Characterization of Weissella viridescens UCO-SMC3 as a Potential Probiotic for the Skin: Its Beneficial Role in the Pathogenesis of Acne Vulgaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Weissella viridescens UCO-SMC3
2.2. Glass Adherence
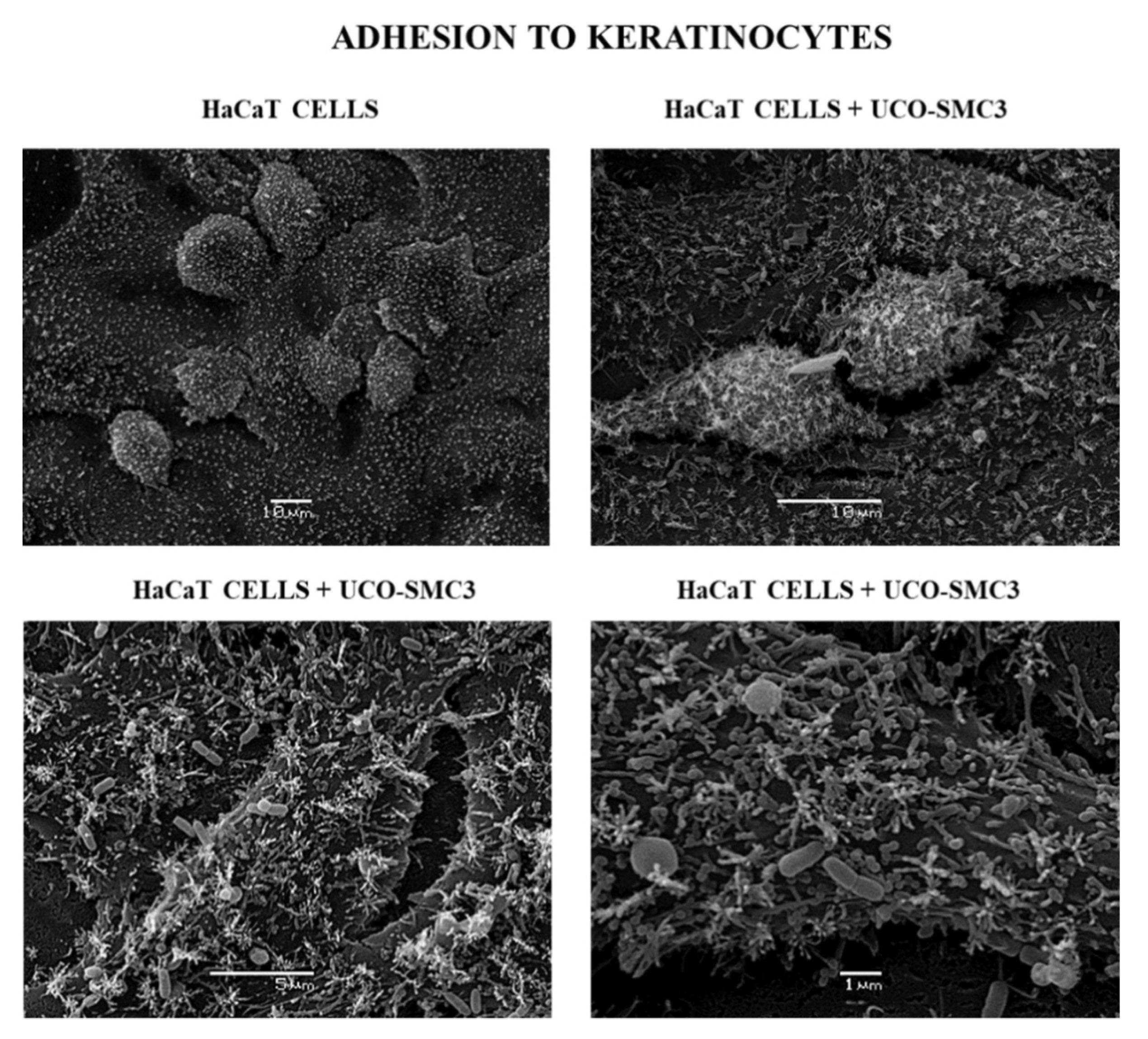
2.3. Culture of HaCat Cells and Adherence Assay
2.4. Resistance to Gastric Conditions
2.5. Antibiotic Susceptibility
2.6. Hemolysis and Gelatinase Activities Detection
2.7. Cytotoxicity Assay on HaCat Cells
2.8. Hydrogen Peroxide (H2O2) Production
2.9. Lactic Acid and Bacteriocin Detection
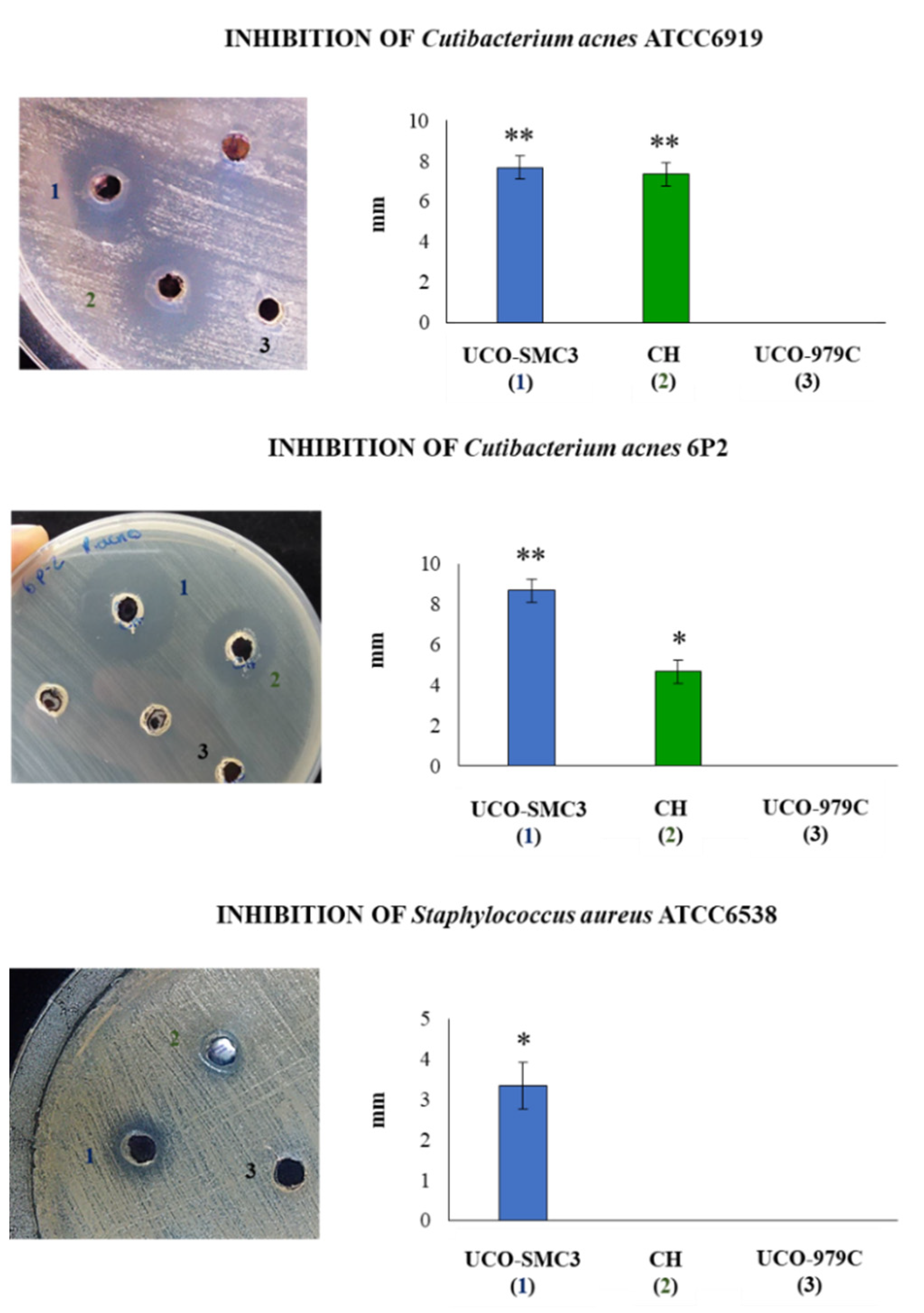
2.10. Microbicidal Activity on C. acnes and S. aureus
2.11. Antagonistic Activity on the Adhesion of C. acnes and S. aureus in HaCat Cells
2.12. Protection Against C. acnes Infection In Vivo
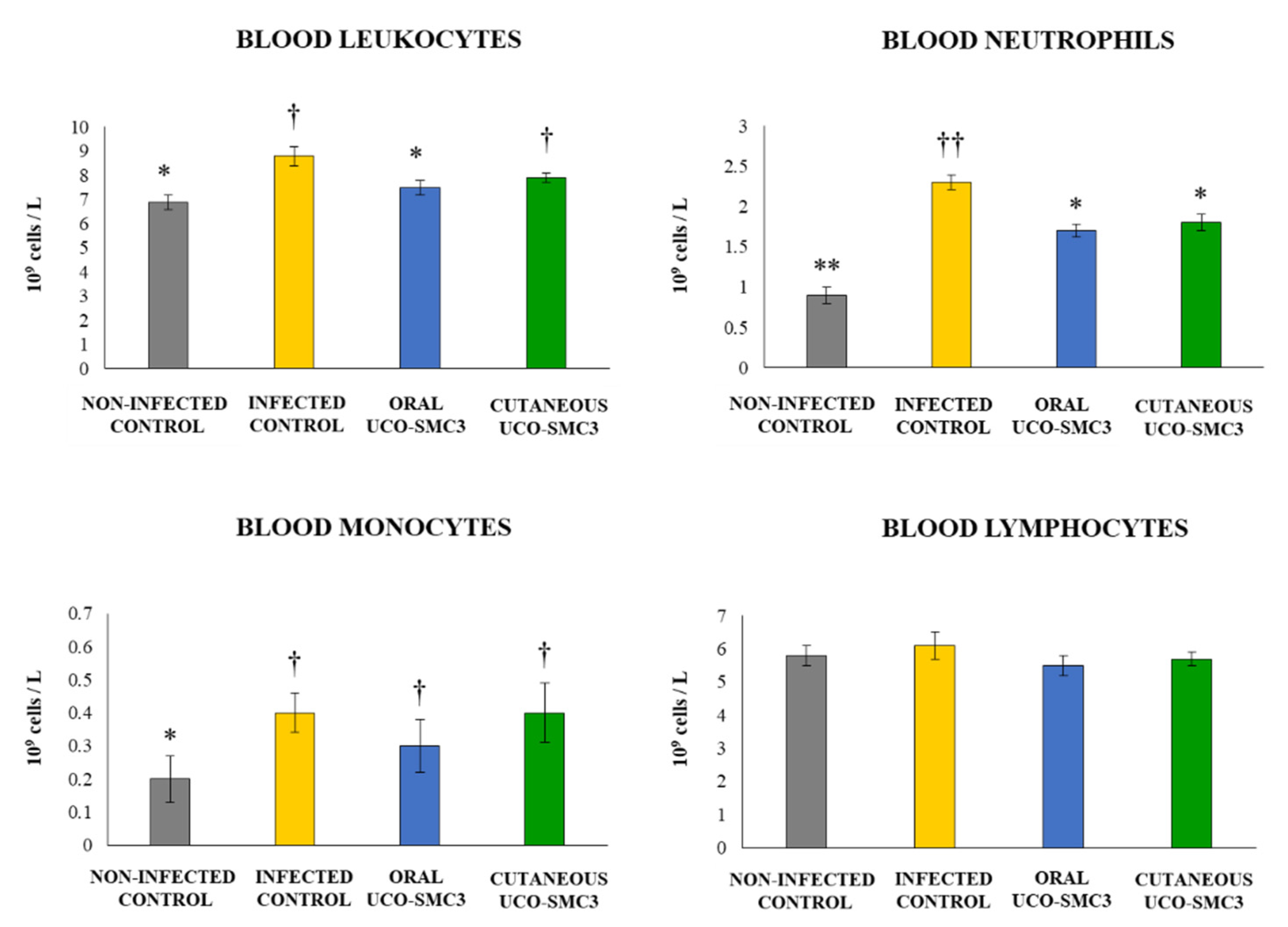
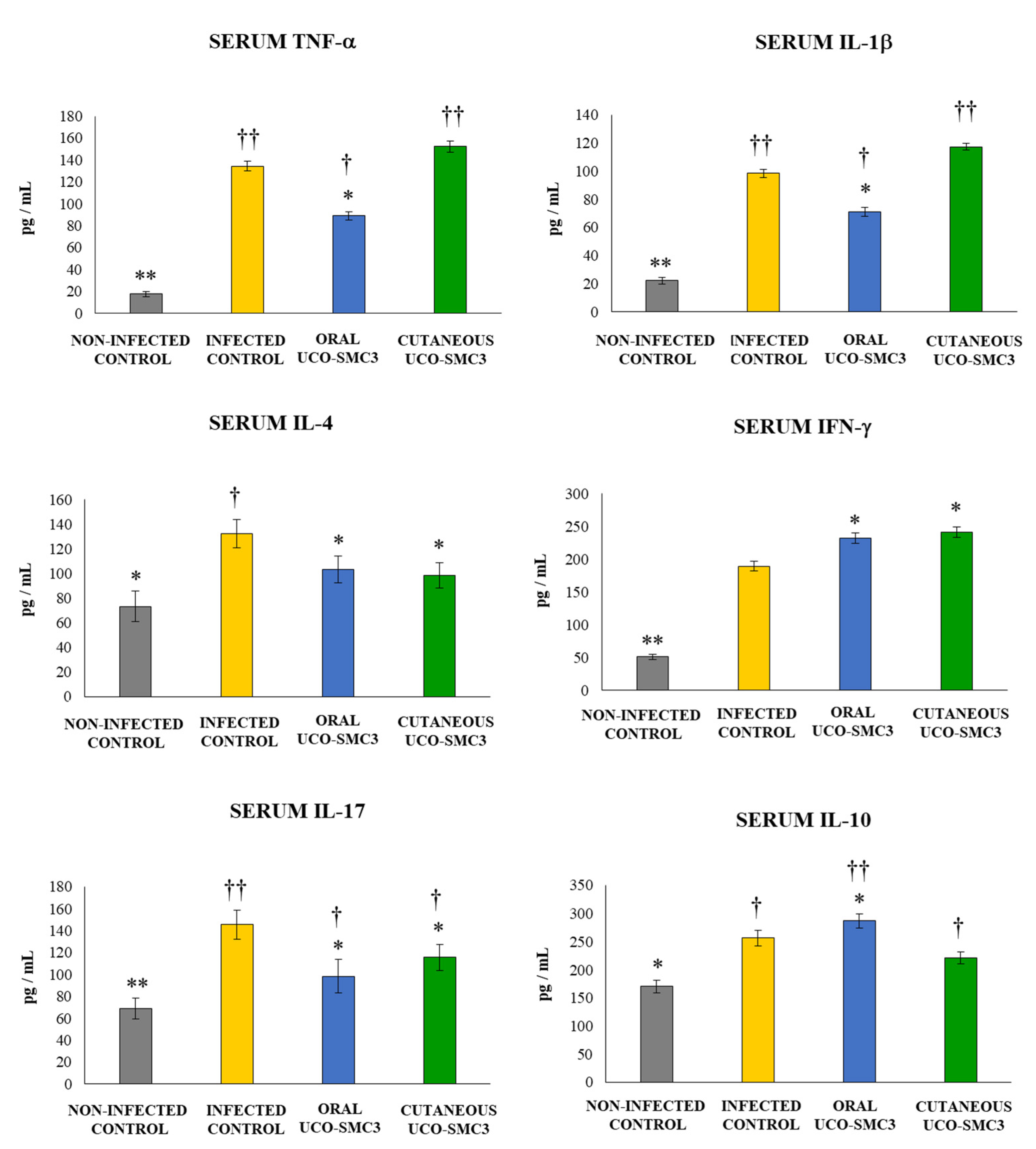
2.13. Determination of Blood Leukocytes Counts and Serum Cytokines
2.14. Flow Cytometry Studies in Lymphoid Nodes
2.15. Development of Novobase II Cream with W. viridescens UCO-SMC3
2.16. Clinical Pilot Trial
2.17. Statistical Analysis
3. Results
3.1. Adherent Capacity of W. viridescens UCO-SMC3
3.2. Resistance of W. viridescens UCO-SMC3 to Gastrointestinal Conditions
3.3. Innocuousness of W. viridescens UCO-SMC3
3.4. Antimicrobial Activity of W. viridescens UCO-SMC3
3.5. Antagonistic Activity of W. viridescens UCO-SMC3 against Skin Pathogens
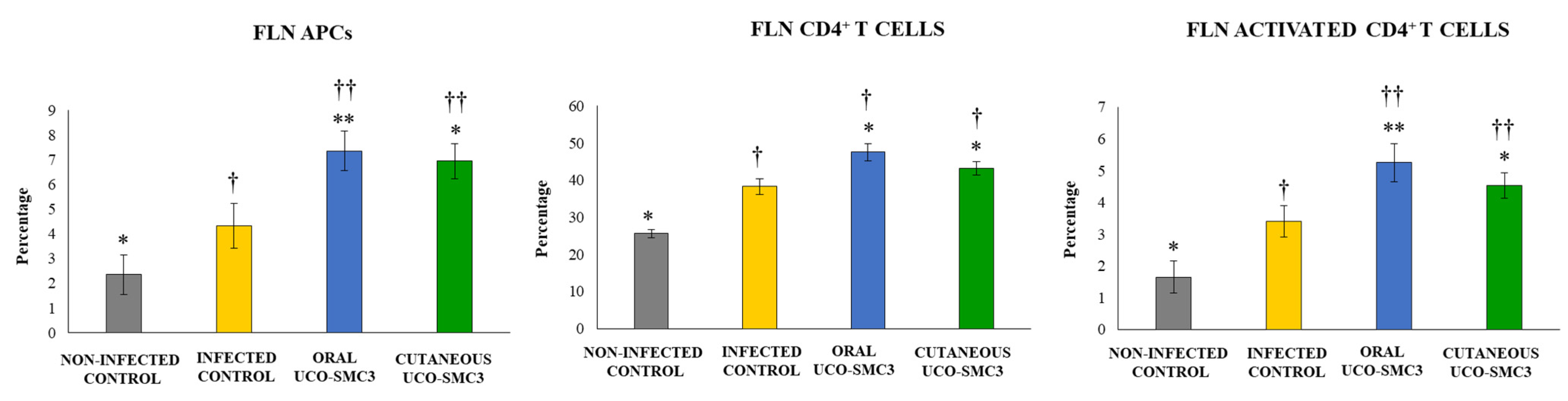
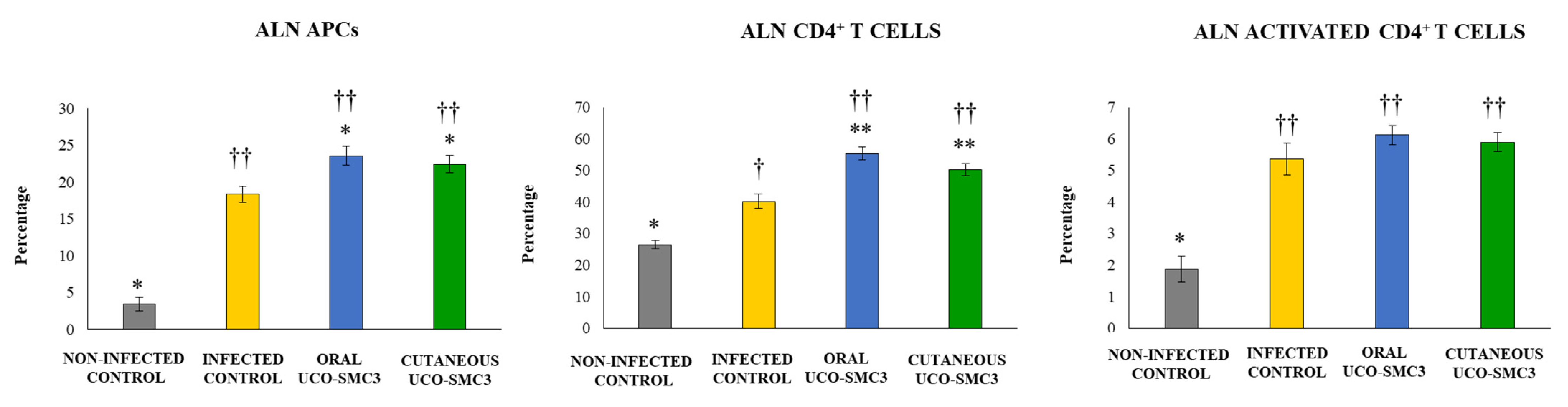
3.6. Immunomodulatory Activity of W. viridescens UCO-SMC3
3.7. Pilot Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lunjani, N.; Ahearn-Ford, S.; Dube, F.S.; Hlela, C.; O’Mahony, L. Mechanisms of microbe-immune system dialogue within the skin. Genes Immun. 2021, 1–13. [Google Scholar] [CrossRef]
- Ramasamy, S.; Barnard, E.; Dawson, T.; Li, H. The role of the skin microbiota in acne pathophysiology. Br. J. Dermatol. 2019, 181, 691–699. [Google Scholar] [CrossRef]
- Yu, Y.; Champer, J.; Agak, G.W.; Kao, W.; Modlin, R.L.; Kim, J. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J. Invest. Dermatol. 2016, 136, 2221–2228. [Google Scholar] [CrossRef]
- Garg, S.; Baveja, S. Combination Therapy in the Management of Atrophic Acne Scars. J. Cutan. Aesthetic Surg. 2014, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Cresce, N.D.; A Davis, S.; Huang, W.W.; Feldman, S.R. The quality of life impact of acne and rosacea compared to other major medical conditions. J. Drugs Dermatol. 2014, 13, 692–697. [Google Scholar]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.-H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium acnes Strain Populations in the Human Skin Microbiome Associated with Acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21 (Suppl. S1), 18–24. [Google Scholar] [CrossRef]
- Thiboutot, D.M. Inflammasome Activation by Propionibacterium acnes: The Story of IL-1 in Acne Continues to Unfold. J. Investig. Dermatol. 2014, 134, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes(Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef]
- Tomida, S.; Nguyen, L.; Chiu, B.-H.; Liu, J.; Sodergren, E.; Weinstock, G.; Li, H. Pan-Genome and Comparative Genome Analyses of Propionibacterium acnes Reveal Its Genomic Diversity in the Healthy and Diseased Human Skin Microbiome. mBio 2013, 4, e00003-13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Pathak, R.; Mary, P.B.; Jha, D.; Sardana, K.; Gautam, H.K. New insights into acne pathogenesis: Exploring the role of acne-associated microbial populations. Dermatol. Sin. 2016, 34, 67–73. [Google Scholar] [CrossRef]
- Johnson, T.; Kang, D.; Barnard, E.; Li, H. Strain-Level Differences in Porphyrin Production and Regulation in Propionibacterium acnes Elucidate Disease Associations. mSphere 2016, 1, e00023-15. [Google Scholar] [CrossRef] [PubMed]
- Bhate, K.; Lin, L.-Y.; Barbieri, J.S.; Leyrat, C.; Hopkins, S.; Stabler, R.; Shallcross, L.; Smeeth, L.; Francis, N.; Mathur, R.; et al. Is there an association between long-term antibiotics for acne and subsequent infection sequelae and antimicrobial resistance? A systematic review. BJGP Open 2021, 5, 181. [Google Scholar] [CrossRef]
- Dreno, B.; Thiboutot, D.; Gollnick, H.; Bettoli, V.; Kang, S.; Leyden, J.J.; Shalita, A.; Torres, V. Antibiotic stewardship in dermatology: Limiting antibiotic use in acne. Eur. J. Dermatol. 2014, 24, 330–334. [Google Scholar] [CrossRef]
- Bowe, W.P.; Logan, A.C. Acne vulgaris, probiotics and the gut-brainskin axis—Back to the future? Gut Pathog. 2011, 3, 1. [Google Scholar] [CrossRef]
- Lee, G.R.; Maarouf, M.; Hendricks, A.J.; E Lee, D.; Shi, V.Y. Topical probiotics: The unknowns behind their rising popularity. Dermatol. Online J. 2019, 25, 13030. [Google Scholar] [CrossRef]
- Dréno, B.; Araviiskaia, E.; Berardesca, E.; Gontijo, G.; Viera, M.S.; Xiang, L.; Martin, R.; Bieber, T. Microbiome in healthy skin, update for dermatologists. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2038–2047. [Google Scholar] [CrossRef]
- Gentili, V.; Bortolotti, D.; Benedusi, M.; Alogna, A.; Fantinati, A.; Guiotto, A.; Turrin, G.; Cervellati, C.; Trapella, C.; Rizzo, R.; et al. HelixComplex snail mucus as a potential technology against O3 induced skin damage. PLoS ONE 2020, 15, e0229613. [Google Scholar] [CrossRef]
- Trapella, C.; Rizzo, R.; Gallo, S.; Alogna, A.; Bortolotti, D.; Casciano, F.; Zauli, G.; Secchiero, P.; Voltan, R. HelixComplex snail mucus exhibits pro-survival, proliferative and pro-migration effects on mammalian fibroblasts. Sci. Rep. 2018, 8, 1–10, Erratum in 2020, 10, 1834. [Google Scholar] [CrossRef]
- Caullan, L.P.; Vila, G.G.; Angulo, E.A.; Calvo, A.; Marcelo, J.A.; Torras, M.A.C. Microbiota from Helix aspersa Müller in Barcelona Area (Spain). Adv. Microbiol. 2014, 4, 604–608. [Google Scholar] [CrossRef]
- Garcia-Cancino, A.; Albarracin, L.; Espinoza-Monje, M.; Campos-Martin, J.; Garcia-Castillo, V.; Nakano, Y.; Ikeda-Ohtsubo, W.; Guitierrez-Zamorano, C.; Morita, H.; Kitazawa, H.; et al. Draft Genome Sequence of Weissella viridescens UCO-SMC3, Isolated from the Slime of Helix aspersa Müller Snails. Microbiol. Resour. Announc. 2019, 8, e01654-18. [Google Scholar] [CrossRef] [PubMed]
- Azcárate-Peril, M.A.; Raya, R.R. Methods for plasmid and genomic DNA isolation from lactobacilli. In Food Microbiology Protocols; Spencer, J.F.T., de Ragout Spencer, A.L., Eds.; Humana Press: Clifton, NJ, USA, 2001; Volume 14, pp. 135–139. [Google Scholar]
- Claudel, J.-P.; Auffret, N.; Leccia, M.-T.; Poli, F.; Corvec, S.; Dréno, B. Staphylococcus epidermidis: A Potential New Player in the Physiopathology of Acne? Dermatology 2019, 235, 287–294. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Navarro, K.; Sanhueza, E.; Pineda, S.; Pastene, E.; Quezada, M.; Henríquez, K.; Karlyshev, A.; Villena, J.; González, C. Characterization of Lactobacillus fermentum UCO-979C, a probiotic strain with a potent anti-Helicobacter pylori activity. Electron. J. Biotechnol. 2017, 25, 75–83. [Google Scholar] [CrossRef]
- Sgouras, D.N.; Maragkoudakis, P.; Petraki, K.; Martinez-Gonzalez, B.; Eriotou, E.; Michopoulos, S.; Kalantzopoulos, G.; Tsakalidou, E.; Mentis, A. In Vitro and In Vivo Inhibition of Helicobacter pylori by Lactobacillus casei Strain Shirota. Appl. Environ. Microbiol. 2004, 70, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, R.; Iliev, I.N.; Chipeva, V.A.; Dimitonova, S.P.; Samelis, J.; Danova, S.T. Identification and in vitro characterization of Lactobacillus plantarum strains from artisanal Bulgarian white brined cheeses. J. Basic Microbiol. 2008, 48, 234–244. [Google Scholar] [CrossRef]
- Peres, C.; Alves, M.; Hernandez-Mendoza, A.; Moreira, L.; Silva, S.; Bronze, M.; Vilas-Boas, L.; Peres, C.; Malcata, F.X. Novel isolates of lactobacilli from fermented Portuguese olive as potential probiotics. LWT Food Sci. Technol. 2014, 59, 234–246. [Google Scholar] [CrossRef]
- Quilodrán, S.; Villena, J.; Valdebenito, J.; Salas, M.; Parra, C.; Ruiz, A.; Kitazawa, H.; García, A. Isolation of lactic acid bacteria from swine milk and characterization of potential probiotic strains with antagonistic effects against swine-associated gastrointestinal pathogens. Can. J. Microbiol. 2016, 62, 514–524. [Google Scholar] [CrossRef]
- Felten, A.; Barreau, C.; Bizet, C.; Lagrange, P.H.; Philippon, A. Lactobacillus species identification, H2O2 production, and antibiotic resistance and correlation with human clinical status. J. Clin. Microbiol. 1999, 370, 729–733. [Google Scholar] [CrossRef]
- Kumar, A.; Ramesh, A. Succession of dominant and antagonistic lactic acid bacteria in fermented cucumber: Insights from a PCR-based approach. Food Microbiol. 2008, 25, 278–287. [Google Scholar]
- Urso, R.; Rantsiou, K.; Cantoni, C.; Comi, G.; Cocolin, L. Sequencing and expresion analisis of the sakacin P bacteriocin producced by a Lactobacillus sakei strain isolated from naturally fermented sausages. Appl. Microbiol. Biotechnol. 2006, 71, 480–485. [Google Scholar] [CrossRef]
- Canzek, A.; Bogovic, B.; Rogelj, I. Chromosomal location of the genetic determinants for bacteriocins produced by Lactobacillus gasseri K7. J. Dairy Res. 2003, 70, 199–203. [Google Scholar] [CrossRef]
- Kizerwetter, M.; Binek, M. Salivaricin B gene its localisation and RFLP analysis in two potentially probiotic Lactobacillus salivarius strains. Bull. Vet. Inst. Pulawy 2010, 54, 513–516. [Google Scholar]
- Pilasombut, K.; Sakpuaram, T.; Wajjwalku, W.; Nitisinprasert, S.; Swetwiwathana, A.; Zendo, T.; Sonomoto, K. Purification and amino acid sequence of a bacteriocins produced by Lactobacillus 809 salivarius K7 isolated from chicken intestine. Songklanakarin J. Sci. Technol. 2006, 28, 121–131. [Google Scholar]
- Gaudana, S.; Dhanani, A.; Bagchi, T. Probiotic attributes of Lactobacillus strains isolated from food and of human origin. Br. J. Nutr. 2010, 103, 1620–1628. [Google Scholar] [CrossRef][Green Version]
- De Young, L.M.; Young, J.M.; Ballaron, S.J.; Spires, D.A.; Puhvel, S.M. Intradermal injection of Propionibacterium acnes: A model of inflammation relevant to acne. J. Invest. Dermatol. 1984, 83, 394–398. [Google Scholar] [CrossRef]
- Jalali, O.; Best, M.; Wong, A.; Schaeffer, B.; Bauer, B.; Johnson, L. Protocatechuic Acid as a Topical Antimicrobial for Surgical Skin Antisepsis: Preclinical Investigations. JB JS Open Access. 2020, 5, e19.00079. [Google Scholar] [CrossRef]
- Hayashi, N.; Akamatsu, H.; Kawashima, M. Acne Study Group. Establishment of grading criteria for acne severity. J. Dermatol. 2008, 35, 255–260. [Google Scholar]
- Witkowski, J.A.; Parish, L.C. The assessment of acne: An evaluation of grading and lesion counting in the measurement of acne. Clin. Dermatol. 2004, 22, 394–397. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Ryu, M.S.; Yang, H.J.; Jeong, S.Y.; Zhang, T.; Yang, H.J.; Kim, M.J.; Park, S. Pediococcus acidilactici intake decreases the clinical severity of atopic dermatitis along with increasing mucin production and improving the gut microbiome in Nc/Nga mice. Biomed. Pharmacother. 2020, 129, 110488. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Jang, Y.J.; Han, D.H.; Jeon, K.; Lee, C.; Han, H.S.; Ko, G. Lactobacillus paracasei KBL382 administration attenuates atopic dermatitis by modulating immune response and gut microbiota. Gut Microbes 2020, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.F.; Cassini-Vieira, P.; Canesso, M.C.C.; Felipetto, M.; Ranfley, H.; Teixeira, M.M.; Nicoli, J.R.; Martins, F.S.; Barcelos, L.S. Lactobacillus rhamnosus CGMCC 1.3724 (LPR) Improves Skin Wound Healing and Reduces Scar Formation in Mice. Probiotics Antimicrob. Proteins 2021, 13, 709–719. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Komatsu, R.; Clua, P.; Indo, Y.; Takagi, M.; Salva, S.; Islam, A.; Alvarez, S.; Takahashi, H.; Garcia-Cancino, A.; et al. Evaluation of the Immunomodulatory Activities of the Probiotic Strain Lactobacillus fermentum UCO-979C. Front. Immunol. 2019, 10, 1376. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Marcial, G.; Albarracín, L.; Tomokiyo, M.; Clua, P.; Takahashi, H.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection. Microorganisms 2020, 8, 479. [Google Scholar] [CrossRef]
- Pearson, R.D.; Steigbigel, R.T.; Davis, H.T.; Chapman, S.W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 1980, 18, 699–708. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Jugeau, S.; Tenaud, I.; Knol, A.; Jarrousse, V.; Quereux, G.; Khammari, A.; Dreno, B. Induction of toll-like receptors by Propionibacterium acnes. Br. J. Dermatol. 2005, 153, 1105–1113. [Google Scholar] [CrossRef]
- Hwang, D.H.; Lee, D.Y.; Koh, P.-O.; Yang, H.R.; Kang, C.; Kim, E. Rosa davurica Pall. Improves Propionibacterium acnes-Induced Inflammatory Responses in Mouse Ear Edema Model and Suppresses Pro-Inflammatory Chemokine Production via MAPK and NF-κB Pathways in HaCaT Cells. Int. J. Mol. Sci. 2020, 21, 1717. [Google Scholar] [CrossRef]
- Zhang, B.; Choi, Y.M.; Lee, J.; An, I.S.; Li, L.; He, C.; Dong, Y.; Bae, S.; Meng, H. Toll-like receptor 2 plays a critical role in pathogenesis of acne vulgaris. Biomed. Dermatol. 2019, 3, 4. [Google Scholar] [CrossRef]
- Akamatsu, H.; Horio, T.; Hattori, K. Increased hydrogen peroxide generation by neutrophils from patients with acne inflammation. Int. J. Dermatol. 2003, 42, 366–369. [Google Scholar] [CrossRef]
- Kistowska, M.; Meier-Schiesser, B.; Proust, T.; Feldmeyer, L.; Cozzio, A.; Kuendig, T.; Contassot, E.; French, L.E. Propionibacterium acnes Promotes Th17 and Th17/Th1 Responses in Acne Patients. J. Investig. Dermatol. 2015, 135, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.E.; Lee, K.; Park, J.; Kwon, P.; Kim, W.; Jung, Y.; Kim, J. Eckol from Eise-nia bicyclis inhibits inflammation through the Akt/NF- κB signaling in propi- onibacterium acnes-induced human keratinocyte hacat cells. J. Food Biochem. 2017, 41, e12312. [Google Scholar] [CrossRef]
- Yan, H.-M.; Zhao, H.-J.; Guo, D.-Y.; Zhu, P.-Q.; Zhang, C.-L.; Jiang, W. Gut microbiota alterations in moderate to severe acne vulgaris patients. J. Dermatol. 2018, 45, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, H.; Zhou, J.; Mou, Y.; Wang, G.; Xiong, X. Patients with Acne Vulgaris Have a Distinct Gut Microbiota in Comparison with Healthy Controls. Acta Derm. Venereol. 2018, 98, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.K.; Haas, K.N.; Sivamani, R.K. Edible Plants and Their Influence on the Gut Microbiome and Acne. Int. J. Mol. Sci. 2017, 18, 1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Kim, K.-P.; Choi, E.; Yim, J.-H.; Choi, C.; Yun, H.-S.; Ahn, H.-Y.; Oh, J.-Y.; Cho, Y. Effects of Lactobacillus plantarum CJLP55 on Clinical Improvement, Skin Condition and Urine Bacterial Extracellular Vesicles in Patients with Acne Vulgaris: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 1368. [Google Scholar] [CrossRef]
- Yu, Y.; Dunaway, S.; Champer, J.; Kim, J.; Alikhan, A. Changing our microbiome: Probiotics in dermatology. Br. J. Dermatol. 2019, 182, 39–46. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Bertona, M.; Picazo, Ó.; Pareja-Galeano, H.; Monfrecola, G.; Emanuele, E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signaling and improves adult acne. Benef. Microbes. 2016, 7, 625–630. [Google Scholar] [CrossRef]
- Jung, G.W.; Tse, J.E.; Guiha, I.; Rao, J. Prospective, Randomized, Open-Label Trial Comparing the Safety, Efficacy, and Tolerability of an Acne Treatment Regimen with and without a Probiotic Supplement and Minocycline in Subjects with Mild to Moderate Acne. J. Cutan. Med. Surg. 2013, 17, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Manzhalii, E.; Hornuss, D.; Stremmel, W. Intestinal-borne dermatoses significantly improved by oral application ofEscherichia coliNissle 1917. World J. Gastroenterol. 2016, 22, 5415–5421. [Google Scholar] [CrossRef]
- Kinoshita, H.; Ohuchi, S.; Arakawa, K.; Watanabe, M.; Kitazawa, H.; Saito, T. Isolation of lactic acid bacteria bound to the porcine intestinal mucosa and an analysis of their moonlighting adhesins. Biosci. Microbiota Food Health 2016, 35, 185–196. [Google Scholar] [CrossRef][Green Version]
- Chuang, L.-T.; Huang, W.-C.; Hou, Y.-C.; Chyuan, J.-H.; Chang, H.; Chang, C.-I.; Tsai, T.-H.; Tsai, P.-J. Suppressive Effect of Two Cucurbitane-Type Triterpenoids from Momordica charantia on Cutibacterium acnes-Induced Inflammatory Responses in Human THP-1 Monocytic Cell and Mouse Models. Molecules 2021, 26, 579. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, B.; Guo, J.; Wang, M.; Cui, H.; Mao, H.; Wang, B.; Yan, F. Active pharmaceutical ingredient poly(ionic liquid)-based microneedles for the treatment of skin acne infection. Acta Biomater. 2020, 115, 136–147. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, K.C.; Kim, M.J.; Jang, Y.H.; Lee, S.-J.; Kim, D.W. Efficacy of Red or Infrared Light-Emitting Diodes in a Mouse Model of Propionibacterium acnes-Induced Inflammation. Ann. Dermatol. 2016, 28, 186–191. [Google Scholar] [CrossRef]
- Jang, Y.H.; Lee, K.C.; Lee, S.-J.; Kim, D.W.; Lee, W.J. HR-1 Mice: A New Inflammatory Acne Mouse Model. Ann. Dermatol. 2015, 27, 257–264. [Google Scholar] [CrossRef]
- Dagnelie, M.; Corvec, S.; Saint-Jean, M.; Nguyen, J.; Khammari, A.; Dréno, B. Cutibacterium acnes phylotypes diversity loss: A trigger for skin inflammatory process. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Agak, G.W.; Kao, S.; Ouyang, K.; Qin, M.; Moon, D.; Butt, A.; Kim, J. Phenotype and antimicrobial activity of Th17 cells induced by propionibacterium acnes strains associated with healthy and acne skin. J. Invest. Dermatol. 2018, 138, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Kupper, T.S. T cells and the skin: From protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019, 19, 490–502. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.-A.; Gearty, S.; Pauli, M.L.; Nosbaum, A.; Gratz, I.; Otto, M.; Moon, J.; Liese, J.; et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 2015, 43, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Nosbaum, A.; Prevel, N.; Truong, H.-A.; Mehta, P.; Ettinger, M.; Scharschmidt, T.C.; Ali, N.H.; Pauli, M.L.; Abbas, A.K.; Rosenblum, M.D. Cutting edge: Regulatory T cells facilitate cutaneous wound healing. J. Immunol. 2016, 196, 2010–2014. [Google Scholar] [CrossRef]
- Sabaté Brescó, M.; Harris, L.G.; Thompson, K.; Stanic, B.; Morgenstern, M.; O’Mahony, L.; Richards, R.G.; Moriarty, T.F. Pathogenic mechanisms and host interactions in Staphylococcus epidermidis device-related infection. Front. Microbiol. 2017, 8, 1401. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Irvine, A.; Foster, T.J. Staphylococcus aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef]
- Goyarts, E.; Muizzuddin, N.; Maes, D.; Giacomoni, P.U. Morphological Changes Associated with Aging: Age Spots and the Microinflammatory Model of Skin Aging. Ann. N. Y. Acad. Sci. 2007, 1119, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kintarak, S.; Whawell, S.A.; Speight, P.M.; Packer, S.; Nair, S.P. Internalization of Staphylococcus aureus by human keratinocytes. Infect. Immun. 2004, 72, 5668–5675. [Google Scholar] [CrossRef] [PubMed]
- Pelyuntha, W.; Chaiyasut, C.; Kantachote, D.; Sirilun, S. Cell-free supernatants from cultures of lactic acid bacteria isolated from fermented grape as biocontrol againstSalmonellaTyphi andSalmonellaTyphimurium virulence via autoinducer-2 and biofilm interference. PeerJ 2019, 7, e7555. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Liu, J.; Liu, M.; Huang, Y.; Wang, K.; Zhou, G. Effects of two Weissella viridescens strains on Listeria monocytogenes growth at different initial inoculum proportions. CyTA J. Food 2018, 16, 299–305. [Google Scholar] [CrossRef]
- Muizzuddin, N.; Maher, W.; Sullivan, M.; Schnittger, S.; Mammone, T. Physiological effect of a probiotic on skin. J. Cosmet. Sci. 2013, 63, 385–395. [Google Scholar]
- Bateni, E.; Tester, R.; Al-Ghazzewi, F.; Bateni, S.; Alvani, K.; Piggott, J. The use of Konjac Glucomannan Hydrolysates (GMH) to improve the health of the skin and reduce acne vulgaris. Am. J. Dermatol. Venereol. 2013, 2, 10–14. [Google Scholar]




| Antibiotic | Concentraion (μg) | W. viridescens UCO-SMC3 |
|---|---|---|
| Streptomycin | 10 | S |
| Etrithromycin | 15 | S |
| Amikacin | 30 | MS |
| Gentamicin | 10 | S |
| Ampicilin | 10 | MS |
| Cefuroxim | 30 | S |
| Penicillin G | 10 | S |
| Sulfatrimethoprim | 25 | R |
| Cefotaxin | 30 | S |
| Amoxicillin | 10 | S |
| Levofloxacin | 5 | S |
| Chloramphenicol | 30 | S |
| Clarithromycin | 15 | S |
| Neomicin | 30 | S |
| Ciprofloxacin | 5 | R |
| Rifampicin | 5 | S |
| Vancomycin | 30 | MS |
| Tetracycline | 30 | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza-Monje, M.; Campos, J.; Alvarez Villamil, E.; Jerez, A.; Dentice Maidana, S.; Elean, M.; Salva, S.; Kitazawa, H.; Villena, J.; García-Cancino, A. Characterization of Weissella viridescens UCO-SMC3 as a Potential Probiotic for the Skin: Its Beneficial Role in the Pathogenesis of Acne Vulgaris. Microorganisms 2021, 9, 1486. https://doi.org/10.3390/microorganisms9071486
Espinoza-Monje M, Campos J, Alvarez Villamil E, Jerez A, Dentice Maidana S, Elean M, Salva S, Kitazawa H, Villena J, García-Cancino A. Characterization of Weissella viridescens UCO-SMC3 as a Potential Probiotic for the Skin: Its Beneficial Role in the Pathogenesis of Acne Vulgaris. Microorganisms. 2021; 9(7):1486. https://doi.org/10.3390/microorganisms9071486
Chicago/Turabian StyleEspinoza-Monje, Marcela, Jorge Campos, Eduardo Alvarez Villamil, Alonso Jerez, Stefania Dentice Maidana, Mariano Elean, Susana Salva, Haruki Kitazawa, Julio Villena, and Apolinaria García-Cancino. 2021. "Characterization of Weissella viridescens UCO-SMC3 as a Potential Probiotic for the Skin: Its Beneficial Role in the Pathogenesis of Acne Vulgaris" Microorganisms 9, no. 7: 1486. https://doi.org/10.3390/microorganisms9071486
APA StyleEspinoza-Monje, M., Campos, J., Alvarez Villamil, E., Jerez, A., Dentice Maidana, S., Elean, M., Salva, S., Kitazawa, H., Villena, J., & García-Cancino, A. (2021). Characterization of Weissella viridescens UCO-SMC3 as a Potential Probiotic for the Skin: Its Beneficial Role in the Pathogenesis of Acne Vulgaris. Microorganisms, 9(7), 1486. https://doi.org/10.3390/microorganisms9071486








