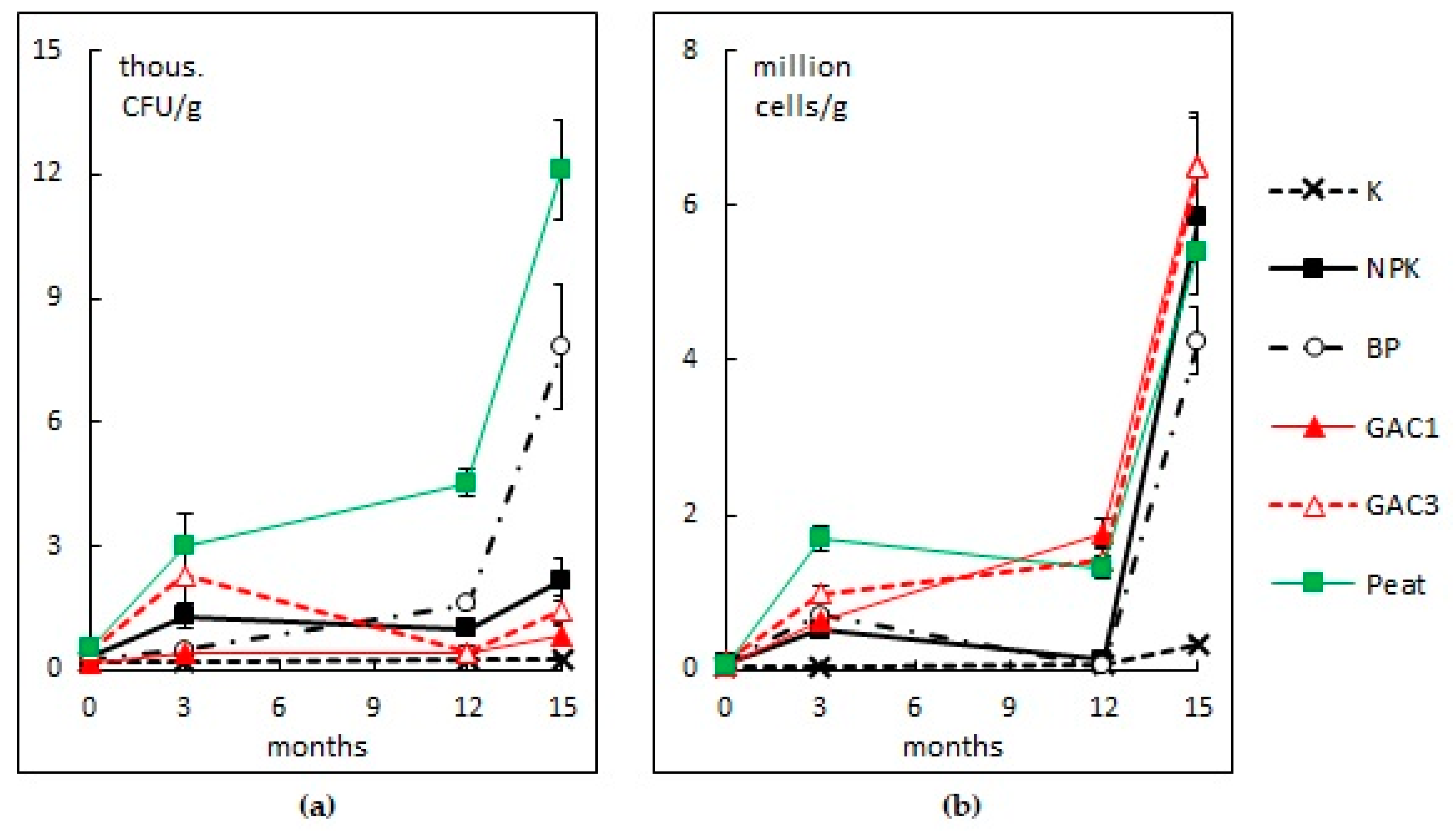
| Variant | Activated Carbon (GAC), g/m2 | N16P16K16, (g N, P2O5, K2O/m2) | Dolomite Flour, g CaCO3/m2 | HOM Association Suspension, L/m2 | Peat, L/m2 |
|---|---|---|---|---|---|
| K | - | - | - | - | - |
| NPK | - | 25 | 200 | - | - |
| BP | - | 25 | 200 | 2 | - |
| GAC1 | 1300 | 25 | 200 | - | - |
| GAC3 | 3900 | 25 | 200 | - | - |
| Peat | - | 25 | 200 | - | 16 |
| Species of Microfungi | Petroleum Degradation, % of the Initial Amount | Degradation Activity, g Petroleum /g Microfungi | Dry Biomass, g |
|---|---|---|---|
| group I—high activity | |||
| Penicillium commune Thom | 98 | 0.414 | 0.596 |
| P. canescens (st.1) Sopp | 98 | 0.372 | 0.867 |
| P. simplicissimum (st.1) (Oudem.) Thom | 96 | 0.353 | 0.687 |
| P. canescens (st.2) Sopp | 95 | 0.335 | 0.758 |
| P. restrictum J.C. Gilman & E.V. Abbott | 95 | 0.330 | 0.738 |
| P. ochrochloron Biourge | 94 | 0.337 | 0.615 |
| P. velutinum J.F.H. Beyma | 93 | 0.253 | 0.335 |
| Ulocladium consortiale (Thum.)E.G.Simmons | 92 | 0.343 | 0.518 |
| P. implicatum Biourge | 92 | 0.327 | 0.530 |
| P. decumbens Thom | 92 | 0.266 | 0.810 |
| P. canescens (st.3) Sopp | 88 | 0.229 | 0.651 |
| P. simplicissimum (st.2) (Oudem.) Thom | 87 | 0.311 | 0.519 |
| P. miczynskii (st.1) K.M. Zaleski | 85 | 0.294 | 0.573 |
| P. spinulosum Thom. | 84 | 0.245 | 0.847 |
| P. jensenii (st.1) K.M. Zaleski | 84 | 0.312 | 0.667 |
| Fusarium solani (Mart.) Sacc. | 84 | 0.310 | 0.251 |
| Alternaria alternata (Fr.) Keissl. | 83 | 0.304 | 0.515 |
| F. oxysporum (st.1) Schltdl. | 80 | 0.311 | 0.268 |
| group II—medium activity | |||
| P. aurantiogriseum (st.1) Dierckx | 79 | 0.269 | 0.620 |
| Rhizopus stolonifer (Ehrenb.) Vuill. | 76 | 0.285 | 0.485 |
| P. adametzii K.M. Zaleski | 75 | 0.267 | 0.716 |
| P. glabrum (Wehmer) Westling | 74 | 0.248 | 0.406 |
| P. spinulosum (st.2) Thom | 74 | 0.281 | 0.463 |
| P. miczynskii (st.2) K.M. Zaleski | 73 | 0.265 | 0.525 |
| Lecanicillium lecanii (Zimm.) Zare & W. Gams | 72 | 0.298 | 0.352 |
| Stachybotrys echinata (Rivolta) G. Sm., | 70 | 0.239 | 0.406 |
| P. canescens (st.4) Sopp. | 70 | 0.295 | 0.466 |
| Trichoderma viride Pers. | 67 | 0.233 | 0.283 |
| P. aurantiogriseum (st.2) Dierckx | 67 | 0.247 | 0.418 |
| P. nalgiovense Laxa | 66 | 0.266 | 0.628 |
| Umbelopsis isabellina (Oudem.) W. Gams | 63 | 0.294 | 0.415 |
| Cephalotrichum stemonitis (Pers.) Nees | 62 | 0.257 | 0.563 |
| Talaromyces stipitatus C.R. Benj. | 62 | 0.270 | 0.361 |
| Chaetomium bostrychodes Zopf | 60 | 0.294 | 0.365 |
| Acremonium egyptiacum (J.F.H. Beyma) W. Gams, | 57 | 0.215 | 0.391 |
| Fusicolla merismoides (Corda) Gräfenhan, Seifert & Schroers | 57 | 0.218 | 0.224 |
| Wallrothiella subiculosa Höhn. | 55 | 0.241 | 0.300 |
| P. multicolor Grig.-Man. & Porad. | 50 | 0.231 | 0.407 |
| group III—low activity | |||
| L. psalliotae (Treschew) Zare & W. Gams | 46 | 0.233 | 0.369 |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert & W. Gams | 44 | 0.281 | 0.402 |
| Umbelopsis longicollis (Dixon-Stew.) Y.N Wang, X.Y. Liu & R.Y. Zheng | 41 | 0.230 | 0.391 |
| Cephalosporium bonordenii Sacc. | 37 | 0.204 | 0.191 |
| Pseudogymnoascus pannorum (Link) Minnis & D.L. Lindner | 33 | 0.233 | 0.185 |
| Scopulariopsis communis (st.1) Bainier | 33 | 0.195 | 0.168 |
| P.thomii Maire | 31 | 0.196 | 0.444 |
| Aspergillus fumigatus Fresen. | 30 | 0.287 | 0.438 |
| P.jensenii K.M. Zaleski | 30 | 0.241 | 0.332 |
| Tr. koningii Oudem. | 29 | 0.162 | 0.116 |
| P.melinii Thom | 29 | 0.274 | 0.419 |
| P. aurantiogriseum (st.3) Dierckx | 28 | 0.245 | 0.461 |
| Phoma eupyrena Sacc. | 28 | 0.236 | 0.513 |
| Torula herbarum (Pers.) Link | 27 | 0.180 | 0.186 |
| Mucor hiemalis Wehmer | 25 | 0.149 | 0.104 |
| Ph. herbarum Westend. | 25 | 0.205 | 0.236 |
| Acr. charticola (Lindau) W. Gams | 24 | 0.213 | 0.234 |
| Tr. polysporum (Link) Rifai | 23 | 0.136 | 0.044 |
| Amorphotheca resinae Parbery | 20 | 0.170 | 0.174 |
| Gibberella fujikuroi (Sawada) Wollenw. | 20 | 0.170 | 0.293 |
| Acr. rutilum W. Gams | 20 | 0.117 | 0.123 |
| Tr. aureoviride Rifai | 19 | 0.162 | 0.165 |
| Clad. cladosporioides (Fresen.) G.A. de Vries | 19 | 0.160 | 0.071 |
| Gibellulopsis nigrescens (Pethybr.) Zare, W. Gams & Summerb. | 17 | 0.222 | 0.183 |
| P.aurantiogriseum(st.4) Dierckx | 17 | 0.122 | 0.327 |
| Aureobasidium microstictum (Bubák) W.B. Cooke | 17 | 0.110 | 0.095 |
| Sc. communis (st.2) Bainier | 15 | 0.109 | 0.165 |
| Gongronella butleri (Lendn.) Peyronel & Dal Vesco | 13 | 0.107 | 0.109 |
| Ph. glomerata (Corda) Wollenw. & Hochapfel | 13 | 0.029 | 0.279 |
| M.circinelloides Tiegh. | 10 | 0.116 | 0.163 |
| Rodotorula sp. | 10 | 0.087 | 0.128 |
| Aur. pullulans (de Bary & Löwenthal) G. Arnaud | 9 | 0.054 | 0.097 |
| Cph. nanum (Ehrenb.)S.Hughes | 9 | 0.050 | 0.090 |
| P. corylophilum Dierckx | 9 | 0.091 | 0.501 |
| Streptothrix luteola Foul. & P.C. Jones, | 8 | 0.107 | 0.311 |
| Tl. flavus (Klocker) Stolk et Samson | 8 | 0.126 | 0.289 |
| P. raistrickii G. Sm. | 7 | 0.084 | 0.423 |
| Asp. repens (Corda) Sacc. | 6 | 0.074 | 0.100 |
| T. allii (Harz) Sacc. | 5 | 0.083 | 0.141 |
| Botrytis cinerea Pers. | 5 | 0.065 | 0.164 |
| F. oxisporum Schltdl. | 4 | 0.053 | 0.178 |
| P. spinulosum Thom | 4 | 0.033 | 0.119 |
| Humicola grisea Traaen | 3 | 0.020 | 0.280 |
| TPH, mg/kg | Organic Matter, % | pH | Humidity, % | Temperature, °C |
|---|---|---|---|---|
| 26,548 ± 2299 | 5.04 ± 0.29 | 5.93 ± 0.03 | 10.2 ± 3.1 | 21.5 ± 1.1 |
| Variant | The Rate Constant of Hydrocarbons Decomposition | TAPC (Days) | T99 (Days) |
|---|---|---|---|
| K | 0.00079 | 4892 | 5816 |
| NPK | 0.00140 | 2699 | 3298 |
| BP | 0.00135 | 2428 | 3420 |
| GAC1 | 0.00168 | 2104 | 2746 |
| GAC3 | 0.00151 | 2501 | 3053 |
| Peat | 0.00235 | 1479 | 1960 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myazin, V.A.; Korneykova, M.V.; Chaporgina, A.A.; Fokina, N.V.; Vasilyeva, G.K. The Effectiveness of Biostimulation, Bioaugmentation and Sorption-Biological Treatment of Soil Contaminated with Petroleum Products in the Russian Subarctic. Microorganisms 2021, 9, 1722. https://doi.org/10.3390/microorganisms9081722
Myazin VA, Korneykova MV, Chaporgina AA, Fokina NV, Vasilyeva GK. The Effectiveness of Biostimulation, Bioaugmentation and Sorption-Biological Treatment of Soil Contaminated with Petroleum Products in the Russian Subarctic. Microorganisms. 2021; 9(8):1722. https://doi.org/10.3390/microorganisms9081722
Chicago/Turabian StyleMyazin, Vladimir A., Maria V. Korneykova, Alexandra A. Chaporgina, Nadezhda V. Fokina, and Galina K. Vasilyeva. 2021. "The Effectiveness of Biostimulation, Bioaugmentation and Sorption-Biological Treatment of Soil Contaminated with Petroleum Products in the Russian Subarctic" Microorganisms 9, no. 8: 1722. https://doi.org/10.3390/microorganisms9081722
APA StyleMyazin, V. A., Korneykova, M. V., Chaporgina, A. A., Fokina, N. V., & Vasilyeva, G. K. (2021). The Effectiveness of Biostimulation, Bioaugmentation and Sorption-Biological Treatment of Soil Contaminated with Petroleum Products in the Russian Subarctic. Microorganisms, 9(8), 1722. https://doi.org/10.3390/microorganisms9081722








