Host Age and Denture Wearing Jointly Contribute to Oral Colonization with Intrinsically Azole-Resistant Yeasts in the Elderly
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort and Sampling
2.2. Fungal Differentiation and Drug Resistance Testing
2.3. Dermatophyte Sampling and Differentiation
2.4. Biofilm Formation on Denture Material
2.5. Statistical Data Analysis
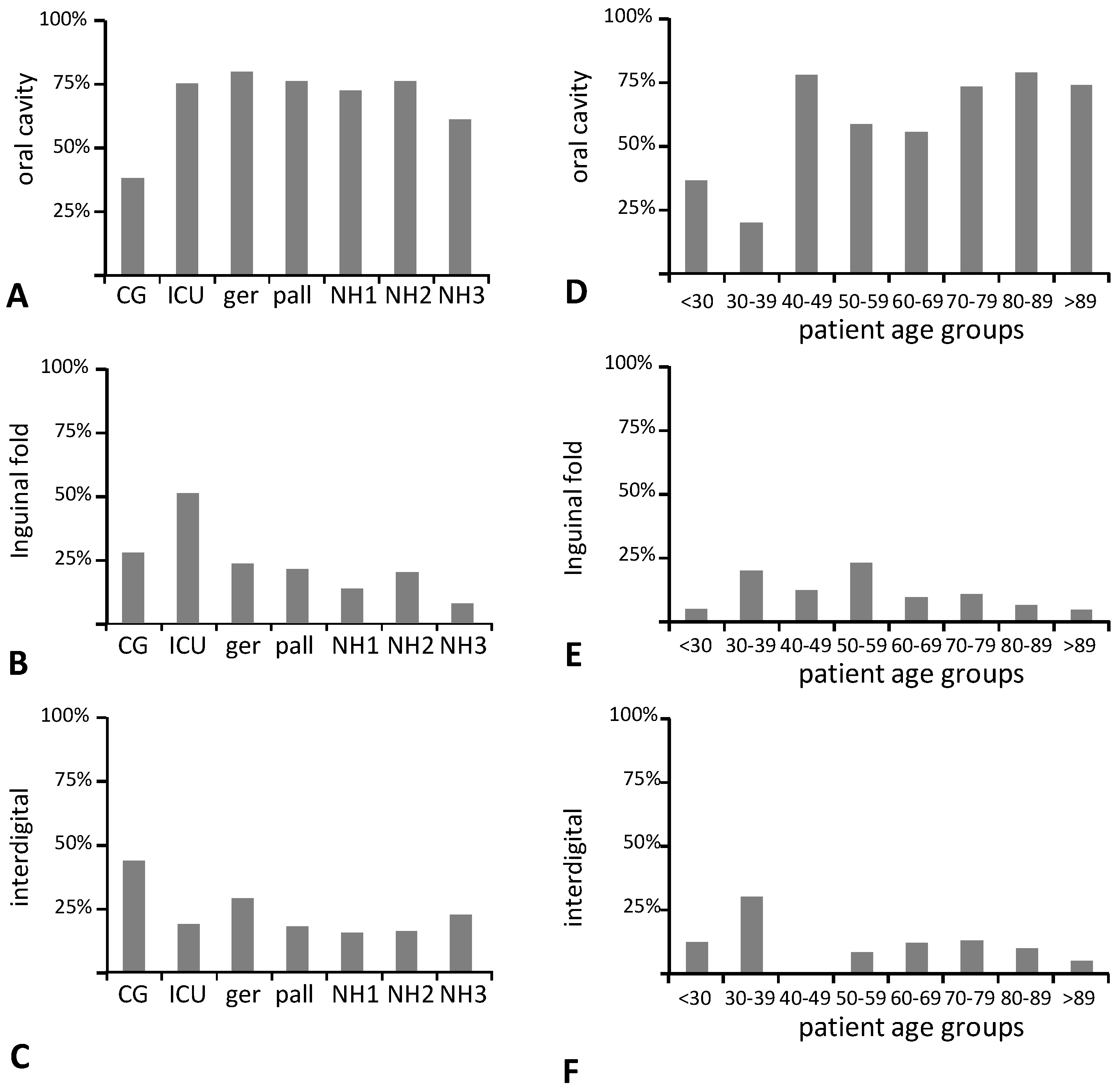
3. Results
3.1. Cohort
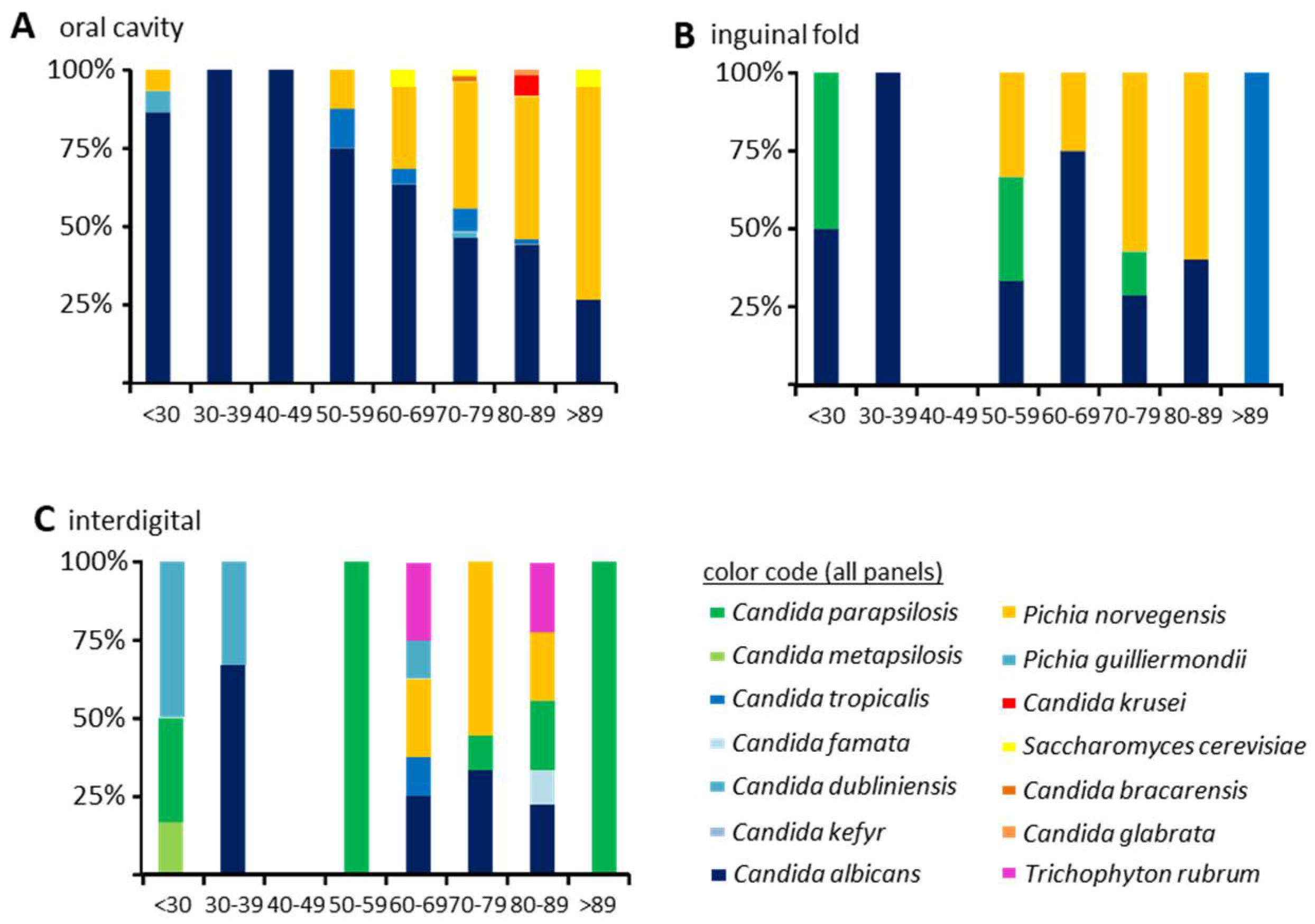
3.2. Fungal Colonization
3.3. Antifungal Drug Susceptibility
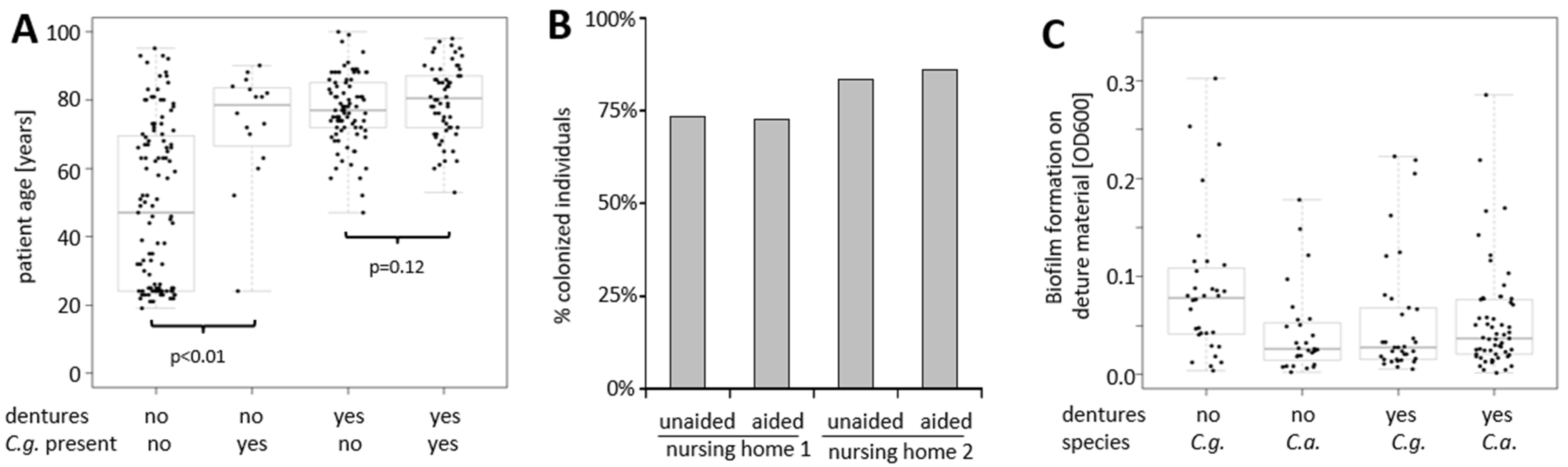
3.4. Increasing Host Age and Wearing of Dentures Are Both Predictors of Oral Colonization with C. glabrata
3.5. Oral Colonization with C. albicans Is Different between Healthy and Diseased Subjects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Luzzati, R.; Cavinato, S.; Deiana, M.L.; Rosin, C.; Maurel, C.; Borelli, M. Epidemiology and outcome of nosocomial candidemia in elderly patients admitted prevalently in medical wards. Aging Clin. Exp. Res. 2015, 27, 131–137. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Chatterjee, S.S.; Rao, K.L.; Zameer, M.M.; Shivaprakash, M.R.; Singhi, S.; Singh, R.; Varma, S.C. Recent experience with fungaemia: Change in species distribution and azole resistance. Scand. J. Infect. Dis. 2009, 41, 275–284. [Google Scholar] [CrossRef]
- Timmermans, B.; De Las Penas, A.; Castano, I.; Van Dijck, P. Adhesins in Candida glabrata. J. Fungi 2018, 4, 60. [Google Scholar] [CrossRef]
- Gomez-Molero, E.; De-la-Pinta, I.; Fernandez-Pereira, J.; Gross, U.; Weig, M.; Quindos, G.; de Groot, P.W.J.; Bader, O. Candida parapsilosis Colony Morphotype Forecasts Biofilm Formation of Clinical Isolates. J. Fungi 2021, 7, 33. [Google Scholar] [CrossRef]
- Gómez-Molero, E.; Willis, J.; Dudakova, A.; Gacser, A.; Weig, M.; Groß, U.; Gabaldón, T.; Bader, O. Phenotypic variability in a coinfection with three independent C. parapsilosis lineages. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Zarco, M.F.; Vess, T.J.; Ginsburg, G.S. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012, 18, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Malani, A.N.; Psarros, G.; Malani, P.N.; Kauffman, C.A. Is age a risk factor for Candida glabrata colonisation? Mycoses 2011, 54, 531–537. [Google Scholar] [CrossRef][Green Version]
- Zaremba, M.L.; Daniluk, T.; Rozkiewicz, D.; Cylwik-Rokicka, D.; Kierklo, A.; Tokajuk, G.; Dabrowska, E.; Pawinska, M.; Klimiuk, A.; Stokowska, W.; et al. Incidence rate of Candida species in the oral cavity of middle-aged and elderly subjects. Adv. Med.Sci. 2006, 51 (Suppl. 1), 233–236. [Google Scholar]
- Vanden Abbeele, A.; de Meel, H.; Ahariz, M.; Perraudin, J.P.; Beyer, I.; Courtois, P. Denture contamination by yeasts in the elderly. Gerodontology 2008, 25, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Renteria, M.; Marquez-Preciado, R.; Ortiz-Magdaleno, M.; Bermeo-Escalona, J.; Sanchez-Vargas, L.O. Frequency of Pathogenic Microorganisms in Removable Orthodontic Appliances and Oral Mucosa in Children. J. Clin. Pediatric Dentistry 2021, 45, 135–139. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Castanheira, M.; Messer, S.A.; Moet, G.J.; Jones, R.N. Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: Report from the SENTRY Antimicrobial Surveillance Program (2008–2009). Diagn. Microbiol. Infect. Dis. 2010, 68, 278–283. [Google Scholar] [CrossRef]
- De Groot, P.W.; Bader, O.; De Boer, A.D.; Weig, M.; Chauhan, N. Adhesins in human fungal pathogens: Glue with plenty of stick. Eukaryot. Cell 2013, 12, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Vale-Silva, L.; Beaudoing, E.; Tran, V.D.T.; Sanglard, D. Comparative Genomics of Two Sequential Candida glabrata Clinical Isolates. G3 2017, 7, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. EUCAST definitive document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol Infect. 2008, 14, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Eisner, J.D.; Kattar, M.M.; Rassoulian-Barrett, S.L.; LaFe, K.; Yarfitz, S.L.; Limaye, A.P.; Cookson, B.T. Identification of medically important yeasts using PCR-based detection of DNA sequence polymorphisms in the internal transcribed spacer 2 region of the rRNA genes. J. Clin. Microbiol. 2000, 38, 2302–2310. [Google Scholar] [CrossRef]
- Heidemann, S.; Monod, M.; Graser, Y. Signature polymorphisms in the internal transcribed spacer region relevant for the differentiation of zoophilic and anthropophilic strains of Trichophyton interdigitale and other species of T. mentagrophytes sensu lato. Br. J. Dermatol. 2010, 162, 282–295. [Google Scholar] [CrossRef]
- Gupta, A.K.; Daigle, D.; Foley, K.A. The prevalence of culture-confirmed toenail onychomycosis in at-risk patient populations. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Furlaneto-Maia, L.; Specian, A.F.; Bizerra, F.C.; de Oliveira, M.T.; Furlaneto, M.C. In vitro evaluation of putative virulence attributes of oral isolates of Candida spp. obtained from elderly healthy individuals. Mycopathologia 2008, 166, 209–217. [Google Scholar] [CrossRef]
- Gacon, I.; Loster, J.E.; Wieczorek, A. Relationship between oral hygiene and fungal growth in patients: Users of an acrylic denture without signs of inflammatory process. Clin. Interv. Aging 2019, 14, 1297–1302. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Kleinegger, C.L.; Lockhart, S.R.; Vargas, K.; Soll, D.R. Frequency, intensity, species, and strains of oral Candida vary as a function of host age. J. Clin. Microbiol. 1996, 34, 2246–2254. [Google Scholar] [CrossRef]
- Daniluk, T.; Tokajuk, G.; Stokowska, W.; Fiedoruk, K.; Sciepuk, M.; Zaremba, M.L.; Rozkiewicz, D.; Cylwik-Rokicka, D.; Kedra, B.A.; Anielska, I.; et al. Occurrence rate of oral Candida albicans in denture wearer patients. Adv. Med Sci. 2006, 51 (Suppl. 1), 77–80. [Google Scholar]
- Macaulay, R.; Akbar, A.N.; Henson, S.M. The role of the T cell in age-related inflammation. Age 2013, 35, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, V.; Rink, L.; Uciechowski, P. The Th17/Treg balance is disturbed during aging. Exp. Gerontol. 2013, 48, 1379–1386. [Google Scholar] [CrossRef]
- do Nascimento, M.P.; Pinke, K.H.; Penitenti, M.; Ikoma, M.R.; Lara, V.S. Aging does not affect the ability of human monocyte-derived dendritic cells to phagocytose Candida albicans. Aging Clin. Exp. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.N.; Brailsford, S.; Broadley, K.; Beighton, D. Oral yeast carriage in patients with advanced cancer. Oral Microbiol. Immunol. 2002, 17, 79–84. [Google Scholar] [CrossRef]
- Duan, M.; Ning, Z.; Fu, Z.; Zhang, J.; Liu, G.; Wei, Q.; Zheng, X. Decreased IL-27 Negatively Correlated with Th17 Cells in Non-Small-Cell Lung Cancer Patients. Mediat. Inflamm. 2015, 2015, 802939. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; Hsueh, E.C.; et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015, 6, 17462–17478. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Duggan, S.; Essig, F.; Hunniger, K.; Mokhtari, Z.; Bauer, L.; Lehnert, T.; Brandes, S.; Hader, A.; Jacobsen, I.D.; Martin, R.; et al. Neutrophil activation by Candida glabrata but not Candida albicans promotes fungal uptake by monocytes. Cell Microbiol. 2015. [Google Scholar] [CrossRef]
- Wagener, J.; MacCallum, D.M.; Brown, G.D.; Gow, N.A. Candida albicans Chitin Increases Arginase-1 Activity in Human Macrophages, with an Impact on Macrophage Antimicrobial Functions. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Keppler-Ross, S.; Douglas, L.; Konopka, J.B.; Dean, N. Recognition of yeast by murine macrophages requires mannan but not glucan. Eukaryot. Cell 2010, 9, 1776–1787. [Google Scholar] [CrossRef]
- De Groot, P.W.J.; Kraneveld, E.A.; Yin, Q.Y.; Dekker, H.L.; Groß, U.; Crielaard, W.; De Koster, C.G.; Bader, O.; Klis, F.M.; Weig, M. The cell wall of the human pathogen Candida glabrata: Differential incorporation of novel adhesin-like wall proteins. Eukaryot. Cell 2008, 7, 1951–1964. [Google Scholar] [CrossRef]
- Halliwell, S.C.; Smith, M.C.; Muston, P.; Holland, S.L.; Avery, S.V. Heterogeneous expression of the virulence-related adhesin Epa1 between individual cells and strains of the pathogen Candida glabrata. Eukaryot. Cell 2012, 11, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jimenez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Castanheira, M.; Lockhart, S.R.; Ahlquist, A.M.; Messer, S.A.; Jones, R.N. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J. Clin. Microbiol. 2012, 50, 1199–1203. [Google Scholar] [CrossRef]
- Coste, A.T.; Kritikos, A.; Li, J.; Khanna, N.; Goldenberger, D.; Garzoni, C.; Zehnder, C.; Boggian, K.; Neofytos, D.; Riat, A.; et al. Emerging echinocandin-resistant Candida albicans and glabrata in Switzerland. Infection 2020, 48, 761–766. [Google Scholar] [CrossRef]
- Fanello, S.; Bouchara, J.P.; Sauteron, M.; Delbos, V.; Parot, E.; Marot-Leblond, A.; Moalic, E.; Le Flohicc, A.M.; Brangerd, B. Predictive value of oral colonization by Candida yeasts for the onset of a nosocomial infection in elderly hospitalized patients. J. Med Microbiol. 2006, 55, 223–228. [Google Scholar] [CrossRef] [PubMed]
| Study Group | Health Status | Total | Mean (+/− SD) Age | Male/Female |
|---|---|---|---|---|
| control group | healthy | 46 | 25 ± 3.6 y | 67%/33% |
| palliative care unit | diseased | 50 | 65 ± 10.0 y | 34%/66% |
| intensive care unit | diseased | 47 | 67 ± 15.6 y | 59%/41% |
| geriatric care unit | diseased | 51 | 80 ± 6.7 y | 44%/56% |
| nursing home #1 | healthy | 37 | 83 ± 8.1 y | 81%/19% |
| nursing home #2 | healthy | 30 | 86 ± 8.6 y | 67%/33% |
| nursing home #3 | healthy | 13 | 81 ± 13.1 y | 54%/46% |
| Yeast Species | Dentures | |
|---|---|---|
| No | Yes | |
| (culture negative) | 81 | 115 |
| C. albicans only | 54 | 38 |
| C. albicans + other yeast | 2 | 4 |
| C. albicans + C. glabrata | 4 | 14 |
| C. albicans + C. glabrata + other yeast(s) | 2 | |
| C. glabrata only | 13 | 41 |
| C. glabrata + other yeast(s) | 1 | 2 |
| other yeast(s) only | 7 | 4 |
| total culture-positive specimen | 81 | 105 |
| % culture positivity | 50% | 48% |
| % positive containing C. albicans | 74% | 50% |
| % positive containing C. glabrata | 31% | 55% |
| Parameter | Level | Presence of C. albicans | Presence of Non-albicans Candida Species | Presence of C. glabrata | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | OR | p, (pmult) | No | Yes | OR | p, (pmult) | No | Yes | OR | p, (pmult) | ||
| age | years | 65.53 +/− 23.84 | 66.14 +/− 20.68 | 0.8227 (0.0908) | 59.81 +/− 24.04 | 76.82 +/− 14.07 | <0.0001 (0.0006) | 60.75 +/− 23.76 | 78.40 +/− 12.33 | <0.0001 (0.0010) | |||
| gender | female | 96(62%) | 60(38%) | 1.22 | 0.4572 | 98(63%) | 58(37%) | 0.80 | 0.4435 | 108(69%) | 48(31%) | 0.77 | 0.3473 |
| male | 67(57%) | 51(43%) | 80(68%) | 38(32%) | 88(75%) | 30(25%) | |||||||
| health status | control | 33(72%) | 13(28%) | <0.0001 (0.0007) | 44(96%) | 2(4%) | <0.0001 (0.0628) | 46(100%) | 0(0%) | <0.0001 (0.0189) | |||
| healthy | 58(72%) | 22(28%) | 39(49%) | 41(51%) | 45(56%) | 35(44%) | |||||||
| diseased | 72(49%) | 76(51%) | 95(64%) | 53(36%) | 105(71%) | 43(29%) | |||||||
| antibacterials | no | 136(61%) | 87(39%) | 1.39 | 0.3432 | 147(66%) | 76(34%) | 1.25 | 0.5172 | 164(74%) | 59(26%) | 1.65 | 0.1259 |
| yes | 27(53%) | 24(47%) | 31(61%) | 20(39%) | 32(63%) | 19(37%) | |||||||
| antimycotics | no | 156(60%) | 104(40%) | 1.50 | 0.5781 | 166(64%) | 94(36%) | 0.30 | 0.1485 (0.9856) | 183(70%) | 77(30%) | 0.18 | 0.1234 (0.9942) |
| yes | 7(50%) | 7(50%) | 12(86%) | 2(14%) | 13(93%) | 1(7%) | |||||||
| independent oral hygiene | yes | 125(61%) | 79(39%) | 1.33 | 0.3254 | 138(68%) | 66(32%) | 1.56 | 0.1461 | 152(75%) | 52(25%) | 1.72 | 0.0672 |
| no | 38(54%) | 32(46%) | 40(57%) | 30(43%) | 44(63%) | 26(37%) | |||||||
| oral dentures present | no | 80(61%) | 51(39%) | 1.13 | 0.6243 (0.9167) | 107(82%) | 24(18%) | 4.49 | <0.0001 (0.2331) | 115(88%) | 16(12%) | 1.47 | <0.0001 (0.1243) |
| yes | 83(58%) | 60(42%) | 71(50%) | 72(50%) | 81(57%) | 62(43%) | |||||||
| health status X age | (0.0185) | (0.0055) | |||||||||||
| health status X antimycotics | (0.9851) | (0.9932) | |||||||||||
| health status X dentures | (0.0219) | (0.0243) | |||||||||||
| age X dentures | (0.9300) | (0.8903) | (0.4091) | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojak, K.-P.; Ungermann, G.F.; Ichsan, I.; Gomez-Molero, E.; Jung, K.; Weig, M.; Nauck, F.; Ziebolz, D.; Gräser, Y.; Nau, R.; et al. Host Age and Denture Wearing Jointly Contribute to Oral Colonization with Intrinsically Azole-Resistant Yeasts in the Elderly. Microorganisms 2021, 9, 1627. https://doi.org/10.3390/microorganisms9081627
Wojak K-P, Ungermann GF, Ichsan I, Gomez-Molero E, Jung K, Weig M, Nauck F, Ziebolz D, Gräser Y, Nau R, et al. Host Age and Denture Wearing Jointly Contribute to Oral Colonization with Intrinsically Azole-Resistant Yeasts in the Elderly. Microorganisms. 2021; 9(8):1627. https://doi.org/10.3390/microorganisms9081627
Chicago/Turabian StyleWojak, Klaus-Peter, Gertrud F. Ungermann, Ichsan Ichsan, Emilia Gomez-Molero, Klaus Jung, Michael Weig, Friedemann Nauck, Dirk Ziebolz, Yvonne Gräser, Roland Nau, and et al. 2021. "Host Age and Denture Wearing Jointly Contribute to Oral Colonization with Intrinsically Azole-Resistant Yeasts in the Elderly" Microorganisms 9, no. 8: 1627. https://doi.org/10.3390/microorganisms9081627
APA StyleWojak, K.-P., Ungermann, G. F., Ichsan, I., Gomez-Molero, E., Jung, K., Weig, M., Nauck, F., Ziebolz, D., Gräser, Y., Nau, R., Groß, U., Alt-Epping, B., & Bader, O. (2021). Host Age and Denture Wearing Jointly Contribute to Oral Colonization with Intrinsically Azole-Resistant Yeasts in the Elderly. Microorganisms, 9(8), 1627. https://doi.org/10.3390/microorganisms9081627









