Visible Lights Combined with Photosensitizing Compounds Are Effective against Candida albicans Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Light Sources and Photosensitizing Compounds
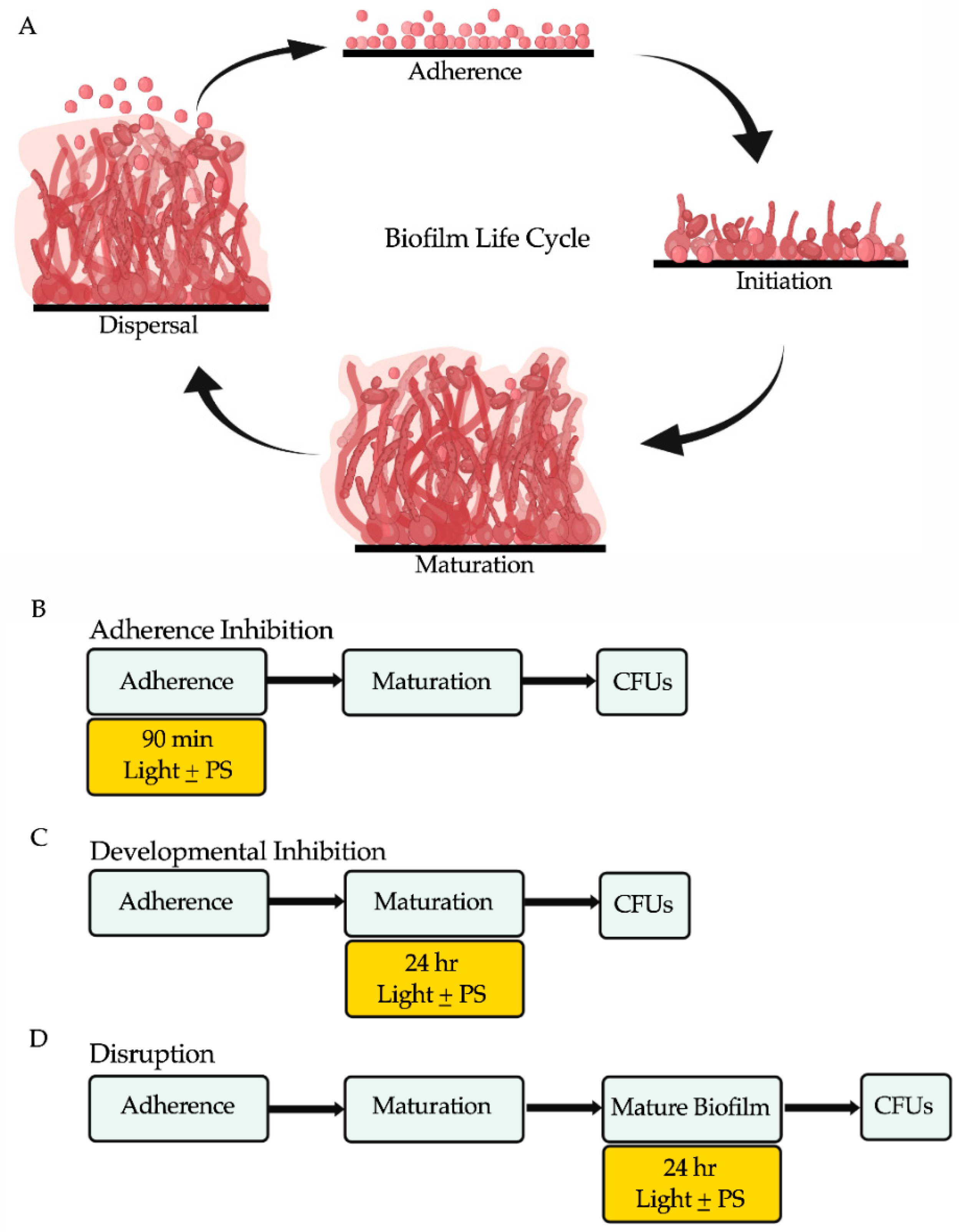
2.3. Biofilm Assays
2.4. Determination of Colony Forming Units (CFUs) from Biofilms
2.5. Viability Staining of Biofilm Cells
2.6. Assessment of Cellular Morphologies of Biofilm Cells
3. Results
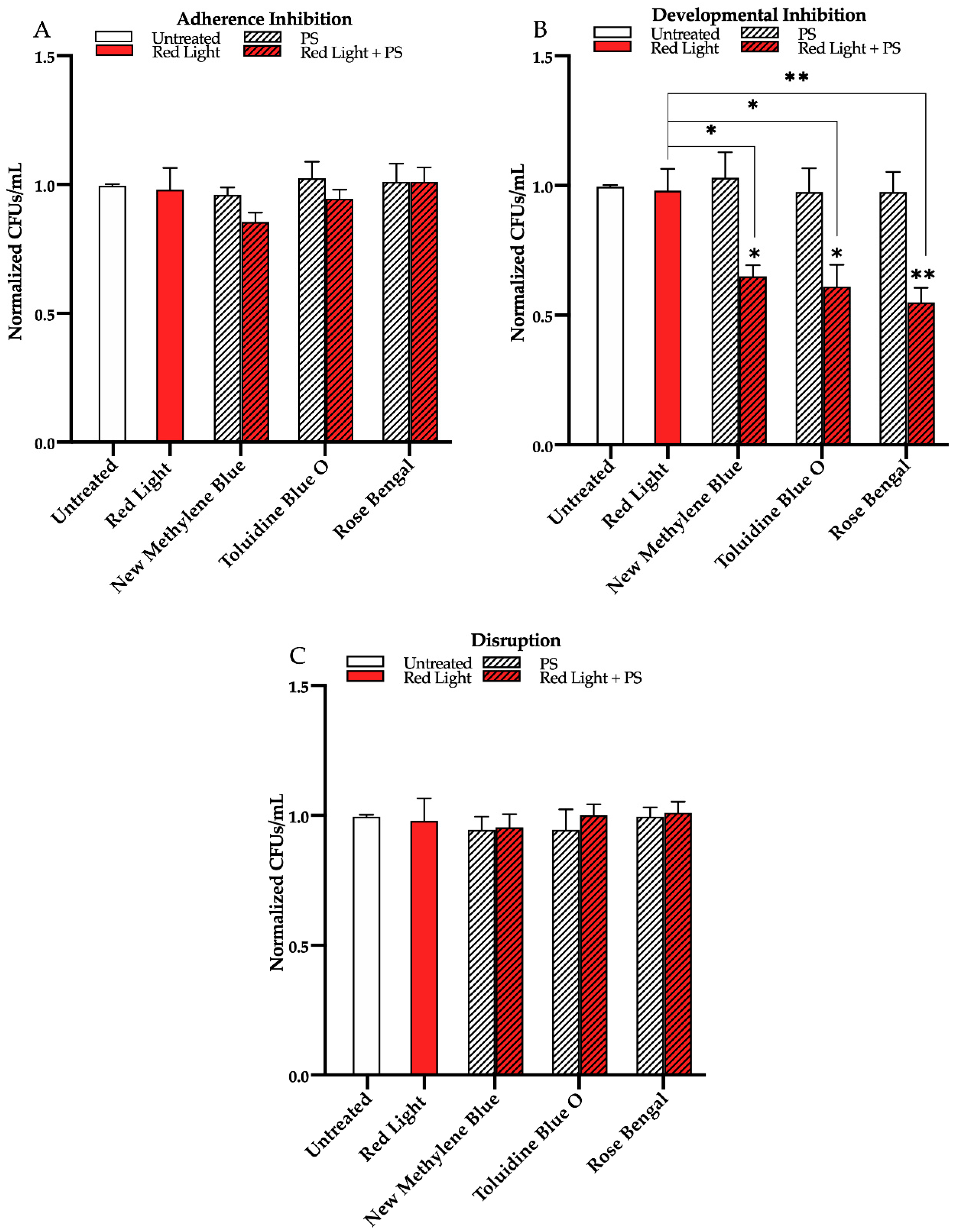
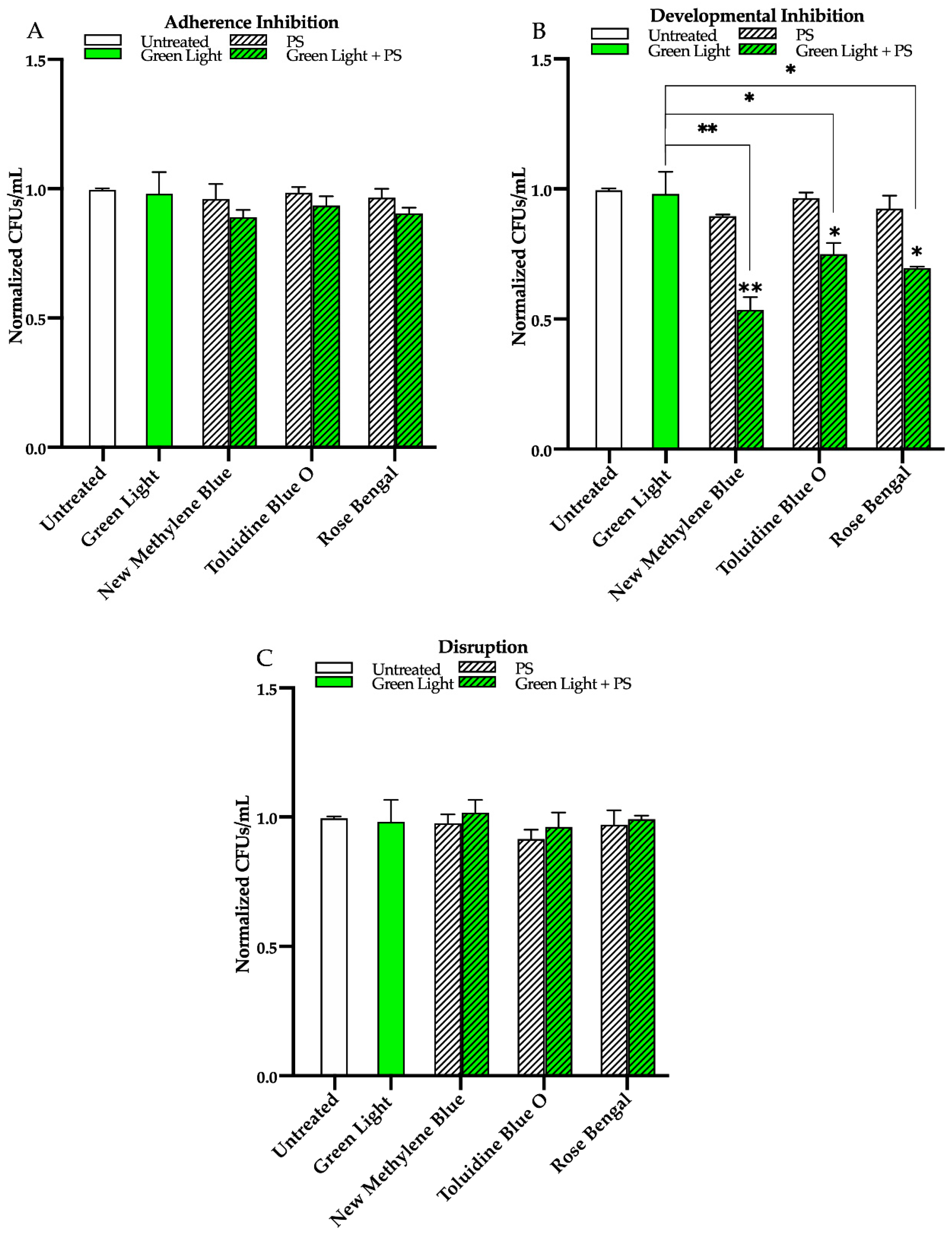
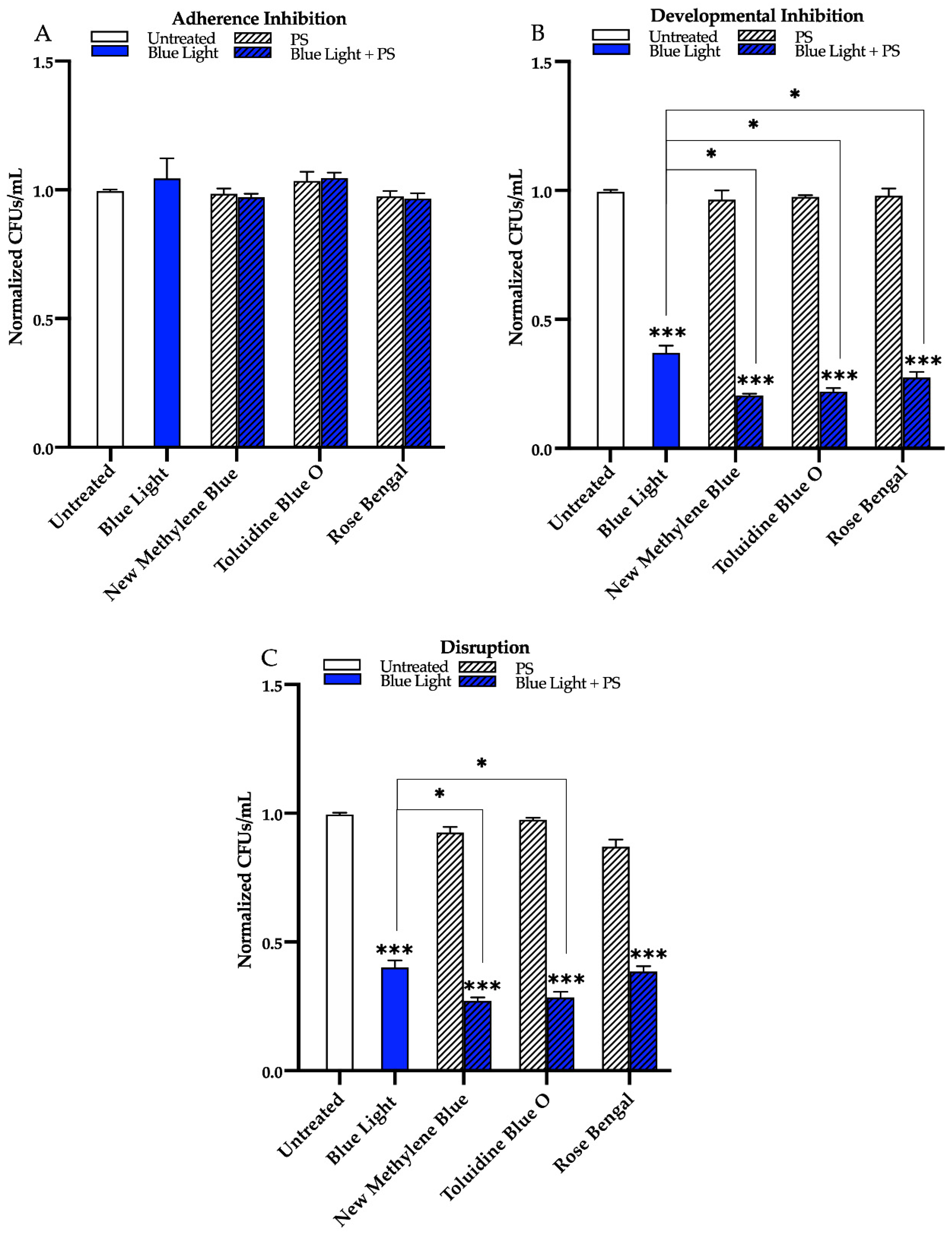
3.1. Effects of Red, Green, and Blue Visible Lights on C. albicans Biofilms
3.2. Effects of Red, Green, and Blue Visible Lights in Combination with Exogenous Photosensitizing Compounds on C. albicans Biofilms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Romo, J.A.; Kumamoto, C.A. On commensalism of Candida. J. Fungi 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kolter, R.; Greenberg, E.P. Microbial sciences: The superficial life of microbes. Nature 2006, 441, 300–302. [Google Scholar] [CrossRef]
- LóPez, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Chaturvedi, A.K.; Srinivasan, A.; Banerjee, M.; Ramasubramaniam, A.K.; Köhler, J.R.; Kadosh, D.; Lopez-Ribot, J.L. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010, 6, e1000828. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 2014, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Shah, A.H.; Rawal, M.K. Mechanism of action and drug resistance. Adv. Exp. Med. Biol. 2016, 892, 327–349. [Google Scholar]
- Nobile, C.J.; Ennis, C.L.; Hartooni, N.; Johnson, A.D.; Lohse, M.B. A selective serotonin reuptake inhibitor, a proton pump inhibitor, and two calcium channel blockers inhibit Candida albicans biofilms. Microorganisms 2020, 8, 756. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Gulati, M.; Craik, C.S.; Johnson, A.D.; Nobile, C.J. Combination of antifungal drugs and protease inhibitors prevent Candida albicans biofilm formation and disrupt mature biofilms. Front. Microbiol. 2020, 11, 1027. [Google Scholar] [CrossRef]
- Williams, D.W.; Kuriyama, T.; Silva, S.; Malic, S.; Lewis, M.A. Candida biofilms and oral candidosis: Treatment and prevention. Periodontology 2000 2011, 55, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Benitez, L.L.; Carver, P.L. Adverse effects associated with long-term administration of azole antifungal agents. Drugs 2019, 79, 833–853. [Google Scholar] [CrossRef]
- Dixon, D.M.; Walsh, T.J. Antifungal Agents. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 76. [Google Scholar]
- Liu, X.; Wang, D.; Yu, C.; Li, T.; Liu, J.; Sun, S. Potential antifungal targets against a Candida biofilm based on an enzyme in the arachidonic acid cascade—A review. Front. Microbiol. 2016, 7, 1925. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Cohen, D.K.; Lee, P.K. Photodynamic Therapy for Non-Melanoma Skin Cancers. Cancers 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, Y.; Park, H.J. Topical PDT in the treatment of benign skin diseases: Principles and new applications. Int. J. Mol. Sci. 2015, 16, 23259–23278. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Phoenix, D.; Laycock, S.L.; Wareing, D.R.; Wright, P.A. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol. Lett. 1998, 160, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.P.; Moreira, L.M.; de Moraes, P.C.; dos Santos, F.V.; de Resende, M.A. Photodynamic therapy for pathogenic fungi. Mycoses 2011, 54, e265–e271. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, P.J.; Gallagher, M.P. Light as a Broad-Spectrum Antimicrobial. Antimicrob. Front. Microbiol. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Bruno, T.J.; Svoronos, P.D.N. CRC Handbook of Fundamental Spectroscopic Correlation Charts, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- St. Denis, T.G.; Dai, T.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light: Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2011, 2, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ghate, V.; Kim, M.J.; Zhou, W.; Khoo, G.H.; Yuk, H.G. Antibacterial efficacy of 405, 460 and 520 nm light emitting diodes on Lactobacillus plantarum, Staphylococcus aureus and Vibrio parahaemolyticus. J. Appl. Microbiol. 2016, 120, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Lim, W.; Jeon, S.; Kim, O.; Koh, J.T.; Kim, C.S.; Choi, H.; Kim, O. In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomed. Laser Surg. 2013, 31, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Huang, Y.Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Kielian, T.; Hamblin, M.R. Blue light eliminates community-acquired methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed. Laser Surg. 2013, 31, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Espada, R.; Wang, Y.; Goh, X.S.; Dai, T. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers Surg. Med. 2020, 52, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, S.; Maclean, M.; Macgregor, S.J.; Anderson, J.G. Comparative sensitivity of Trichophyton and Aspergillus conidia to inactivation by violet-blue light exposure. Photomed. Laser Surg. 2016, 34, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Guffey, J.S.; Payne, W.; Buchanan, B.; Daugherty, J.; Meurer, L.; Hensley, P. Susceptibility of Trichophyton mentagrophytes to visible light wavelengths. Adv. Skin Wound Care 2017, 30, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, L.E.; McKenzie, K.; Maclean, M.; MacGregor, S.J.; Anderson, J.G. Lethal effects of high-intensity violet 405-nm light on Saccharomyces cerevisiae, Candida albicans, and on dormant and germinating spores of Aspergillus niger. Fungal Biol. 2013, 117, 519–527. [Google Scholar] [CrossRef]
- Trzaska, W.J.; Wrigley, H.E.; Thwaite, J.E.; May, R.C. Species-specific antifungal activity of blue light. Sci. Rep. 2017, 7, 4605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist. Updates 2017, 33–35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Halstead, F.D.; Thwaite, J.E.; Burt, R.; Laws, T.R.; Raguse, M.; Moeller, R.; Webber, M.A.; Oppenheim, B.A. Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms. Appl. Environ. Microbiol. 2016, 82, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 2014, 5, 405. [Google Scholar] [CrossRef]
- De Sousa, N.T.; Santos, M.F.; Gomes, R.C.; Brandino, H.E.; Martinez, R.; de Jesus Guirro, R.R. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Photomed. Laser Surg. 2015, 33, 278–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Chen, J.; Wang, Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Hooper, D.C.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence 2016, 7, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Z.; Peng, Y.; Guo, Y.; Yao, M.; Dong, J. Application of 460 nm visible light for the elimination of Candida albicans in vitro and in vivo. Mol. Med. Rep. 2018, 18, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Dai, T. The antimicrobial effect of blue light: What are behind? Virulence 2017, 8, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; Rosseti, I.B.; Carvalho, M.L.; da Silva, B.G.M.; Alberto-Silva, C.; Costa, M.S. Photodynamic Antimicrobial Chemotherapy (PACT), using Toluidine blue O inhibits the viability of biofilm produced by Candida albicans at different stages of development. Photodiagn. Photodyn. Ther. 2018, 21, 182–189. [Google Scholar] [CrossRef]
- Panariello, B.H.D.; Garcia, B.A.; Duarte, S. Daily phototherapy with red light to regulate Candida albicans biofilm growth. J. Vis. Exp. 2019, e59326. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.Y.; Na, J.I.; Huh, C.H.; Park, K.C. The effect of photodynamic therapy using indole-3-acetic acid and green light on acne vulgaris. Ann. Dermatol. 2012, 24, 56–60. [Google Scholar] [CrossRef]
- Boral, H.; Metin, B.; Döğen, A.; Seyedmousavi, S.; Ilkit, M. Overview of selected virulence attributes in Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Trichophyton rubrum, and Exophiala dermatitidis. Fungal Genet. Biol. 2018, 111, 92–107. [Google Scholar] [CrossRef]
- Vural, E.; Winfield, H.L.; Shingleton, A.W.; Horn, T.D.; Shafirstein, G. The effects of laser irradiation on Trichophyton rubrum growth. Lasers Med. Sci. 2008, 23, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.G.; Felipe, M.P.; Costa, M.S. The photodynamic effect of methylene blue and toluidine blue on Candida albicans is dependent on medium conditions. J. Microbiol. 2009, 47, 619–623. [Google Scholar] [CrossRef]
- Romano, R.A.; Pratavieira, S.; Silva, A.P.D.; Kurachi, C.; Guimarães, F.E.G. Light-driven photosensitizer uptake increases Candida albicans photodynamic inactivation. J. Biophotonics 2017, 10, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Crossley, K.B. Methylene Blue—A Therapeutic Dye for All Seasons? J. Chemother. 2002, 14, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Jajarm, H.H.; Falaki, F.; Sanatkhani, M.; Ahmadzadeh, M.; Ahrari, F.; Shafaee, H. A comparative study of toluidine blue-mediated photodynamic therapy versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus: A randomized clinical controlled trial. Lasers Med. Sci. 2015, 30, 1475–1480. [Google Scholar] [CrossRef]
- Ali, M.F. Topical delivery and photodynamic evaluation of a multivesicular liposomal rose bengal. Lasers Med. Sci. 2011, 26, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wiench, R.; Skaba, D.; Stefanik, N.; Kępa, M.; Gilowski, Ł.; Cieślar, G.; Kawczyk-Krupka, A. Assessment of sensitivity of selected Candida strains on antimicrobial photodynamic therapy using diode laser 635 nm and toluidine blue—In vitro research. Photdiagn. Photodyn. Ther. 2019, 27, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Rasteiro, V.M.; Pereira, C.A.; Rossoni, R.D.; Junqueira, J.C.; Jorge, A.O. The effects of rose bengal- and erythrosine-mediated photodynamic therapy on Candida albicans. Mycoses 2012, 55, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Bil de Arce, V.J.; Tegos, G.P.; Hamblin, M.R. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob. Agents Chemother. 2011, 55, 5710–5717. [Google Scholar] [CrossRef]
- Freire, F.; Costa, A.C.; Pereira, C.A.; Beltrame Junior, M.; Junqueira, J.C.; Jorge, A.O. Comparison of the effect of rose bengal-and eosin Y-mediated photodynamic inactivation on planktonic cells and biofilms of Candida albicans. Lasers Surg. Med. 2014, 29, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.M.; French, S.; Kohn, L.A.; Chen, V.; Johnson, A.D. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 2010, 42, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.; Miraglia, G.J.; Smith, D.A.; Basch, H.I.; Pansy, F.E.; Trejo, W.H.; Donovick, R. Biological characterization of prasinomycin, a phosphorus-containing antibiotic. Appl. Microbiol. 1968, 16, 603–608. [Google Scholar] [CrossRef]
- Gulati, M.; Lohse, M.B.; Ennis, C.L.; Gonzalez, R.E.; Perry, A.M.; Bapat, P.; Arevalo, A.V.; Rodriguez, D.L.; Nobile, C.J. In vitro culturing and screening of Candida albicans biofilms. Curr. Protoc. Microbiol. 2018, 50, e60. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Gulati, M.; Valle Arevalo, A.; Fishburn, A.; Johnson, A.D.; Nobile, C.J. Assessment and optimizations of Candida albicans in vitro biofilm assays. Antimicrob. Agents Chemother. 2017, 61, e02749-16. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, T.; Samaranayake, Y.H.; Fang, H.H.; Yip, H.K.; Samaranayake, L.P. The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia 2005, 159, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Durantini, E.N. New insights into the antimicrobial blue light inactivation of Candida albicans. Virulence 2016, 7, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updates 2012, 15, 223–236. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Viveiros, J.; Yang, C.; Ahmadi, A.; Ganz, R.A.; Tolkoff, M.J. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob. Agents Chemother. 2005, 49, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- Winckler, K.D. Special section: Focus on anti-microbial photodynamic therapy (PDT). J. Photochem. Photobiol. B Biol. 2007, 86, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.; Koshi, E.; Philip, K.; Mohan, A. Antimicrobial photodynamic therapy: An overview. J. Indian Soc. Periodontol. 2011, 15, 323–327. [Google Scholar] [PubMed]
- Maisch, T. Resistance in antimicrobial photodynamic inactivation of bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526. [Google Scholar] [CrossRef]
- Al-Fattani, M.A.; Douglas, L.J. Penetration of Candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 2004, 48, 3291–3297. [Google Scholar] [CrossRef] [PubMed]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Al-Fattani, M.A.; Douglas, L.J. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J. Med. Microbiol. 2006, 55 Pt 8, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.N.; Cuevasanta, E.; Orrico, F.; Lopez, A.C.; Thomson, L.; Denicola, A. Diffusion and transport of reactive species across cell membranes. Adv. Exp. Med. Biol. 2019, 1127, 3–19. [Google Scholar]
- Wolcott, R.; Costerton, J.W.; Raoult, D.; Cutler, S.J. The polymicrobial nature of biofilm infection. Clin. Microbiol. Infect. 2013, 19, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single-and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef]
- Orazi, G.; O’Toole, G.A. “It takes a village”: Mechanisms underlying antimicrobial recalcitrance of polymicrobial biofilms. J. Bacteriol. 2019, 202, e00530-19. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Briggs, T.; Blunn, G.; Hislop, S.; Ramalhete, R.; Bagley, C.; Mckenna, D.; Coathup, M. Antimicrobial photodynamic therapy-a promising treatment for prosthetic joint infections. Lasers Med. Sci. 2018, 33, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Shany-Kdoshim, S.; Polak, D.; Houri-Haddad, Y.; Feuerstein, O. Killing mechanism of bacteria within multi-species biofilm by blue light. J. Oral Microbiol. 2019, 11, 1628577. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Hamblin, M.R. Visible Blue Light is Capable of Inactivating Candida albicans and Other Fungal Species. Photomed. Laser Surg. 2017, 35, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Espada, R.; Liu, X.; Goh, X.S.; Dai, T. Antimicrobial blue light inactivation of polymicrobial biofilms. Front. Microbiol. 2019, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Mota, M.; Fernandes, C.; Sequeira, D.; Palma, P.; Caramelo, F.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Gonçalves, T.; Santos, J.M. Is the chlorophyll derivative Zn(II)e6Me a good photosensitizer to be used in root canal disinfection? Photodiagn. Photodyn. Ther. 2018, 22, 205–211. [Google Scholar] [CrossRef]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial metabolism and antibiotic efficacy. Cell Metab. 2019, 30, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Bojsen, R.; Regenberg, B.; Folkesson, A. Saccharomyces cerevisiae biofilm tolerance towards systemic antifungals depends on growth phase. BMC Microbiol. 2014, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Lafleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Wimpenny, J.; Manz, W.; Szewzyk, U. Heterogeneity in biofilms. FEMS Microbiol. Rev. 2000, 24, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Lewis, K. Persister Cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.P.; Singh-Babak, S.D.; Hartooni, N.; Nobile, C.J. Biofilms and antifungal resistance. In Antifungals: From Genomics to Resistance and the Development of Novel Agents; Caister Academic Press: Caister, UK, 2015; pp. 71–90. [Google Scholar]
- Oppezzo, O.J.; Forte Giacobone, A.F. Lethal effect of photodynamic treatment on persister bacteria. Photochem. Photobiol. 2018, 94, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Huang, Y.Y.; Hamblin, M.R. Photodynamic therapy for localized infections-state of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Palma, P.J.; Baptista, I.P.; Gonçalves, T.; Santos, J.M. An insight into advanced approaches for photosensitizer optimization in endodontics—A critical review. J. Funct. Biomater. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Gonçalves, T.; Palma, P.J.; Santos, J.M.; Jefferies, S. Photodynamic antimicrobial chemotherapy for root canal system asepsis: A narrative literature review. Int. J. Dent. 2015, 2015, 269205. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bapat, P.; Singh, G.; Nobile, C.J. Visible Lights Combined with Photosensitizing Compounds Are Effective against Candida albicans Biofilms. Microorganisms 2021, 9, 500. https://doi.org/10.3390/microorganisms9030500
Bapat P, Singh G, Nobile CJ. Visible Lights Combined with Photosensitizing Compounds Are Effective against Candida albicans Biofilms. Microorganisms. 2021; 9(3):500. https://doi.org/10.3390/microorganisms9030500
Chicago/Turabian StyleBapat, Priyanka, Gurbinder Singh, and Clarissa J. Nobile. 2021. "Visible Lights Combined with Photosensitizing Compounds Are Effective against Candida albicans Biofilms" Microorganisms 9, no. 3: 500. https://doi.org/10.3390/microorganisms9030500
APA StyleBapat, P., Singh, G., & Nobile, C. J. (2021). Visible Lights Combined with Photosensitizing Compounds Are Effective against Candida albicans Biofilms. Microorganisms, 9(3), 500. https://doi.org/10.3390/microorganisms9030500







