Evaluation of Adhesive Characteristics of L. plantarum and L. reuteri Isolated from Weaned Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culturing Conditions
2.2. Aggregative Abilities of LAB Strains
2.3. Determination of Bacterial Hydrophobicity
2.4. Cell Line and Culture Conditions
2.5. Bacterial Adhesion Assay
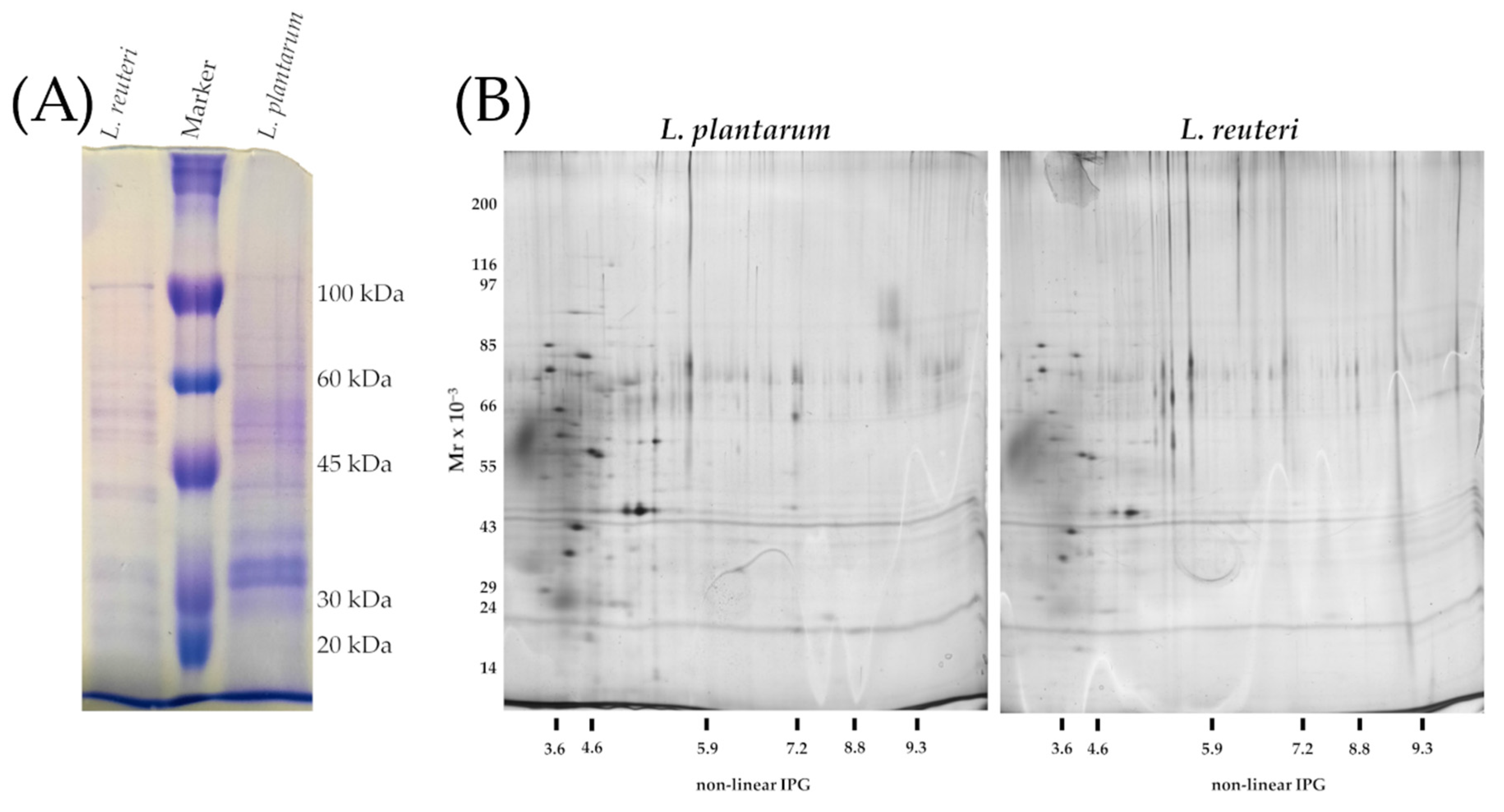
2.6. Isolation and One and Two-Dimensional Gel Electrophoresis of S-Layer Proteins from L. plantarum and L. reuteri
2.7. Statistical Analysis
3. Results
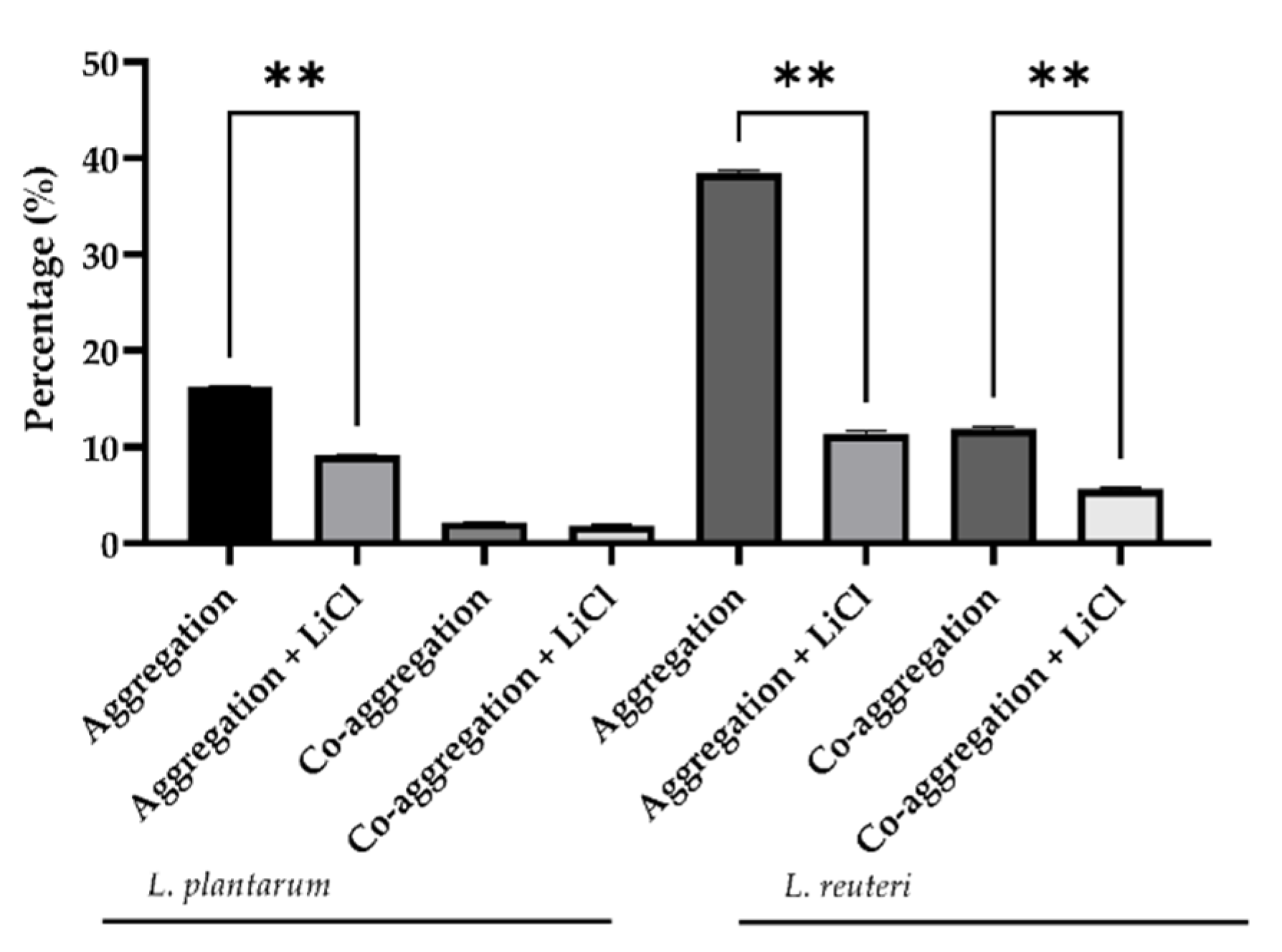
3.1. Aggregation Abilities
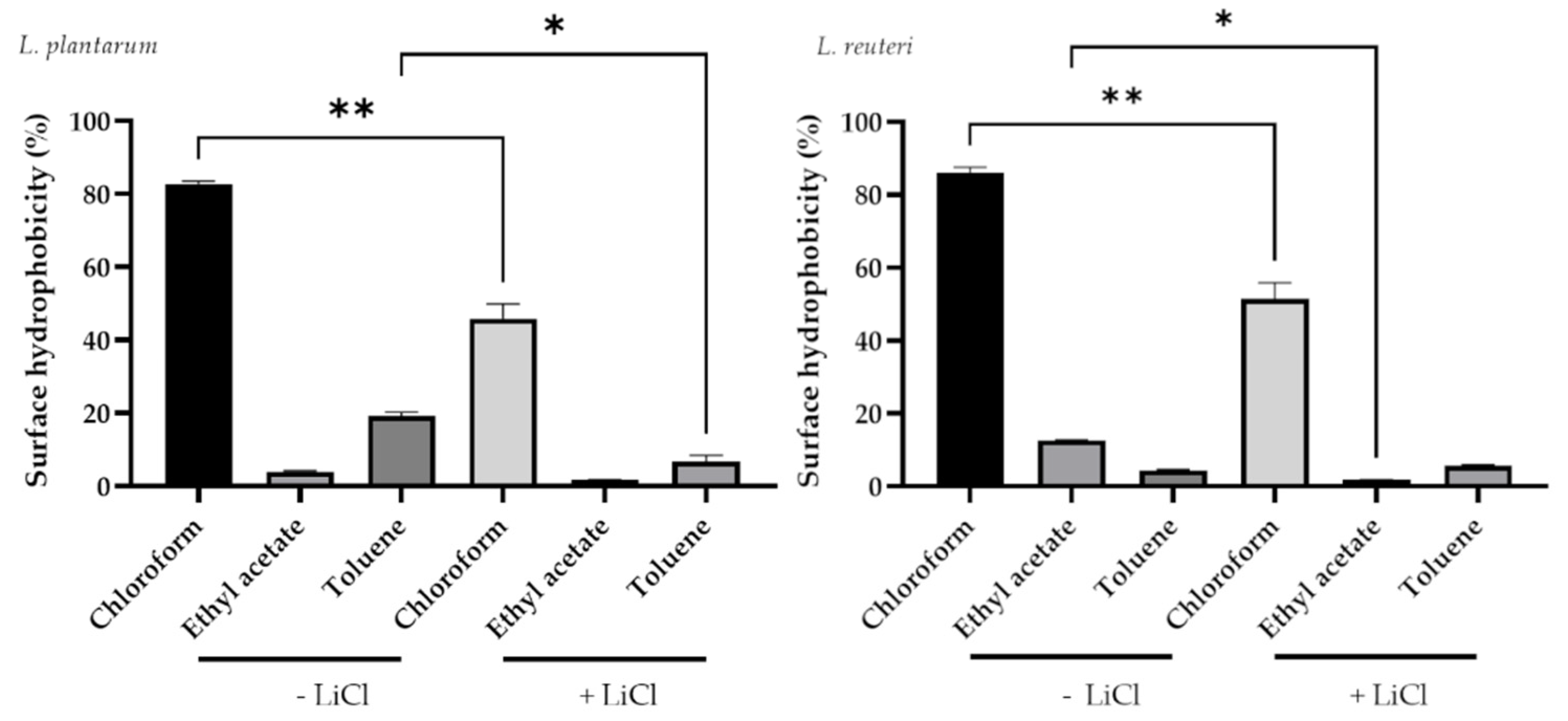
3.2. Determination of Bacterial Hydrophobicity
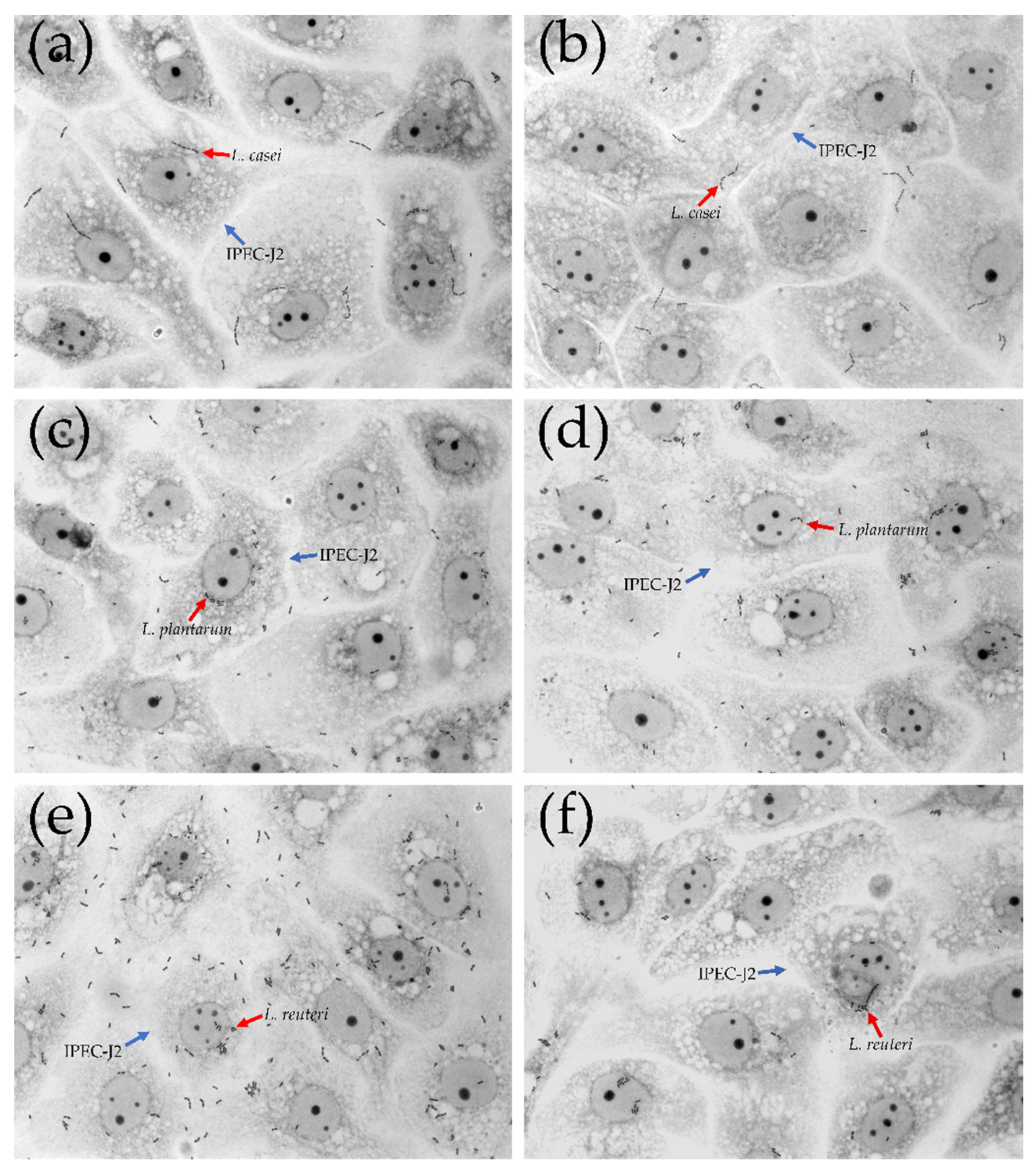
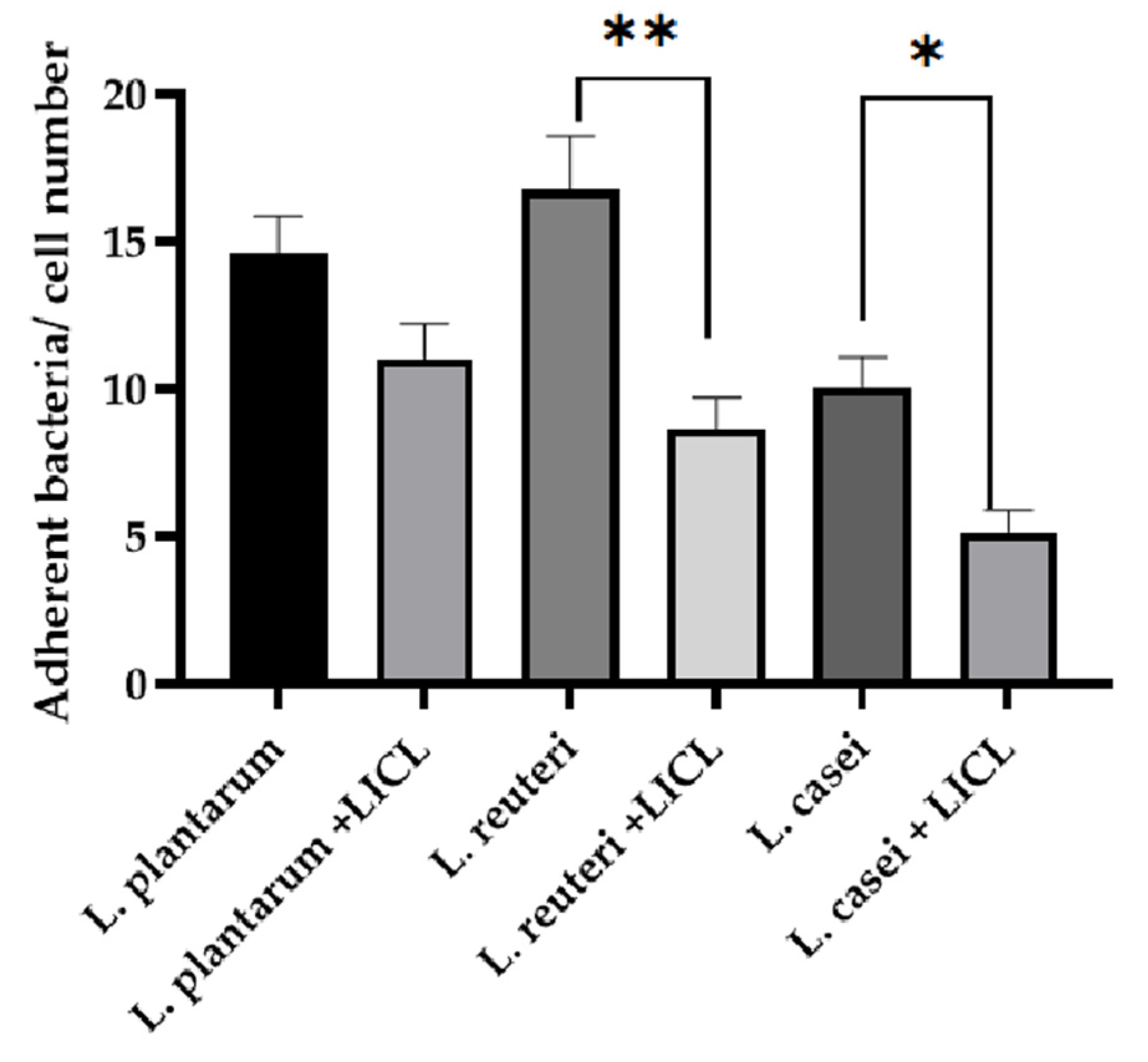
3.3. Bacterial Adhesion Assay
3.4. One and Two-Dimensional Gel Electrophoresis of Surface Proteins from Lactobacilli Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valeriano, V.D.; Balolong, M.; Kang, D.-K.; Valeriano, V.D.; Balolong, M.; Kang, D.-K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef]
- Niu, Q.; Li, P.; Hao, S.; Zhang, Y.; Kim, S.W.; Li, H.; Ma, X.; Gao, S.; He, L.; Wu, W. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Hou, C.; Zeng, X.; Yang, F.; Liu, H.; Qiao, S. Study and use of the probiotic Lactobacillus reuteri in pigs: A review. J. Anim. Sci. Biotechnol. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Zhou, H.; Wang, L.; Ding, S.; Wang, Y.; Song, D.; Li, A. Effects of microencapsulated Lactobacillus plantarum and fructooligosaccharide on growth performance, blood immune parameters, and intestinal morphology in weaned piglets. Food Agric. Immunol. 2017, 29, 84–94. [Google Scholar] [CrossRef]
- Regulation EC 1831/2003. of the European Parliament and of the Council, of 22 September 2003 on Additives for Use in Animal Nutrition (Text with EEA Relevance); Official Journal of the European Union: Brussels, Belgium, 2003.
- Walter, J. Ecological Role of Lactobacilli in the Gastrointestinal Tract: Implications for Fundamental and Biomedical Research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef]
- Hekmat, S.; Soltani, H.; Reid, G. Growth and survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in yogurt for use as a functional food. Innov. Food Sci. Emerg. Technol. 2009, 10, 293–296. [Google Scholar] [CrossRef]
- Van Tassell, M.L.; Miller, M.J. Lactobacillus Adhesion to Mucus. Nutrients 2011, 3, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Malik, R.K.; Kaur, G. Cell surface proteins play an important role in probiotic activities of Lactobacillus reuteri. Nutrire 2016, 41, 1–10. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Callegari, M.L.; Reggi, S.; Caprarulo, V.; Giromini, C.; Spalletta, A.; Coranelli, S.; Rossi, C.A.S.; Rossi, L. Lac-tobacillus plantarum and Lactobacillus reuteri as Functional Feed Additives to Prevent Diarrhoea in Weaned Piglets. Animals 2021, 11, 1766. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anno, M.; Sotira, S.; Rebucci, R.; Reggi, S.; Castiglioni, B.; Rossi, L. In vitro evaluation of antimicrobial and antioxidant activities of algal extracts. Ital. J. Anim. Sci. 2020, 19, 103–113. [Google Scholar] [CrossRef]
- Kos, B.; Šušković, J.; Vuković, S.; Šimpraga, M.; Frece, J.; Matošić, S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Bellon-Fontaine, M.-N.; Rault, J.; Van Oss, C. Microbial adhesion to solvents: A novel method to determine the electron-donor/electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surf. B Biointerfaces 1996, 7, 47–53. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Liu, J.; Zhao, Y.; Gao, K.; Zhang, J. Adhesive ability means inhibition activities for lactobacillus against pathogens and S-layer protein plays an important role in adhesion. Anaerobe 2013, 22, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Chen, X.; Jiang, X.; Zhao, R.; Yuan, Y.; Chen, D.; Zhang, C.; Lu, M.; Lu, Z.; Gu, R. In vitro studies of adhesion prop-erties of six lactic acid bacteria isolated from the longevous population of China. RSC Adv. 2020, 10, 24234–24240. [Google Scholar] [CrossRef]
- Singh, T.P.; Kaur, G.; Kapila, S.; Malik, R.K. Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Front. Microbiol. 2017, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Bonjoch, N.P.; Tamayo, P.R. Protein Content Quantification by Bradford Method. In Handbook of Plant Ecophysiology Techniques; Springer: Berlin/Heidelberg, Germany, 2006; pp. 283–295. [Google Scholar]
- Moscatelli, A.; Gagliardi, A.; Maneta-Peyret, L.; Bini, L.; Stroppa, N.; Onelli, E.; Landi, C.; Scali, M.; Idilli, A.I.; Moreau, P. Characterisation of detergent-insoluble membranes in pollen tubes of Nicotiana tabacum (L.). Biol. Open 2015, 4, 378–399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Sinha, P.; Poland, J.; Schnölzer, M.; Rabilloud, T. A new silver staining apparatus and procedure for matrix-assisted laser desorption/ionization-time of flight analysis of proteins after two-dimensional electrophoresis. Proteomics Int. Ed. 2001, 1, 835–840. [Google Scholar] [CrossRef]
- Sotira, S.; Dell’Anno, M.; Caprarulo, V.; Hejna, M.; Pirrone, F.; Callegari, M.L.; Tucci, T.V.; Rossi, L. Effects of Tributyrin Supplementation on Growth Performance, Insulin, Blood Metabolites and Gut Microbiota in Weaned Piglets. Animals 2020, 10, 726. [Google Scholar] [CrossRef]
- Zaninelli, M.; Rossi, L.; Costa, A.; Tangorra, F.; Agazzi, A.; Savoini, G. Monitoring of goats’ health status by on-line analysis of milk electrical conductivity. Large Anim. Rev. 2015, 21, 81–86. [Google Scholar]
- Dell’Anno, M.; Hejna, M.; Sotira, S.; Caprarulo, V.; Reggi, S.; Pilu, R.; Miragoli, F.; Callegari, M.L.; Panseri, S.; Rossi, L. Evaluation of leonardite as a feed additive on lipid metabolism and growth of weaned piglets. Anim. Feed Sci. Technol. 2020, 266, 114519. [Google Scholar] [CrossRef]
- FAO; WHO. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 2001, 5, 1–10. [Google Scholar]
- Muñoz-Provencio, D.; Llopis, M.; Antolín, M.; De Torres, I.; Guarner, F.; Pérez-Martínez, G.; Monedero, V. Adhesion proper-ties of Lactobacillus casei strains to resected intestinal fragments and components of the extracellular matrix. Arch. Microbiol. 2009, 191, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Śliżewska, K.; Chlebicz-Wójcik, A.; Nowak, A. Probiotic Properties of New Lactobacillus strains Intended to Be Used as Feed Additives for Monogastric Animals. In Probiotics and Antimicrobial Proteins; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–17. [Google Scholar]
- Krausova, G.; Hyrslova, I.; Hynstova, I. In Vitro Evaluation of Adhesion Capacity, Hydrophobicity, and Auto-Aggregation of Newly Isolated Potential Probiotic Strains. Fermentation 2019, 5, 100. [Google Scholar] [CrossRef]
- Rossi, L.; Vagni, S.; Polidori, C.; Alborali, G.L.; Baldi, A.; Dell’Orto, V. Experimental Induction of Escherichia coli Diarrhoea in Weaned Piglets. Open J. Veter.-Med. 2012, 2, 1–8. [Google Scholar] [CrossRef]
- Rossi, L.; Pinotti, L.; Agazzi, A.; Dell’Orto, V.; Baldi, A. Plant bioreactors for the antigenic hook-associated flgK protein expression. Ital. J. Anim. Sci. 2014, 13, 2939. [Google Scholar] [CrossRef]
- Rossi, L.; Dell’Orto, V.; Vagni, S.; Sala, V.; Reggi, S.; Baldi, A. Protective effect of oral administration of transgenic tobacco seeds against verocytotoxic Escherichia coli strain in piglets. Veter. Res. Commun. 2014, 38, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.; Yu, H.; Ai, L.; Wu, Z.; Guo, B.; Chen, W. Aggregation and adhesion properties of 22 Lactobacillus strains. J. Dairy Sci. 2013, 96, 4252–4257. [Google Scholar] [CrossRef] [PubMed]
- Reggi, S.; Giromini, C.; Dell’Anno, M.; Baldi, A.; Rebucci, R.; Rossi, L. In Vitro Digestion of Chestnut and Quebracho Tannin Extracts: Antimicrobial Effect, Antioxidant Capacity and Cytomodulatory Activity in Swine Intestinal IPEC-J2 Cells. Animals 2020, 10, 195. [Google Scholar] [CrossRef]
- Vergauwen, H.; Tambuyzer, B.; Jennes, K.; DeGroote, J.; Wang, W.; De Smet, S.; Michiels, J.; Van Ginneken, C. Trolox and Ascorbic Acid Reduce Direct and Indirect Oxidative Stress in the IPEC-J2 Cells, an In Vitro Model for the Porcine Gastrointestinal Tract. PLoS ONE 2015, 10, e0120485. [Google Scholar] [CrossRef]
- Brosnahan, A.J.; Brown, D.R. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Veter. Microbiol. 2012, 156, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Tallon, R.; Arias, S.; Bressollier, P.; Urdaci, M. Strain-and matrix-dependent adhesion of Lactobacillus plantarum is mediated by proteinaceous bacterial compounds. J. Appl. Microbiol. 2007, 102, 442–451. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Genet. 2010, 8, 171–184. [Google Scholar] [CrossRef]
- Lähteinen, T.; Malinen, E.; Koort, J.M.; Mertaniemi-Hannus, U.; Hankimo, T.; Karikoski, N.; Pakkanen, S.; Laine, H.; Sillanpää, H.; Söderholm, H. Probiotic properties of Lactobacillus isolates originating from porcine intestine and feces. Anaerobe 2010, 16, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.-Y.; Yueh, P.-Y.; Yu, B.; Zhao, X.; Liu, J.-R. Expression of Lactobacillus reuteri Pg4 Collagen-Binding Protein Gene in Lactobacillus casei ATCC 393 Increases Its Adhesion Ability to Caco-2 Cells. J. Agric. Food Chem. 2010, 58, 12182–12191. [Google Scholar] [CrossRef]
- Minelli, E.B.; Benini, A.; Marzotto, M.; Sbarbati, A.; Ruzzenente, O.; Ferrario, R.; Hendriks, H.; Dellaglio, F. Assessment of novel probiotic Lactobacillus casei strains for the production of functional dairy foods. Int. Dairy J. 2004, 14, 723–736. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, J.; Yan, L.; Chen, W.; Liu, X.-M.; Zhang, H.-P. In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. LWT 2009, 42, 1640–1646. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Zhao, J.; Xia, Y.; Lai, P.F.-H.; Ai, L. A surface protein from Lactobacillus plantarum increases the adhe-sion of lactobacillus strains to human epithelial cells. Front. Microbiol. 2018, 9, 2858. [Google Scholar] [CrossRef] [PubMed]
- Hynönen, U.; Palva, A. Lactobacillus surface layer proteins: Structure, function and applications. Appl. Microbiol. Biotechnol. 2013, 97, 5225–5243. [Google Scholar] [CrossRef]
- Siezen, R.; Boekhorst, J.; Muscariello, L.; Molenaar, D.; Renckens, B.; Kleerebezem, M. Lactobacillus plantarum gene clusters encoding putative cell-surface protein complexes for carbohydrate utilization are conserved in specific gram-positive bacteria. BMC Genom. 2006, 7, 1–13. [Google Scholar] [CrossRef]
- Pretzer, G.; Snel, J.; Molenaar, D.; Wiersma, A.; Bron, P.A.; Lambert, J.; de Vos, W.M.; van der Meer, R.; Smits, M.A.; Kleer-ebezem, M. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J. Bacteriol. 2005, 187, 6128–6136. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.; Roos, S.; Jonsson, H.; Rud, I.; Grimmer, S.; Van Pijkeren, J.-P.; Britton, R.A.; Axelsson, L. Role of Lactobacillus reuteri cell and mucus-binding protein A (CmbA) in adhesion to intestinal epithelial cells and mucus in vitro. Microbiology 2014, 160, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wei, H.; Yuan, J.; Li, Q.; Li, Y.; Li, N.; Li, J. Identification of a Surface Protein from Lactobacillus reuteri JCM1081 That Adheres to Porcine Gastric Mucin and Human Enterocyte-Like HT-29 Cells. Curr. Microbiol. 2008, 57, 33–38. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Anno, M.; Giromini, C.; Reggi, S.; Cavalleri, M.; Moscatelli, A.; Onelli, E.; Rebucci, R.; Sundaram, T.S.; Coranelli, S.; Spalletta, A.; et al. Evaluation of Adhesive Characteristics of L. plantarum and L. reuteri Isolated from Weaned Piglets. Microorganisms 2021, 9, 1587. https://doi.org/10.3390/microorganisms9081587
Dell’Anno M, Giromini C, Reggi S, Cavalleri M, Moscatelli A, Onelli E, Rebucci R, Sundaram TS, Coranelli S, Spalletta A, et al. Evaluation of Adhesive Characteristics of L. plantarum and L. reuteri Isolated from Weaned Piglets. Microorganisms. 2021; 9(8):1587. https://doi.org/10.3390/microorganisms9081587
Chicago/Turabian StyleDell’Anno, Matteo, Carlotta Giromini, Serena Reggi, Mariagrazia Cavalleri, Alessandra Moscatelli, Elisabetta Onelli, Raffaella Rebucci, Tamil Selvi Sundaram, Simona Coranelli, Ambra Spalletta, and et al. 2021. "Evaluation of Adhesive Characteristics of L. plantarum and L. reuteri Isolated from Weaned Piglets" Microorganisms 9, no. 8: 1587. https://doi.org/10.3390/microorganisms9081587
APA StyleDell’Anno, M., Giromini, C., Reggi, S., Cavalleri, M., Moscatelli, A., Onelli, E., Rebucci, R., Sundaram, T. S., Coranelli, S., Spalletta, A., Baldi, A., & Rossi, L. (2021). Evaluation of Adhesive Characteristics of L. plantarum and L. reuteri Isolated from Weaned Piglets. Microorganisms, 9(8), 1587. https://doi.org/10.3390/microorganisms9081587








