Potential Probiotic Bacillus subtilis Isolated from a Novel Niche Exhibits Broad Range Antibacterial Activity and Causes Virulence and Metabolic Dysregulation in Enterotoxic E. coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Growth Conditions
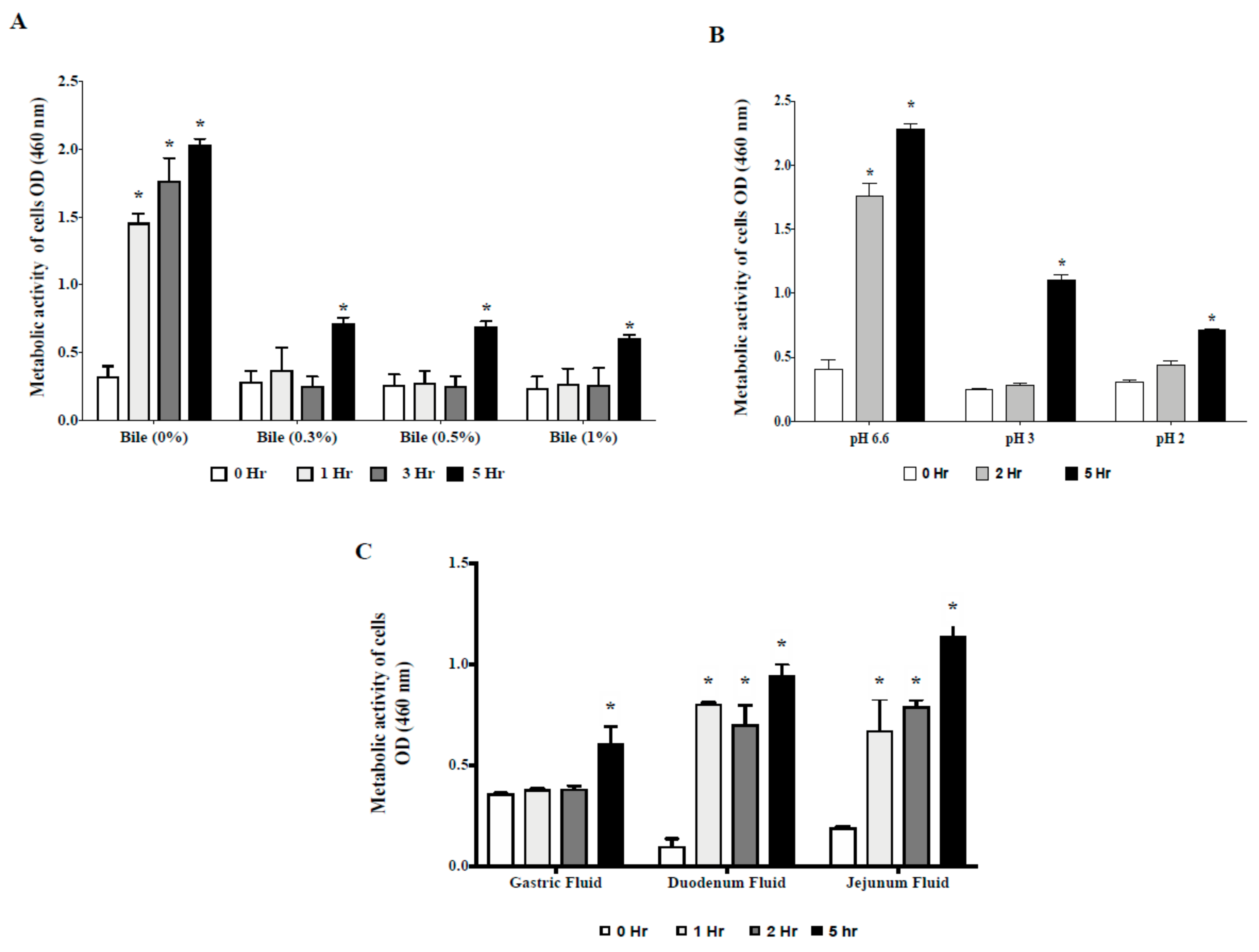
2.2. Survivability in Gastrointestinal Tract (GIT) Environment
2.2.1. Tolerance to Acid and Bile Salts
2.2.2. Tolerance to Swine GIT Fluids
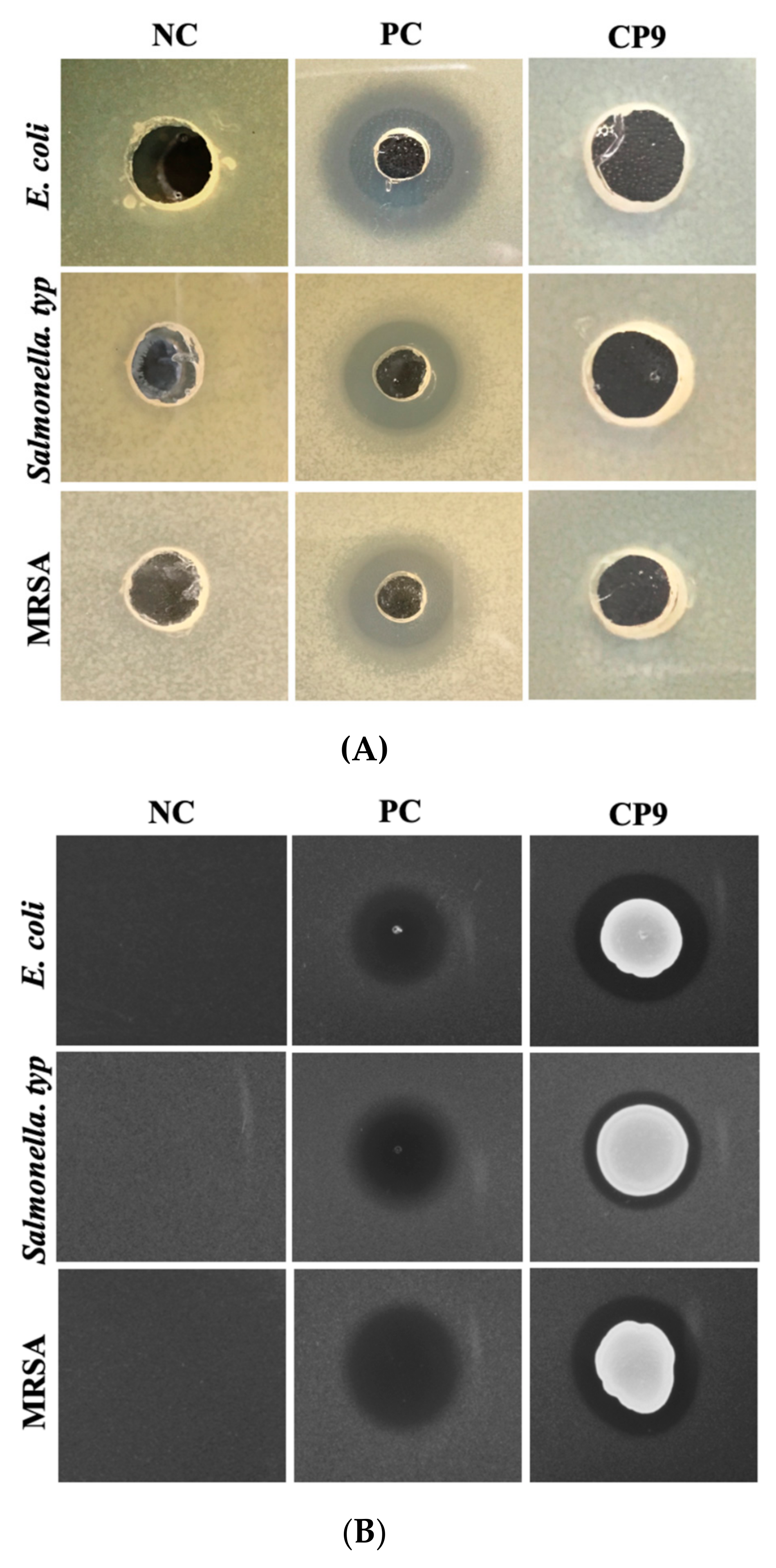
2.3. Evaluation of Antagonistic Activity of CP9 against ETEC, Salmonella Typ., and MRSA
2.3.1. Agar Radial Diffusion Assay
2.3.2. Agar Spot Assay
2.3.3. Bacterial Co-Culture Assay
2.3.4. Cell Line Culture Conditions
2.3.5. Cell Cytotoxicity Assay
2.3.6. Cell Surface Adhesion Assay
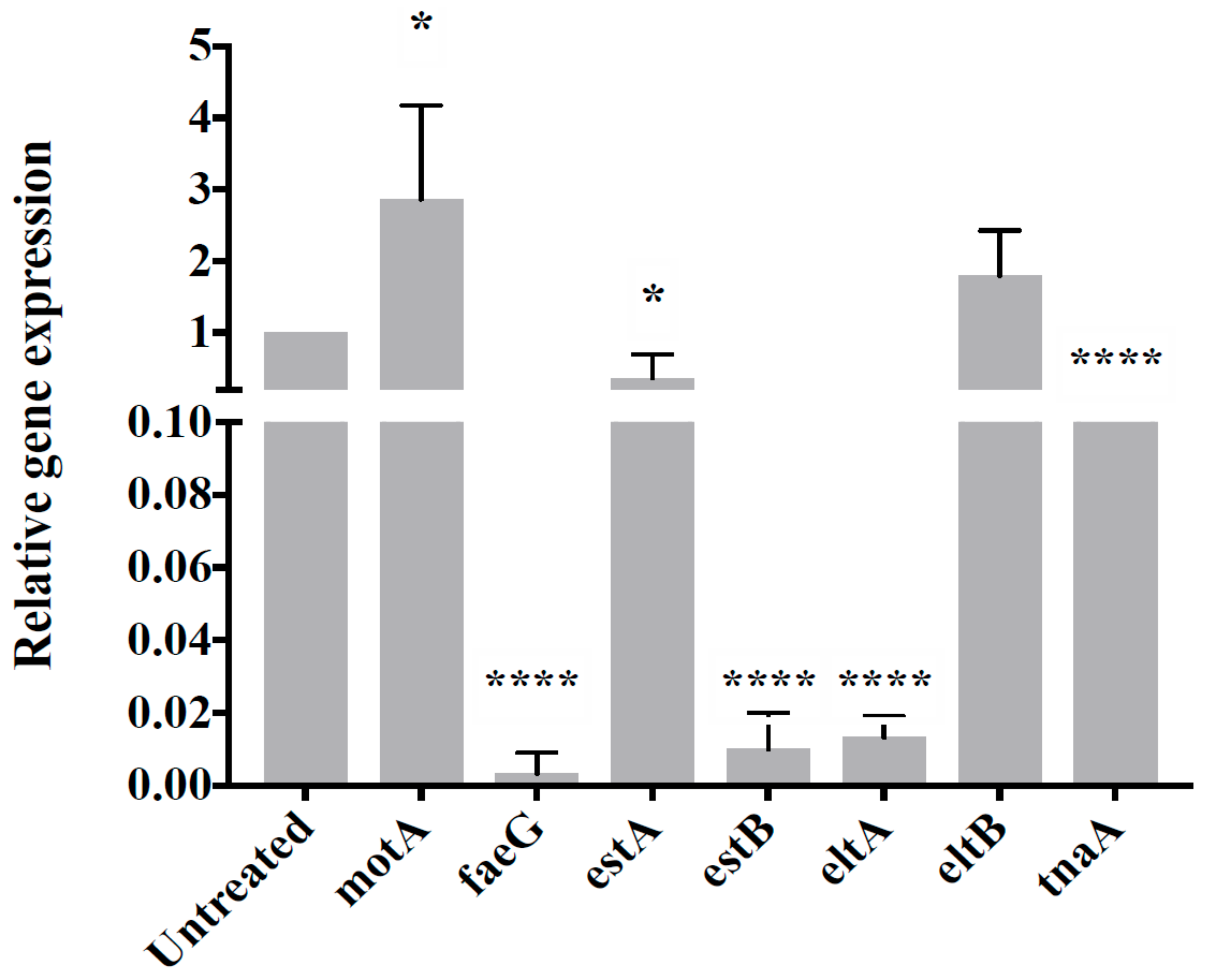
2.3.7. Gene Expression Analysis
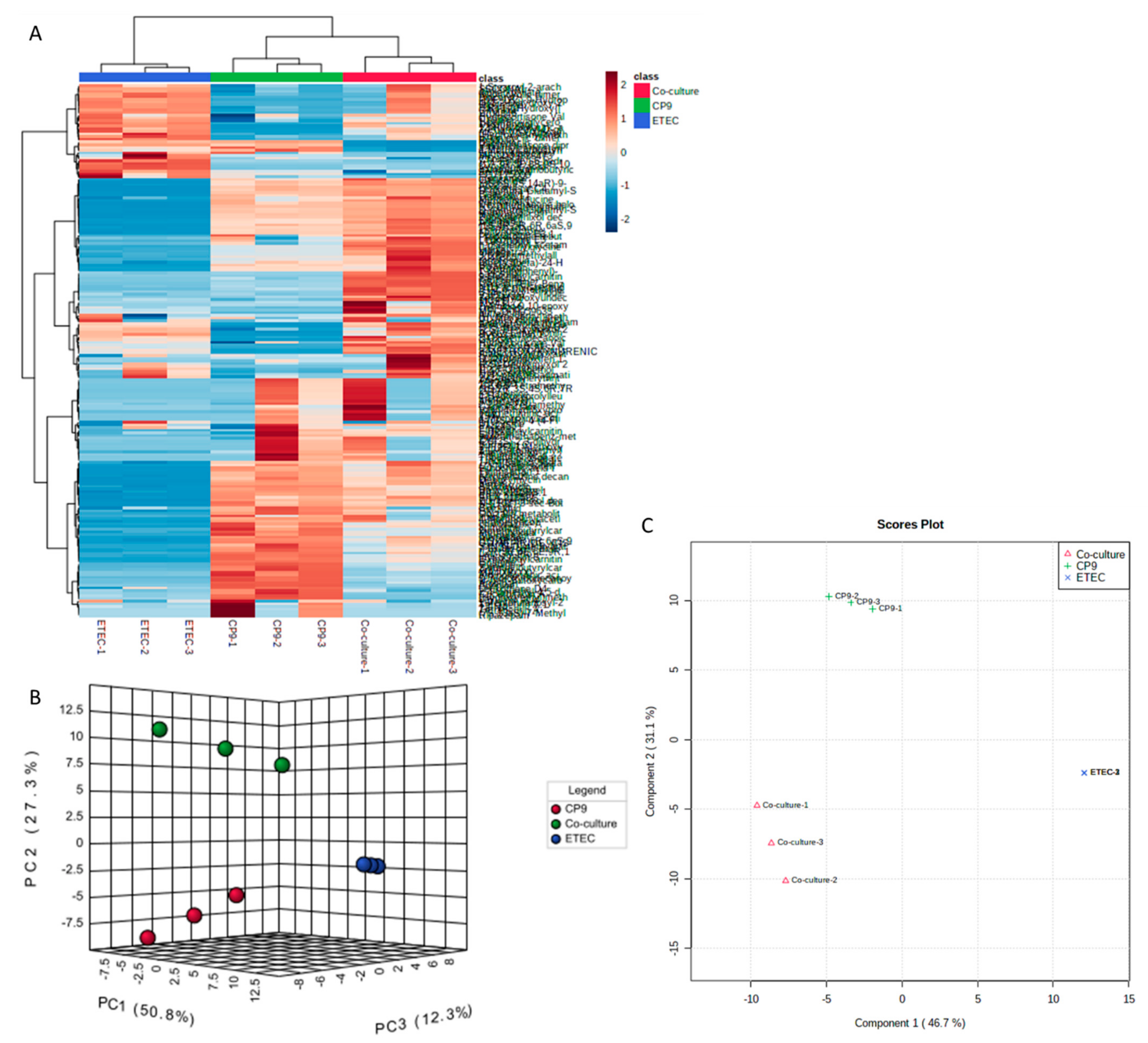
2.4. Metabolomic Analyses
Sample Preparation and LC/MS Procedure
2.5. Statistical Analyses
3. Results
3.1. CP9 Survives Gastrointestinal Environment
3.2. CP9 Adherence and Toxicity on IPEC Cells
3.3. CP9 Exhibits Anti-Pathogenic Activity via Contact Inhibition
3.4. CP9 Downregulates Virulence Genes Expression in ETEC
3.5. Secreted Metabolic Repertoires of the CP9 and ETEC Co-Culture Vary Significantly Than Their Monocultures
3.6. CP9 and ETEC Co-Culture Induces Emergence of New Metabolites with Antimicrobial Properties
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valdes, A.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Anadón, A.; Irma, A.; Martínez-Larrañaga, M.R.; Maria, A.M. Prebiotics and Probiotics in Feed and Animal Health. In Nutraceuticals in Veterinary Medicine; Springer International Publishing: Cham, Switzerland, 2019; pp. 261–285. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Ahn, J. Probiotic-mediated competition, exclusion and displacement in biofilm formation by food-borne pathogens. Lett. Appl. Microbiol. 2013, 56, 307–313. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Factories 2020, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Sardi, J.D.C.O.; Pitangui, N.D.S.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Bron, P.A.; van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Genet. 2011, 10, 66–78. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Saulnier, D.M.; Pot, B.; Versalovic, J. How can probiotics and prebiotics impact mucosal immunity? Gut Microbes 2010, 1, 293–300. [Google Scholar] [CrossRef]
- Trush, E.A.; Poluektova, E.A.; Beniashvilli, A.G.; Shifrin, O.S.; Poluektov, Y.M.; Ivashkin, V.T. The Evolution of Human Probiotics: Challenges and Prospects. Probiotics Antimicrob. Proteins 2020, 12, 1291–1299. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Veiga, P.; Suez, J.; Derrien, M.; Elinav, E. Moving from probiotics to precision probiotics. Nat. Microbiol. 2020, 5, 878–880. [Google Scholar] [CrossRef]
- Evivie, S.E.; Huo, G.-C.; Igene, J.O.; Bian, X. Some current applications, limitations and future perspectives of lactic acid bacteria as probiotics. Food Nutr. Res. 2017, 61, 1318034. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.; Rosa, F.; Rossi, N.; Martin, T.; Mohney, R.P.; Li, W.; De Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; De Foy, J.-M.P.; Dequenne, I.; De Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Medlock, G.L.; Carey, M.; McDuffie, D.G.; Mundy, M.; Giallourou, N.; Swann, J.R.; Kolling, G.L.; Papin, J.A. Inferring Metabolic Mechanisms of Interaction within a Defined Gut Microbiota. Cell Syst. 2018, 7, 245–257. [Google Scholar] [CrossRef]
- Hove, P.R.; Nora, J.N.; Siu, H.J.C.; Shea, M.B.; Hannah, B.H.; Elizabeth, P.R. Metabolomics and Proteomics of L. Rhamnosus GG and E. Coli Nissle Probiotic Supernatants Identify Distinct Pathways That Mediate Growth Suppression of Antimicrobial-Resistant Pathogens. BioRxiv 2020. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Hong, J.-H.; Jeong, Y.S.; Jung, H.K. Evaluation of two Bacillus subtilis strains isolated from Korean fermented food as probiotics against loperamide-induced constipation in mice. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 797–806. [Google Scholar] [CrossRef]
- Mazkour, S.; Shekarforoush, S.S.; Basiri, S.; Nazifi, S.; Yektaseresht, A.; Honarmand, M. Effects of two probiotic spores of Bacillus species on hematological, biochemical, and inflammatory parameters in Salmonella Typhimurium infected rats. Sci. Rep. 2020, 10, 8035. [Google Scholar] [CrossRef]
- Gaete, A.; Mandakovic, D.; González, M. Isolation and Identification of Soil Bacteria from Extreme Environments of Chile and Their Plant Beneficial Characteristics. Microorganisms 2020, 8, 1213. [Google Scholar] [CrossRef]
- Bang, C.; Dagan, T.; Deines, P.; Dubilier, N.; Duschl, W.J.; Fraune, S.; Hentschel, U.; Hirt, H.; Hülter, N.F.; Lachnit, T.; et al. Metaorganisms in extreme environments: Do microbes play a role in organismal adaptation? Zoology 2018, 127, 1–19. [Google Scholar] [CrossRef]
- Akhtar, N.; Cai, H.; Kiarie, E.G.; Li, J. A novel Bacillus sp. with rapid growth property and high enzyme activity that allows efficient fermentation of soybean meal for improving digestibility in growing pigs. J. Appl. Microbiol. 2021, Submitted. [Google Scholar]
- Ramos, C.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Maystrenko, A.; Feng, Y.; Akhtar, N.; Li, J. The Addition of a Synthetic LPS-Targeting Domain Improves Serum Stability While Maintaining Antimicrobial, Antibiofilm, and Cell Stimulating Properties of an Antimicrobial Peptide. Biomolecules 2020, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Astill, J.; Kulkarni, R.R.; Read, L.R.; Najarian, A.; Farber, J.M.; Sharif, S. In vitro assessment of immunomodulatory and anti-Campylobacter activities of probiotic lactobacilli. Sci. Rep. 2019, 9, 17903. [Google Scholar] [CrossRef] [PubMed]
- Kemme, M.; Heinzel-Wieland, R. Quantitative Assessment of Antimicrobial Activity of PLGA Films Loaded with 4-Hexylresorcinol. J. Funct. Biomater. 2018, 9, 4. [Google Scholar] [CrossRef]
- Alcoforado, D.; Juliana, B.H.; Sarah, J.C. Quantitative Determination of Anti-Bacterial Activity during Bacterial Co-Culture. In Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2017; Volume 1615, pp. 517–524. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, Y.; Wang, S.; Liu, H.; Zhang, D.; Zhang, W.; Wang, Y.; Ji, H. Swine-Derived Probiotic Lactobacillus plantarum Inhibits Growth and Adhesion of Enterotoxigenic Escherichia coli and Mediates Host Defense. Front. Microbiol. 2018, 9, 1364. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.W.; El-Nezami, H.; Shah, N.P. The protective effects of enriched citrulline fermented milk with Lactobacillus helveticus on the intestinal epithelium integrity against Escherichia coli infection. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Medellin-Peña, M.J.; Wang, H.; Johnson, R.; Anand, S.; Griffiths, M.W. Probiotics Affect Virulence-Related Gene Expression in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 4259–4267. [Google Scholar] [CrossRef]
- Bonetti, A.; Tugnoli, B.; Rossi, B.; Giovagnoni, G.; Piva, A.; Grilli, E. Nature-Identical Compounds and Organic Acids Reduce E. coli K88 Growth and Virulence Gene Expression In Vitro. Toxins 2020, 12, 468. [Google Scholar] [CrossRef]
- Wang, W.; He, J.; Pan, D.; Wu, Z.; Guo, Y.; Zeng, X.; Lian, L. Metabolomics analysis of Lactobacillus plantarum ATCC 14917 adhesion activity under initial acid and alkali stress. PLoS ONE 2018, 13, e0196231. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vlieg, J.E.V.H. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015, 23, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R. Survival of Probiotic Lactobacilli in Acidic Environments Is Enhanced in the Presence of Metabolizable Sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef]
- Wilks, J.C.; Kitko, R.D.; Cleeton, S.H.; Lee, G.E.; Ugwu, C.S.; Jones, B.D.; BonDurant, S.S.; Slonczewski, J.L. Acid and Base Stress and Transcriptomic Responses in Bacillus subtilis. Appl. Environ. Microbiol. 2009, 75, 981–990. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Denayrolles, M.; Ripert, G.; Desfougères, T.; Lobach, A.R.; Simon, R.; Pélerin, F.; Jüsten, P.; Urdaci, M.C. Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regul. Toxicol. Pharmacol. 2017, 83, 54–65. [Google Scholar] [CrossRef]
- Alayande, K.A.; Aiyegoro, O.A.; Ateba, C.N. Probiotics in Animal Husbandry: Applicability and Associated Risk Factors. Sustainability 2020, 12, 1087. [Google Scholar] [CrossRef]
- Rhayat, L.; Maresca, M.; Nicoletti, C.; Perrier, J.; Brinch, K.S.; Christian, S.; Devillard, E.; Eckhardt, E. Effect of Bacillus subtilis Strains on Intestinal Barrier Function and Inflammatory Response. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Gu, M.J.; Song, S.K.; Park, S.M.; Lee, I.K.; Yun, C.-H. Bacillus subtilis Protects Porcine Intestinal Barrier from Deoxynivalenol via Improved Zonula Occludens-1 Expression. Asian Australas. J. Anim. Sci. 2014, 27, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Cummings, J.H. Probiotics and prebiotics: Can regulating the activities of intestinal bacteria benefit health? BMJ 1999, 318, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Tazehabadi, M.H.; Algburi, A.; Popov, I.V.; Ermakov, A.M.; Chistyakov, V.A.; Prazdnova, E.V.; Weeks, R.; Chikindas, M.L. Probiotic Bacilli Inhibit Salmonella Biofilm Formation Without Killing Planktonic Cells. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Chalasani, A.G.; Lal, R.; Roy, U. A Broad-Spectrum Antimicrobial Activity ofBacillus subtilisRLID 12.1. Sci. World J. 2014, 2014, 968487. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stubbendieck, R.M.; Straight, P.D. Multifaceted Interfaces of Bacterial Competition. J. Bacteriol. 2016, 198, 2145–2155. [Google Scholar] [CrossRef]
- Garcia, E.C. Contact-dependent interbacterial toxins deliver a message. Curr. Opin. Microbiol. 2018, 42, 40–46. [Google Scholar] [CrossRef]
- Garcia, E.C.; Perault, A.I.; Marlatt, S.A.; Cotter, P.A. Interbacterial signaling via Burkholderia contact-dependent growth inhibition system proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 8296–8301. [Google Scholar] [CrossRef]
- Lopez, D.; Kolter, R. Extracellular signals that define distinct and coexisting cell fates inBacillus subtilis. FEMS Microbiol. Rev. 2010, 34, 134–149. [Google Scholar] [CrossRef]
- Kaundal, S.; Deep, A.; Kaur, G.; Thakur, K.G. Molecular and Biochemical Characterization of YeeF/YezG, a Polymorphic Toxin-Immunity Protein Pair From Bacillus subtilis. Front. Microbiol. 2020, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Holberger, L.E.; Garza-Sánchez, F.; Lamoureux, J.; Low, D.A.; Hayes, C.S. A novel family of toxin/antitoxin proteins inBacillusspecies. FEBS Lett. 2011, 586, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Ikryannikova, L.; Kurbatov, L.; Gorokhovets, N.; Zamyatnin, A. Contact-Dependent Growth Inhibition in Bacteria: Do Not Get Too Close! Int. J. Mol. Sci. 2020, 21, 7990. [Google Scholar] [CrossRef]
- Anyanful, A.; Dolan-Livengood, J.M.; Lewis, T.; Sheth, S.; DeZalia, M.N.; Sherman, M.A.; Kalman, L.V.; Benian, G.M.; Kalman, D. Paralysis and killing of Caenorhabditis elegans by enteropathogenic Escherichia coli requires the bacterial tryptophanase gene. Mol. Microbiol. 2005, 57, 988–1007. [Google Scholar] [CrossRef]
- Zhou, M.; Duan, Q.; Zhu, X.; Guo, Z.; Li, Y.; Hardwidge, P.R.; Zhu, G. Both flagella and F4 fimbriae from F4ac+ enterotoxigenic Escherichia coli contribute to attachment to IPEC-J2 cells in vitro. Vet. Res. 2013, 44, 30. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef]
- Wilson, M.L.; Macnab, R.M. Overproduction of the MotA protein of Escherichia coli and estimation of its wild-type level. J. Bacteriol. 1988, 170, 588–597. [Google Scholar] [CrossRef]
- Jurtshuk, P. Good info about different types and pathways: Bacterial Metabolism. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21413278 (accessed on 23 May 2021).
- Ward, B. Bacterial Energy Metabolism. In Molecular Medical Microbiology, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 1. [Google Scholar] [CrossRef]
- Hansen, S.K.; Rainey, P.; Haagensen, J.A.J.; Molin, S. Evolution of species interactions in a biofilm community. Nat. Cell Biol. 2007, 445, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Freilich, S.; Zarecki, R.; Eilam, O.; Segal, E.S.; Henry, C.S.; Kupiec, M.; Gophna, U.; Sharan, R.; Ruppin, E. Competitive and cooperative metabolic interactions in bacterial communities. Nat. Commun. 2011, 2, 589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Rubin, E.J. Feast or famine: The host-pathogen battle over amino acids. Cell. Microbiol. 2013, 15, 1079–1087. [Google Scholar] [CrossRef]
- Li, G.; Young, K.D. A new suite of tnaA mutants suggests that Escherichia coli tryptophanase is regulated by intracellular sequestration and by occlusion of its active site. BMC Microbiol. 2015, 15, 1–17. [Google Scholar] [CrossRef]
- Orozco-Gómez, D.I.; Sosa-Hernández, J.E.; Gallardo-Navarro, Ó.A.; Santana-Solano, J.; Santillán, M. Bistable behaviour and medium-dependent post-translational regulation of the tryptophanase operon regulatory pathway in Echerichia coli. Sci. Rep. 2019, 9, 5451. [Google Scholar] [CrossRef]
- Di Martino, P.; Fursy, R.; Bret, L.; Sundararaju, B.; Phillips, R. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Can. J. Microbiol. 2003, 49, 443–449. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sperandio, V. Indole Signaling at the Host-Microbiota-Pathogen Interface. mBio 2019, 10, e01031-19. [Google Scholar] [CrossRef] [PubMed]
- Bommarius, B.; Anyanful, A.; Izrayelit, Y.; Bhatt, S.; Cartwright, E.; Wang, W.; Swimm, A.; Benian, G.M.; Schroeder, F.; Kalman, D. A Family of Indoles Regulate Virulence and Shiga Toxin Production in Pathogenic E. coli. PLoS ONE 2013, 8, e54456. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, S.; Kareenhalli, V.V.; Giri, L.; Mitra, K. Experimental investigation into indole production using passaging of E. coli and B. subtilis along with unstructured modeling and parameter estimation using dynamic optimization: An integrated framework. Biochem. Eng. J. 2020, 163, 107743. [Google Scholar] [CrossRef]
- Melander, R.J.; Minvielle, M.J.; Melander, C. Controlling bacterial behavior with indole-containing natural products and derivatives. Tetrahedron 2014, 70, 6363–6372. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Nunn, W.D.; Kelly, D.L.; Stumfall, M.Y. Regulation of fatty acid synthesis during the cessation of phospholipid biosynthesis in Escherichia coli. J. Bacteriol. 1977, 132, 526–531. [Google Scholar] [CrossRef]
- Parsons, J.B.; Rock, C.O. Bacterial lipids: Metabolism and membrane homeostasis. Prog. Lipid Res. 2013, 52, 249–276. [Google Scholar] [CrossRef] [PubMed]
- Meadows, J.A.; Wargo, M.J. Carnitine in bacterial physiology and metabolism. Microbiology 2015, 161, 1161–1174. [Google Scholar] [CrossRef]
- Bjørndal, B.; Alterås, E.K.; Lindquist, C.; Svardal, A.; Skorve, J.; Berge, R.K. Associations between fatty acid oxidation, hepatic mitochondrial function, and plasma acylcarnitine levels in mice. Nutr. Metab. 2018, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, D.; Jiang, Y. Function, Detection and Alteration of Acylcarnitine Metabolism in Hepatocellular Carcinoma. Metabolites 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and Gut Microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef]
- Shah, P.; Swiatlo, E. A multifaceted role for polyamines in bacterial pathogens. Mol. Microbiol. 2008, 68, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Castanie-Cornet, M.-P.; Foster, J.W.; Crawford, J.A.; Brinkley, C.; Kaper, J.B. An activator of glutamate decarboxylase genes regulates the expression of enteropathogenic Escherichia coli virulence genes through control of the plasmid-encoded regulator, Per. Mol. Microbiol. 2008, 41, 1133–1150. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.-S.; Sponder, G.; Aschenbach, J.R.; Kerner, K.; Bauerfeind, R.; Deiner, C. The GadX regulon affects virulence gene expression and adhesion of porcine enteropathogenic Escherichia coli in vitro. Vet. Anim. Sci. 2017, 3, 10–17. [Google Scholar] [CrossRef]
- Lu, P.; Ma, D.; Chen, Y.; Guo, Y.; Chen, G.-Q.; Deng, H.; Shi, Y. L-glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia. Cell Res. 2013, 23, 635–644. [Google Scholar] [CrossRef]
- Leung, H.B.; Doering, A.M.; Cohen, S.S. Effect of 9-β- d -Arabinofuranosyladenine on Polymer Synthesis in a Polyauxotrophic Strain of Escherichia coli. J. Bacteriol. 1966, 92, 558–564. [Google Scholar] [CrossRef]
- Xi, H.; Schneider, B.L.; Reitzer, L. Purine Catabolism in Escherichia coli and Function of Xanthine Dehydrogenase in Purine Salvage. J. Bacteriol. 2000, 182, 5332–5341. [Google Scholar] [CrossRef] [PubMed]
- Röhl, F.; Rabenhorst, J.; Zahner, H. Biological properties and mode of action of clavams. Arch. Microbiol. 1987, 147, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.E.; Fujishima, Y.; Goh, K.-C.; Schofield, C.J. Enzymes of valclavam biosynthesis. Tetrahedron Lett. 1994, 35, 2783–2786. [Google Scholar] [CrossRef]
- McGowan, S.J.; Barrie, W.B.; George, P.C.S. Bacterial production of carbapenems and clavams: Evolution of β-Lactam antibiotic pathways. Trends Microbiol. 1998, 6, 203–208. [Google Scholar] [CrossRef]
- Nigam, P.K.; Sehgal, U. Leukotrienes. Indian J. Dermatol. Venereol. Leprol. 1989, 55, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Garreta, A.; Benincasa, M.; Fusté, M.C.; Busquets, M.; Manresa, A. Bacterial lipoxygenases, a new subfamily of enzymes? A phylogenetic approach. Appl. Microbiol. Biotechnol. 2013, 97, 4737–4747. [Google Scholar] [CrossRef] [PubMed]
- Parlesak, A. Alcohol, Altered Gut Permeability, and Endotoxins. In Comprehensive Handbook of Alcohol Related Pathology; Elsevier Inc.: Amsterdam, The Netherlands, 2005; Volume 2. [Google Scholar] [CrossRef]
- Dean, C.R.; Ward, O.P. Nature of Escherichia coli cell lysis by culture supernatants of Bacillus species. Appl. Environ. Microbiol. 1991, 57, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Perez, F.; Nataro, J.P. Bacterial serine proteases secreted by the autotransporter pathway: Classification, specificity, and role in virulence. Cell. Mol. Life Sci. 2014, 71, 745–770. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]


| VIP Scores | |||||
|---|---|---|---|---|---|
| Group | Metabolite | Comp. 1 | Comp. 2 | p Value | Pathway/Function |
| Co-culture only | 9- Decenoylcarnitine | 1.3611 | 1.3578 | 5.69 × 10−8 | Fatty acid/energy Metabolism |
| Carnosine | 1.412 | 1.3994 | 1.47 × 10−6 | Fatty acid/energy Metabolism | |
| 5-Methoxy-3- indoleaceate | 1.3661 | 1.3571 | 2.08 × 10−6 | Tryptophan metabolism and antimicrobial metabolite | |
| Indole | 1.5536 | 1.5057 | 1.70 × 10−6 | Tryptophan metabolism and antimicrobial metabolite | |
| Valclavam | 1.3586 | 1.3543 | 4.26 × 10−6 | Antimicrobial metabolite | |
| 3-[(3- Hydroxyundecanoyl)oxy]-4- (trimethylammonio) butanoate | 1.3429 | 1.3343 | 0.00092045 | Fatty acid/energy Metabolism | |
| n-phenethyl acetamide | 1.4326 | 1.4083 | 0.0011712 | Antibacterial secondary metabolite | |
| LT9970000/ Furmecyclox | 1.4475 | 1.4108 | 0.0029438 | Drug | |
| Uric Acid | 1.039 | 1.0652 | 0.0034036 | Nitrogen metabolism/Amino acid and protein synthesis | |
| Putrescine | 1.0175 | 1.0174 | 0.0071156 | Polyamine/Cell growth and metabolism/ Virulence | |
| MFCD00059633/ 3- Hydroxymyristic Acid | 1.0336 | 1.0203 | 0.023742 | Bacterial metabolite/fatty acid metabolism | |
| CP9 and overexpressed in Co-culture | C8-Carnitine | 1.4194 | 1.4042 | 1.22 × 10−9 | Fatty acid/energy Metabolism |
| L-Cysteinylglycine disulfide | 1.5642 | 1.5136 | 2.15 × 10−9 | Di-peptides/ Glutathione metabolism | |
| N(1)- acetylspermidine | 1.4443 | 1.4248 | 1.82 × 10−8 | Polyamine metabolite/Cell- Cell signalling/ Virulence | |
| 5,6- Dihydrothymidine | 1.4407 | 1.3837 | 4.66 × 10−8 | Nucleoside analogues | |
| Leukotriene C4 | 1.4209 | 1.3659 | 7.24 × 10−8 | Arachidonic Acid metabolite/ antimicrobial | |
| Naloxegol | 1.4683 | 1.4096 | 3.51 × 10−7 | Drug | |
| gamma-Glu-gln | 1.4715 | 1.4125 | 3.67 × 10−7 | Glutathione metabolism | |
| Aderbasib | 1.5448 | 1.4852 | 1.11 × 10−6 | Drug | |
| Spironolactone | 1.5097 | 1.4494 | 2.27 × 10−6 | Drug | |
| 3-[(2,6- Dimethylheptanoyl)oxy]-4- (trimethylammonio) butanoate | 1.4724 | 1.4402 | 5.38 × 10−6 | Fatty acid/energy Metabolism | |
| Leukotriene E3 | 1.262 | 1.247 | 6.88 × 10−6 | Arachidonic Acid metabolite/ antimicrobial | |
| Carnosine.1 | 1.4944 | 1.4362 | 0.00012389 | Fatty acid/energy Metabolism | |
| Melagatran | 1.5172 | 1.4729 | 0.00015017 | Serine protease inhibitor | |
| (3beta,5beta)-24- Hydroxy-24- oxocholan-3-yl beta-D-glucopyranosiduronic acid | 1.4674 | 1.4101 | 0.0010426 | Secondary bacterial bile acid metabolite/ antibacterial metabolite | |
| ETEC and over/ under expressed in Co-culture | Kynurenic acid (↓) | 1.2825 | 1.2397 | 7.61 × 10−8 | Tryptophan metabolism |
| gamma- Aminobutyric acid (↓) | 1.2656 | 1.2242 | 9.81 × 10−8 | Spore germination/bile and low pH resistance | |
| 8- Methoxykynurenic acid (↑) | 1.3368 | 1.3216 | 1.05 × 10−5 | Tryptophan metabolism | |
| Gln-Gln (↑) | 1.2812 | 1.2803 | 0.00010342 | L-Glutamine Di- peptide/acid resistance | |
| (1Z,3R,5E,8S,9S, 10R)-N-[(Z)-2-(3- Chloro-4- hydroxyphenyl) vinyl]-3,9- dihydroxy-2,4- dimethoxy-6,8,10- trimethyl-7-oxo-5- tetradecenimidic acid (↑) | 1.3569 | 1.3068 | 4.29 × 10−7 | Unknown | |
| Arabinosylhypoxanthine (↑) | 1.1994 | 1.1915 | 1.07 × 10−7 | Purine metabolism/E. coli cellular growth and virulence in mixed culture | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudan, S.; Flick, R.; Nong, L.; Li, J. Potential Probiotic Bacillus subtilis Isolated from a Novel Niche Exhibits Broad Range Antibacterial Activity and Causes Virulence and Metabolic Dysregulation in Enterotoxic E. coli. Microorganisms 2021, 9, 1483. https://doi.org/10.3390/microorganisms9071483
Sudan S, Flick R, Nong L, Li J. Potential Probiotic Bacillus subtilis Isolated from a Novel Niche Exhibits Broad Range Antibacterial Activity and Causes Virulence and Metabolic Dysregulation in Enterotoxic E. coli. Microorganisms. 2021; 9(7):1483. https://doi.org/10.3390/microorganisms9071483
Chicago/Turabian StyleSudan, Sudhanshu, Robert Flick, Linda Nong, and Julang Li. 2021. "Potential Probiotic Bacillus subtilis Isolated from a Novel Niche Exhibits Broad Range Antibacterial Activity and Causes Virulence and Metabolic Dysregulation in Enterotoxic E. coli" Microorganisms 9, no. 7: 1483. https://doi.org/10.3390/microorganisms9071483
APA StyleSudan, S., Flick, R., Nong, L., & Li, J. (2021). Potential Probiotic Bacillus subtilis Isolated from a Novel Niche Exhibits Broad Range Antibacterial Activity and Causes Virulence and Metabolic Dysregulation in Enterotoxic E. coli. Microorganisms, 9(7), 1483. https://doi.org/10.3390/microorganisms9071483






