Bacillus pumilus and Bacillus subtilis Promote Early Maturation of Cecal Microbiota in Broiler Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Birds, Diet and Experimental Design
2.2. Sample Collection and DNA Extraction
2.3. Sequencing and Data Analysis of Cecal Microbial Community
3. Results
3.1. Sequencing Data
3.2. Probiotics Improve the Cecal Microbial Alpha Diversity in Young Chickens
3.3. Probiotics Affect the Cecal Microbial Beta Diversity in Young Chickens
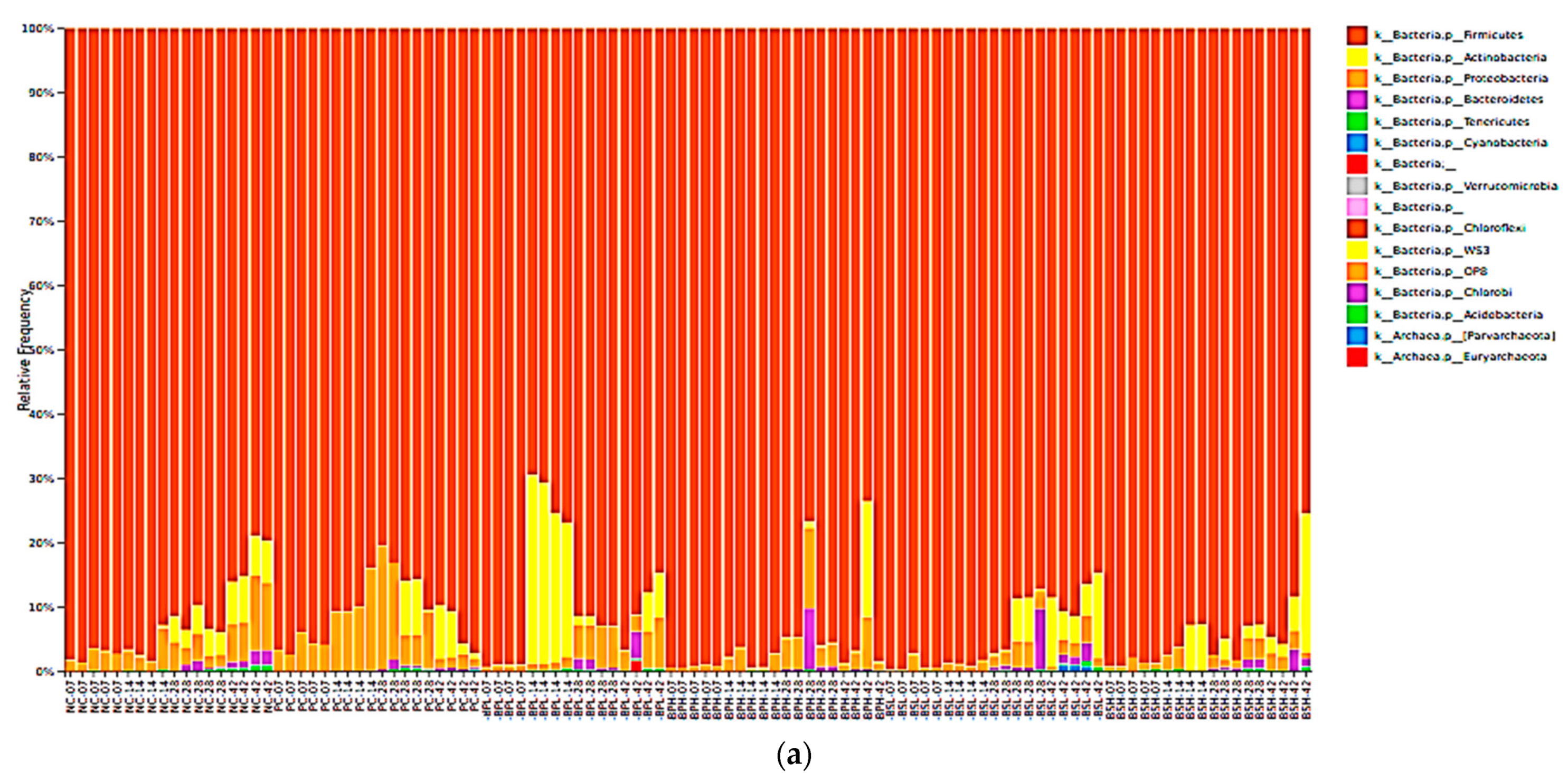
3.4. Probiotics Change Composition of Cecal Microbiota in Broiler Chickens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Gateway to Poultry Production and Products. 2021. Available online: http://www.fao.org/poultry-production-products/production/en/ (accessed on 5 September 2021).
- Food and Agriculture Organization. 2015. Available online: http://www.fao.org/3/y4252e/y4252e07.htm (accessed on 1 April 2021).
- Grant, A.; Gay, C.G.; Lillehoj, H.S. Bacillus spp. as directed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018, 47, 339–351. [Google Scholar] [CrossRef]
- Chen, Q.; Tong, C.; Ma, S.; Zhou, L.; Zhao, X. Involvement of microRNAs in probiotics-induced reduction of the cecal inflammation by salmonella typhimurium. Front. Immunol. 2017, 8, 704. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, L.; Lv, Y.; Chen, Q.; Feng, J.; Zhao, X. Lactobacillus plantarum restores intestinal permeability disrupted by Salmonella infection in newly hatched chicks. Sci. Rep. 2018, 8, 2229. [Google Scholar] [CrossRef]
- Marcolla, C.S.; Alvarado, C.S.; Willing, B.P. Early life microbial exposure shapes subsequent animal health. Can. J. Anim. Sci. 2019, 99, 661–677. [Google Scholar] [CrossRef]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabst, O.; Cerovic, V.; Hornef, M. Secretory IgA in the coordination of establishment and maintenance of the microbiota. Trends Immunol. 2016, 37, 5. [Google Scholar] [CrossRef] [PubMed]
- Sterlin, D.; Fadlallah, J.; Slack, E.; Gorochov, G. The antibody/microbiota interface in health and disease. Mucosal. Immunol. 2020, 13, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ocejo, M.; Oporto, B.; Hurtado, A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow growing chickens throughout their productive lifespan. Sci. Rep. 2019, 9, 2506. [Google Scholar] [CrossRef] [Green Version]
- Hartog, D.G.; Vries-Reilingh, G.D.; Wehrmaker, A.M.; Savelkoul, H.F.J.; Parmentier, H.K.; Lammers, A. Intestinal immune maturation is accompanied by temporal changes in the composition of the microbiota. Benef. Microbes. 2016, 7, 677–685. [Google Scholar] [CrossRef]
- Bilal, M.; Si, W.; Barbe, F.; Chevaux, E.; Sienkiewicz, O.; Zhao, X. Effects of novel probiotic strains of Bacillus pumilus and Bacillus subtilis on production, gut health and immunity of broiler chickens raised under sub-optimal conditions. Poult. Sci. 2021, 100, 100871. [Google Scholar] [CrossRef]
- Pereira, R.; Bortoluzzi, C.; Durrer, A.; Fagundes, N.S.; Pedroso, A.A.; Rafael, J.M.; Perim, J.E.; Zavarize, K.C.; Napty, G.S.; Andreote, F.D.; et al. Performance and intestinal microbiota of chickens receiving probiotic in the feed and submitted to antibiotic therapy. J. Anim. Physiol. Anim. Nutr. 2019, 103, 72–86. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Olszak, T.; An, D.; Zeissig, S.; Vera, M.P.; Richter, J.; Franke, A.; Glickman, J.N.; Siebert, R.; Baron, R.M.; Kasper, D.L.; et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012, 336, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparrini, A.J.; Wang, B.; Sun, X.; Kennedy, E.A.; Hernandez-Leyva, A.; Ndao, I.M. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat. Microbiol. 2019, 4, 2285–2297. [Google Scholar] [CrossRef]
- Jurburg, S.D.; Brouwer, M.S.M.; Ceccarelli, D.; Goot, J.; Jansman, A.J.M.; Bossers, A. Patterns of community assembly in the developing chicken microbiome reveal rapid primary succession. MicrobiologyOpen 2019, 8, e821. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.; Fothergill, J.; Bernardeau, M.; Wigley, P. Development of the caecal microbiota in three broiler breeds. Front. Vet. Sci. 2019, 6, 201. [Google Scholar] [CrossRef]
- Oakley, B.B.; Buhr, R.; Ritz, C.W.; Kiepper, B.H.; Berrang, M.E.; Seal, B.S. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet. Res. 2014, 360, 282. [Google Scholar] [CrossRef] [Green Version]
- Kubasova, T.; Kollarcikova, M.; Crhanova, M.; Karasova, D.; Cejkova, D.; Sebkova, A. Contact with adult hen affects development of caecal microbiota in newly hatched chicks. PLoS ONE 2019, 14, e0212446. [Google Scholar] [CrossRef]
- Meijerink, N.; Kers, J.G.; Velkers, F.C.; van Haarlem, D.A.; Lamot, D.M.; de Oliveira, J.E.; Smidt, H.; Stegeman, J.A.; Rutten, V.P.M.G.; Jansen, C.A. Early life inoculation with adult-derived microbiota accelerates maturation of intestinal microbiota and enhances NK cell activation in broiler chickens. Front. Vet. Sci. 2020, 7, 584561. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.; Al-Ghalith, G.A. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Prepr. 2018, 6, e27295v2. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Morton, T.J.; Marotz, C.; Washburne, A.; Silverman, J.; Zaramela, L.S.; Edlund, A.; Zengler, K.; Knight, R. Establishing microbial composition measurement standards with reference frames. Nat. Comm. 2019, 10, 2719. [Google Scholar] [CrossRef] [Green Version]
- Fedarko, M.W.; Martino, C.; Morton, J.T.; Gonz’alez, A.; Rahman, G.; Marotz, C.A.; Minich, J.J.; Allen, E.E.; Knight, R. Visualizing ’omic feature rankings and log-ratios using Qurro. NAR Genom. Bioinform. 2020, 2, 2. [Google Scholar] [CrossRef]
- Engelbrektson, A.; Korzenik, J.R.; Pittler, A.; Sanders, M.E.; Klaenhammer, T.R.; Leyer, G. Probiotics to minimize the disruption of faecal microbiota in healthy subjects undergoing antibiotic therapy. J. Med. Microbiol. 2009, 58, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, B.; Kim, J.W.; Kim, B. Changes in the functional potential of the gut microbiome following probiotic supplementation during helicobacter pylori treatment. John Wiley Sons Ltd. Helicobacter. 2016, 21, 493–503. [Google Scholar] [CrossRef]
- Trela, J.; Kieronczyk, B.; Hautekiet, V.; Józefiak, D. Combination of Bacillus licheniformis and salinomycin: Effect on the growth performance and GIT microbial populations of broiler chickens. Animals 2020, 10, 889. [Google Scholar] [CrossRef]
- Feye, K.M.; Baxter, F.M.A.; Tellez-Isaias, G.; Kogut, M.H.; Ricke, S.C. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poult. Sci. 2020, 99, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Watterlot, L.; Lakhdari, O.; Bermu, L.G.; Bridonneau, C.; Furet, J.P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patient. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, A.V. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef]
- Massacci, F.R.; Lovito, C.; Tofani, S.; Tentellini, M.; Genovese, D.A.; De Leo, A.; Papa, P.; Magistrali, C.F.; Manuali, E.; Trabalza-Marinucci, M.; et al. Dietary Saccharomyces cerevisiae boulardii CNCM I-1079 positively affects performance and intestinal ecosystem in broilers during a Campylobacter jejuni Infection. Microorganisms 2019, 7, 596. [Google Scholar] [CrossRef] [Green Version]
- Saliu, E.M.; Vahjen, W.; Zentek, J. Types and prevalence of extended spectrum beta lactamase producing Enterobacteriaceae in poultry. Anim. Health Res. Rev. 2017, 18, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Byndloss, X.M. Microbial management: Colonocyte metabolism plays an essential role in balancing the gut microbiota. Science 2020, 369, 6500. [Google Scholar] [CrossRef]
- Salazar, N.; Gueimonde, M.; Hernandez-Barranco, A.M.; Ruas-Madiedo, P.; de los Reyes-Gavila, C.G. Exopolysaccharides produced by intestinal Bifidobacterium strains act as fermentable substrates for human intestinal bacteria. Appl. Environ. Microbiol. 2008, 74, 4737–4745. [Google Scholar] [CrossRef] [Green Version]
- Rios-Covian, D.; Cuesta, I.; Alvarez-Buylla, J.R.; Ruas-Madiedo, P.; Gueimonde, M.; Reyes-Gavilán, C.G. Bacteroides fragilis metabolises exopolysaccharides produced by Bifidobacteria. BMC Microbiol. 2016, 16, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, X.; Linglong, P.; Weixia, D.; Hong, W. Protective effects of Bifidobacterium on intestinal barrier function in LPS-induced enterocyte barrier injury of Caco-2 monolayers and in a rat NEC model. PLoS ONE 2016, 11, e0161635. [Google Scholar] [CrossRef]
- Lopez, P.; Gonzalez-Rodriguez, I.; Gueimonde, M.; Margolles, A.; Suarez, A. Immune response to Bifidobacterium bifidum strains support Treg/Th17 plasticity. PLoS ONE 2011, 6, e24776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, Y.; Verstegen, M.W.A.; Tamminga, S.; Williams, B.A. The role of the commensal gut microbial community in broiler chickens. World’s Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Kadykalo, S.; Roberts, T.; Thompson, M.; Wilson, W.; Lang, M.; Espeisse, O. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents 2018, 51, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Simjee, S.; Heffron, A.; Pridmore, A.; Shryock, T.R. Reversible monensin adaptation in Enterococcus faecium, Enterococcus faecalis and Clostridium perfringens of cattle origin: Potential impact on human food safety. J. Antimicrob. Chemother. 2012, 67, 2388–2395. [Google Scholar] [CrossRef] [Green Version]
- Dec, M.; Puchalski, A.; Stępień-Pyśniak, D.; Marek, A.; Urban-Chmiel, R. Susceptibility of chicken Lactobacillus bacteria to coccidiostats. J. Vet. Med. Sci. 2020, 24, 333–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.M.; Soratto, T.A.T.; Cardinal, K.M.; Wagner, G.; Hauptli, L.; Lima, A.L.F. Modulation of the intestinal microbiota of broilers supplemented with monensin or functional oils in response to challenge by Eimeria spp. PLoS ONE 2020, 15, e0237118. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, J.L.; Kim, H.B.; Isaacson, R.E.; Tu, Z.J.; Johnson, T.J. Modulations of the Chicken Cecal Microbiome and Metagenome in Response to Anticoccidial and Growth Promoter Treatment. PLoS ONE 2011, 6, e27949. [Google Scholar] [CrossRef] [PubMed]

| a. Effects of dietary treatments on microbial richness (observed operational taxonomic units). | ||||||||
| Groups/Time | Mean Day-7 | SEM | Mean Day-14 | SEM | Mean Day-28 | SEM | Mean Day-42 | SEM |
| NC | 110 3 | 11.9 | 100 b,3 | 13.3 | 188 2 | 11.9 | 241 1 | 13.3 |
| PC | 92 2 | 11.9 | 109 b,2 | 13.3 | 188 1 | 11.9 | 202 1 | 13.3 |
| BPL | 116 3 | 13.3 | 172 a,2 | 13.3 | 231 1 | 13.3 | 228 1 | 13.3 |
| BPH | 118 2 | 11.9 | 163 a,1,2 | 11.9 | 202 1 | 11.9 | 198 1 | 13.3 |
| BSL | 135 2 | 11.9 | 172 a,2 | 13.3 | 234 1 | 11.9 | 249 1 | 11.9 |
| BSH | 106 2 | 11.9 | 177 a,1 | 13.3 | 221 1 | 11.9 | 199 1 | 13.3 |
| b. Effects of dietary treatments on microbial evenness (Pielou index) at days 7, 14, 28, and 42. | ||||||||
| Groups/Time | Mean Day-7 | SEM | Mean Day-14 | SEM | Mean Day-28 | SEM | Mean Day-42 | SEM |
| NC | 0.77 ab,1 | 0.025 | 0.66 ab,2 | 0.029 | 0.75 ab,1,2 | 0.025 | 0.69 1,2 | 0.029 |
| PC | 0.65 c,2 | 0.025 | 0.63 bc,2 | 0.029 | 0.77 a,1 | 0.025 | 0.61 2 | 0.029 |
| BPL | 0.77 ab,1 | 0.029 | 0.52 c,2 | 0.029 | 0.74 ab,1 | 0.029 | 0.73 1 | 0.029 |
| BPH | 0.68 bc,1,2 | 0.025 | 0.77 a,1 | 0.025 | 0.68 ab,2 | 0.025 | 0.64 2 | 0.029 |
| BSL | 0.76 ab,1 | 0.025 | 0.68 ab,1,2 | 0.029 | 0.71 ab,12 | 0.025 | 0.63 2 | 0.025 |
| BSH | 0.80 a,1 | 0.025 | 0.72 ab,1,2 | 0.029 | 0.66 b,2,3 | 0.025 | 0.61 3 | 0.029 |
| c. Effects of dietary treatments on microbial diversity (Shannon index) at days 7, 14, 28, and 42. | ||||||||
| Groups/Time | Mean Day-7 | SEM | Mean Day-14 | SEM | Mean Day-28 | SEM | Mean Day-42 | SEM |
| NC | 5.30 a,1 | 0.218 | 4.39 bcd,2 | 0.244 | 5.67 1 | 0.218 | 5.46 ab,1 | 0.244 |
| PC | 4.24 b,2 | 0.218 | 4.25 cd,2 | 0.244 | 5.77 1 | 0.218 | 4.69 ab,2 | 0.244 |
| BPL | 5.25 a,1 | 0.244 | 3.88 d,2 | 0.244 | 5.81 1 | 0.244 | 5.69 a,1 | 0.244 |
| BPH | 4.67 ab,2 | 0.218 | 5.66 a,1 | 0.218 | 5.17 1,2 | 0.218 | 4.89 ab,1,2 | 0.244 |
| BSL | 5.37 a | 0.218 | 5.04 abc | 0.244 | 5.59 | 0.218 | 5.02 ab | 0.218 |
| BSH | 5.39 a | 0.218 | 5.36 ab | 0.244 | 5.11 | 0.218 | 4.65 b | 0.244 |
| a. Pairwise microbial dissimilarity between groups at day 7. | ||||||
| Treatment Groups | NC | PC | BPL | BPH | BSL | BSH |
| NC | 1 | 0.04 | 0.04 | 0.03 | 0.04 | 0.04 |
| PC | 1 | 0.04 | 0.03 | 0.04 | 0.04 | |
| BPL | 1 | 0.03 | 0.04 | 0.04 | ||
| BPH | 1 | 0.03 | 0.06 | |||
| BSL | 1 | 0.04 | ||||
| BSH | 1 | |||||
| b. Pairwise microbial dissimilarity between groups at day 14. | ||||||
| Treatment Groups | NC | PC | BPL | BPH | BSL | BSH |
| NC | 1 | 0.07 | 0.07 | 0.04 | 0.07 | 0.03 |
| PC | 1 | 0.06 | 0.03 | 0.08 | 0.04 | |
| BPL | 1 | 0.03 | 0.06 | 0.03 | ||
| BPH | 1 | 0.03 | 0.03 | |||
| BSL | 1 | 0.03 | ||||
| BSH | 1 | |||||
| c. Pairwise microbial dissimilarity between groups at day 28 | ||||||
| Treatment Groups | NC | PC | BPL | BPH | BSL | BSH |
| NC | 1 | 0.24 | 0.03 | 0.06 | 0.03 | 0.03 |
| PC | 1 | 0.18 | 0.14 | 0.11 | 0.14 | |
| BPL | 1 | 0.24 | 0.04 | 0.04 | ||
| BPH | 1 | 0.24 | 0.79 | |||
| BSL | 1 | 0.09 | ||||
| BSH | 1 | |||||
| d. Pairwise microbial dissimilarity between groups at day 42. | ||||||
| Treatment Groups | NC | PC | BPL | BPH | BSL | BSH |
| NC | 1 | 0.04 | 0.29 | 0.13 | 0.13 | 0.14 |
| PC | 1 | 0.04 | 0.22 | 0.17 | 0.64 | |
| BPL | 1 | 0.56 | 0.13 | 0.41 | ||
| BPH | 1 | 0.28 | 0.56 | |||
| BSL | 1 | 0.78 | ||||
| BSH | 1 | |||||
| e. Pairwise microbial dissimilarity within groups between days 7 and 14, 14 and 28, and 28 and 42. | ||||||
| Groups/Time Points | Day 7 vs. Day 14 | Day 14 vs. Day 28 | Day 28 vs. Day 42 | |||
| NC | 0.04 | 0.03 | 0.03 | |||
| PC | 0.03 | 0.14 | 0.03 | |||
| BPL | 0.05 | 0.04 | 0.27 | |||
| BPH | 0.04 | 0.03 | 0.52 | |||
| BSL | 0.03 | 0.03 | 0.03 | |||
| BSH | 0.03 | 0.05 | 0.53 | |||
| a. Effects of dietary treatments on the relative abundance of cecal microbiota at days 7 and 14. | |||||||||||||
| Treatments (Day-7) 1 | Treatments (Day-14) 1 | ||||||||||||
| Family | Genus | PC | BPH | BPL | BSH | BSL | NC | PC | BPH | BPL | BSH | BSL | NC |
| Ruminococcaceae | Ruminococcus | −2.9 | −3.5 | −2.7 | −3.0 | −3.5 | −2.5 | −3.5 b | −2.7 a | −2.6 a | −2.7 a | −2.6 a | −3.6 b |
| Oscillospira | −4.3 d | −3.8 c | −2.9 a | −3.4 b | −2.8 a | −3.5 bc | −3.9 bc | −3.5 abc | −2.9 a | −2.8 a | −3.2 ab | −4.1 c | |
| Faecalibacterium | −6.4 b | −1.2a | −6.0 b | −6.9 b | −6.5 b | −6.3 b | −0.7 a | −3.7 cd | −2.9 b | −3.6 c | −4.0 d | −6.2 e | |
| Butyricicoccus | −4.2 b | −4.8 b | * | −2.2 a | −4.6 b | −4.4 b | −6.5 c | −4.2 ab | −4.4 ab | −3.7 a | −4.0 a | −5.1 b | |
| Subdoligranulum | * | * | * | * | * | * | −7.0 c | −3.3 b | 0.28 a | −2.7 b | −4.2 b | −3.4 b | |
| Lachnospiraceae | Ruminococcus | 0.15 | −0.21 | 0.24 | −0.01 | 0.29 | −0.05 | −0.1 d | 0.3 c | −2.0 e | −0.1 d | 0.9 b | 1.7 a |
| Blautia | −0.9 b | −1.6 a | −1.4 a | −0.4 bc | −0.3 c | −0.7 bc | −2.7 c | −1.2 b | −2.7 c | −0.7 ab | 0.1 a | −0.1 a | |
| Coprococcus | −0.6 b | −2.5 b | −1.3 b | −0.9 b | −0.7 b | −1.6 ab | −2.6 c | −1.0 b | −3.7 d | −1.5 b | −1.1 b | −0.4 a | |
| Dorea | −3.4 | −4.3 | −4.1 | −3.4 | −3.6 | −4.3 | −5.2 d | −3.4 b | −2.6 a | −3.8 bc | −4.4 c | −4.1 c | |
| Clostridium | −5.4 | −6.1 | −5.4 | −5.9 | −5.7 | −5.4 | −5.3 a | −6.1 ab | −7.3 b | −4.8 a | −6.5 ab | −5.2 a | |
| Lactobacillaceae | Lactobacillus | −1.01 | −1.81 | −0.04 | −1.18 | −0.74 | −1.08 | * | −2.7 b | −4.8 c | −0.9 a | −5.1 c | −5.2 c |
| 2 Erysipelotrichaceae | - | −2.6 | −3.2 | −2.3 | −1.9 | −2.0 | −2.6 | −4.0 b | −2.5 a | −4.4 b | −2.7 a | −2.6 a | −2.8 a |
| Enterococcaceae | Enterococcus | −0.4 ab | −1.8 abc | 0.2 a | −1.8 bc | −3.0 c | −1.2 a–c | −3.3 a | −4.5 b | −5.2 c | −4.4 b | −6.0 d | −4.0 b |
| Bifidobacteriaceae | Bifidobacterium | * | * | * | * | * | * | −6.7 b | −6.6 b | −0.6 a | −3.9 b | −5.6 b | −4.6 b |
| Alcaligenaceae | Sutterella | * | * | * | * | * | * | −2.2 ab | −4.0 ab | −4.0 ab | −5.0 bc | −7.2 c | −1.0 a |
| 2 Enterobacteriaceae | - | −0.7a | −3.7c | −2.8 bc | −2.9 bc | −3.7c | −1.9 b | −1.6 a | −5.3 c | −5.0 c | −5.6 c | −2.9 b | −2.5 b |
| b. Effects of dietary treatments on relative abundance of cecal microbiota at days 28 and 42. | |||||||||||||
| Treatments (Day-28) 1 | Treatments (Day-42) 1 | ||||||||||||
| Family | Genus | PC | BPH | BPL | BSH | BSL | NC | PC | BPH | BPL | BSH | BSL | NC |
| Ruminococcaceae | Ruminococcus | −1.8 ab | −1.8 ab | −1.1a | −2.1 b | −2.6 b | −2.3 b | −3.0 b | −2.6 ab | −2.1 a | −2.9 b | −2.8 b | −3.0 b |
| Oscillospira | −2.7a | −2.6a | −2.4a | −2.5a | −2.8a | −3.4 b | −2.6 | −2.9 | −2.6 | −3.2 | −2.6 | −2.8 | |
| Faecalibacterium | −1.66 b | 0.03a | −0.68 ab | −0.07a | −0.46 ab | −1.7 b | 0.6 a | 0.2 ab | −0.4 b | 0.3 ab | 0.5 ab | 0.01 ab | |
| Butyricicoccus | −4.0 | −3.8 | −4.5 | −4.5 | −3.6 | −4.5 | −4.9 ab | −4.3 ab | −3.6 a | −5.1 b | −4.6 ab | −3.9 ab | |
| Subdoligranulum | −4.1 | −4.8 | −4.5 | −4.6 | −6.3 | −4.9 | −4.1 ab | −5.7 b | −5.1 b | −2.8 a | −5.3 b | −5.2 b | |
| Lachnospiraceae | Ruminococcus | −0.9 | −1.7 | −1.2 | −1.3 | −1.2 | −0.5 | −1.7 ab | −1.3 a | −1.3 a | −1.6 ab | −2.1 b | −1.6 ab |
| Blautia | −1.5 ab | −2.9 c | −1.2 ab | −2.5bc | −1.4 ab | −1.0 a | −2.9 ab | −2.4 ab | −2.0 a | −2.5 ab | −2.6 ab | −3.2 b | |
| Coprococcus | −2.6bc | −3.3c | −2.1 ab | −2.2 ab | −2.5b | −1.7 a | −3.5 b | −3.4 b | −2.4 ab | −3.0 ab | −3.5 b | −2.3 a | |
| Dorea | −3.8 | −3.6 | −3.6 | −3.7 | −3.3 | −3.6 | −3.1 a | −4.7 b | −3.8 ab | −4.4 b | −3.1 a | −4.3 b | |
| Clostridium | −3.9 | −4.5 | −4.2 | −4.5 | −4.2 | −4.1 | −4.3 | −3.8 | −3.6 | −4.2 | −3.5 | −3.5 | |
| Lactobacillaceae | Lactobacillus | −4.5 | −3.5 | −4.1 | −4.6 | −3.9 | −3.4 | −4.4 b | −2.2 a | −2.3 a | −4.0 ab | −4.5 b | −3.6 ab |
| 2 Erysipelotrichaceae | - | −3.1 | −3.9 | −3.8 | −3.6 | −3.2 | −3.5 | −4.6 | −3.1 | −3.2 | −4.1 | −3.8 | −3.9 |
| Enterococcaceae | Enterococcus | −4.1 | −5.6 | −5.3 | −5.6 | −5.2 | −3.9 | −5.5 ab | −5.7 ab | −4.5 a | −6.8 b | −6.1 ab | −6.4 b |
| Bifidobacteriaceae | Bifidobacterium | −2.4 | −6.0 | −5.4 | −5.0 | −4.1 | −3.1 | −3.0 | −5.1 | −3.0 | −2.4 | −2.1 | −1.9 |
| Alcaligenaceae | Sutterella | −3.7c | −3.7c | −1.9 ab | −3.4c | −2.8bc | −1.4 a | −4.5 c | −3.4 bc | −2.4 ab | −3.8 c | −3.8 c | −1.7 a |
| 2 Enterobacteriaceae | - | −1.2a | −3.0 b | −4.2 cd | −5.1d | −4.3 cd | −3.8 bc | −4.5 | −5.6 | −3.8 | −4.3 | −4.7 | −5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilal, M.; Achard, C.; Barbe, F.; Chevaux, E.; Ronholm, J.; Zhao, X. Bacillus pumilus and Bacillus subtilis Promote Early Maturation of Cecal Microbiota in Broiler Chickens. Microorganisms 2021, 9, 1899. https://doi.org/10.3390/microorganisms9091899
Bilal M, Achard C, Barbe F, Chevaux E, Ronholm J, Zhao X. Bacillus pumilus and Bacillus subtilis Promote Early Maturation of Cecal Microbiota in Broiler Chickens. Microorganisms. 2021; 9(9):1899. https://doi.org/10.3390/microorganisms9091899
Chicago/Turabian StyleBilal, Muhammad, Caroline Achard, Florence Barbe, Eric Chevaux, Jennifer Ronholm, and Xin Zhao. 2021. "Bacillus pumilus and Bacillus subtilis Promote Early Maturation of Cecal Microbiota in Broiler Chickens" Microorganisms 9, no. 9: 1899. https://doi.org/10.3390/microorganisms9091899
APA StyleBilal, M., Achard, C., Barbe, F., Chevaux, E., Ronholm, J., & Zhao, X. (2021). Bacillus pumilus and Bacillus subtilis Promote Early Maturation of Cecal Microbiota in Broiler Chickens. Microorganisms, 9(9), 1899. https://doi.org/10.3390/microorganisms9091899







