Diversity and Biocontrol Potential of Cultivable Endophytic Bacteria Associated with Halophytes from the West Aral Sea Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Site Description
2.2. Sterilization of Plant Materials
2.3. Isolation of Endophytic Bacteria
2.4. DNA Extraction, PCR Amplification and 16S rRNA Gene Sequencing
2.5. Accession Numbers
2.6. Screening of Bacterial Isolates Producing Protease, Lipase, and Cellulose
2.7. Screening of Endophytic Bacterial Isolates for Their Antifungal Activity
2.8. Statistical Analysis
3. Results
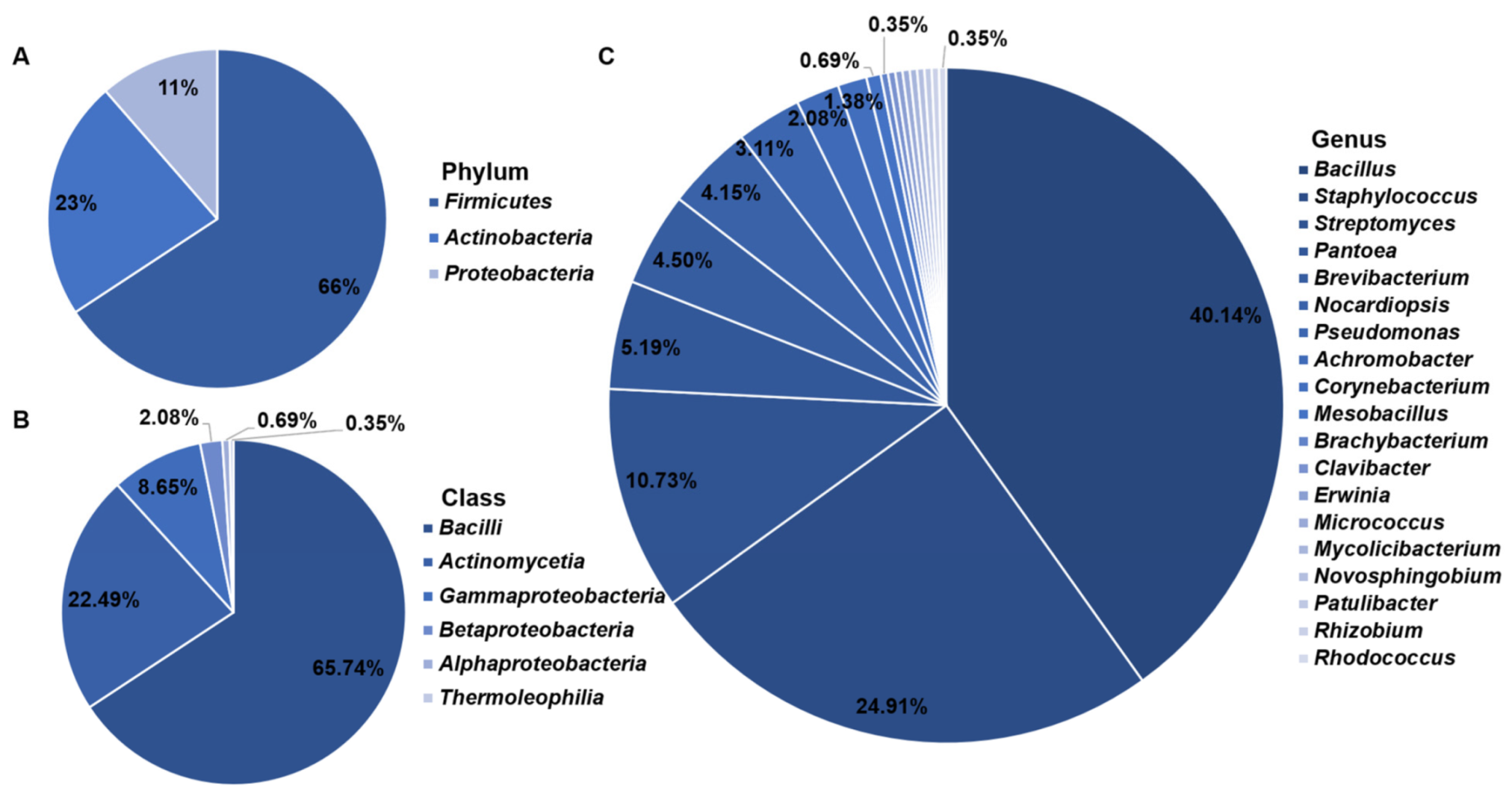
3.1. Isolation and Identification of Cultivable Endophytic Bacteria
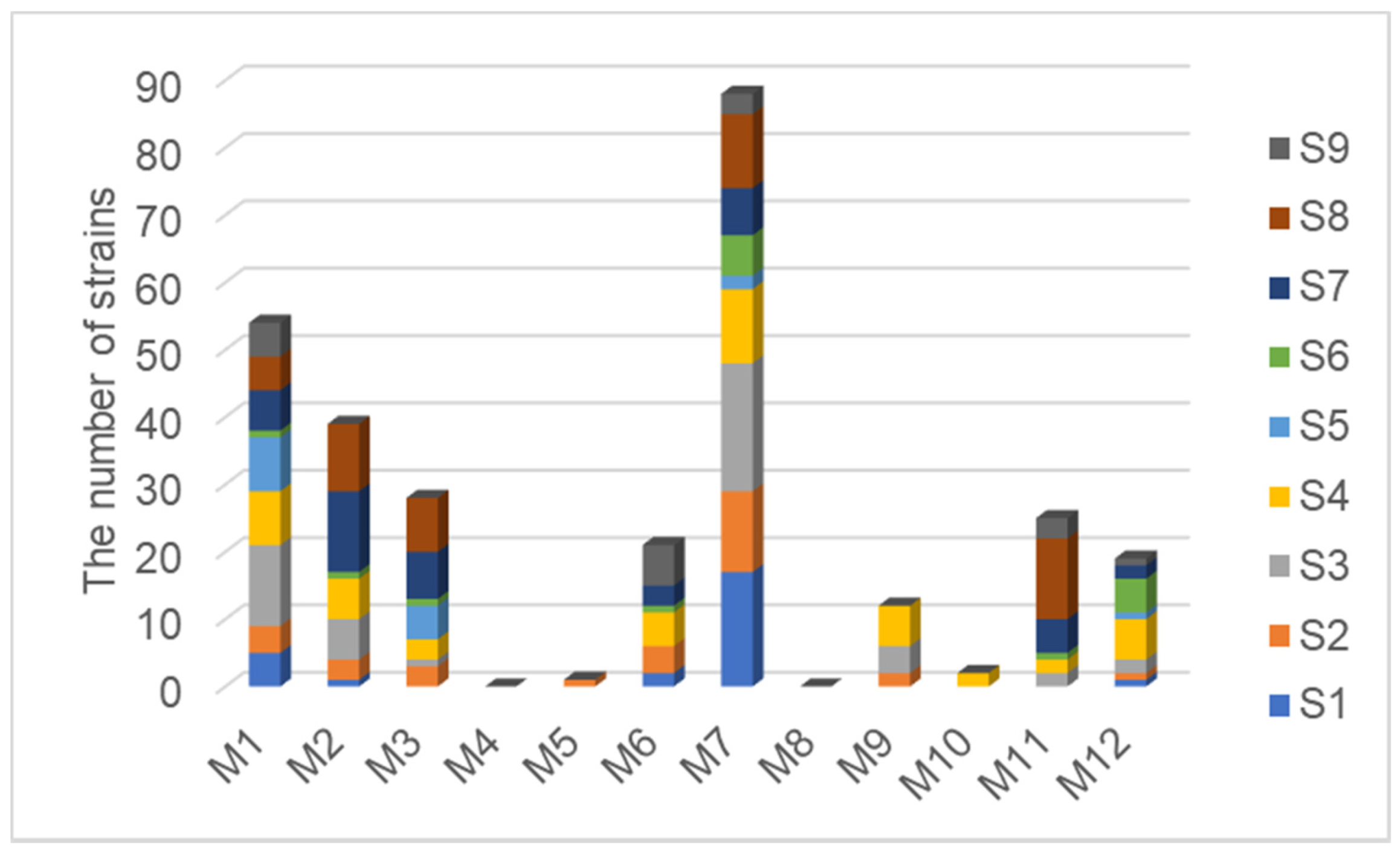
3.2. Comparison of Isolation Effects between Different Media
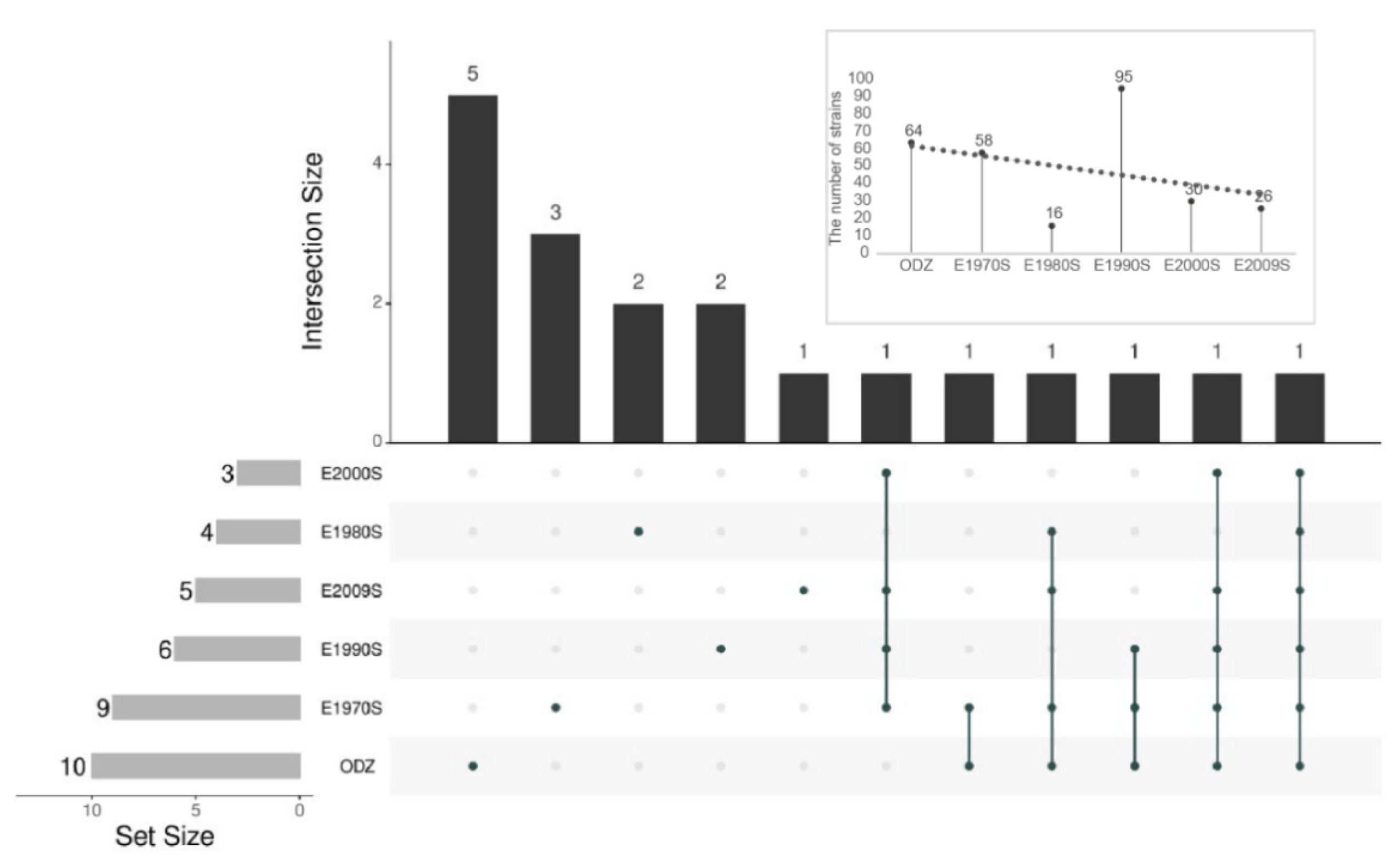
3.3. Comparison of the Isolated Effect of Endophytic Bacteria Isolated from Halophytes between Different Exposed Zones
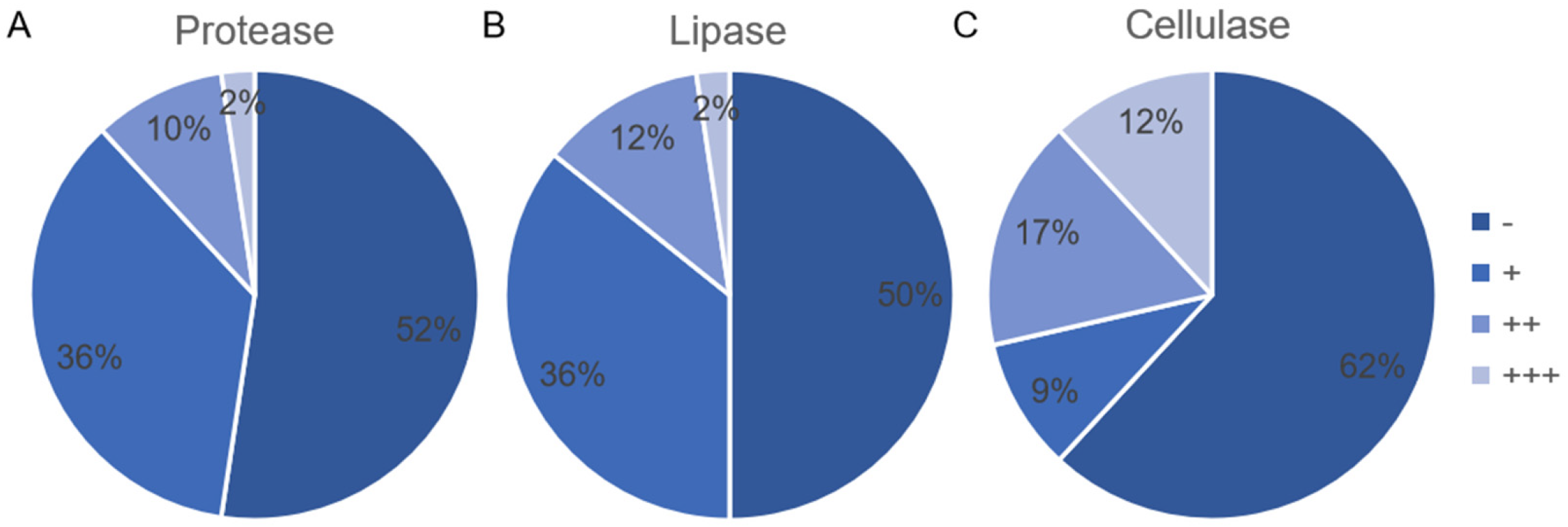
3.4. Production of Protease, Lipase, and Cellulose by Endophytic Bacterial Isolates
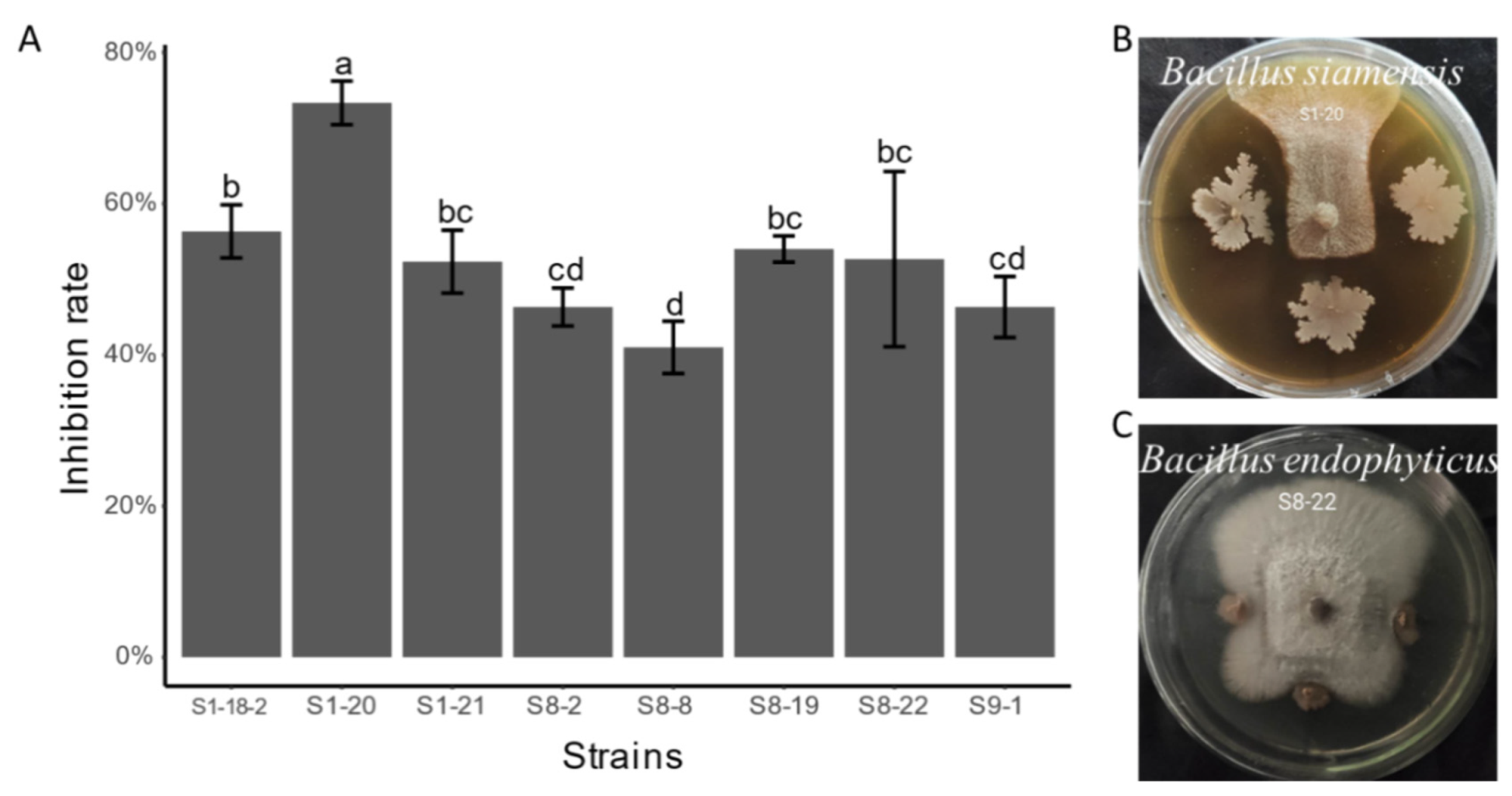
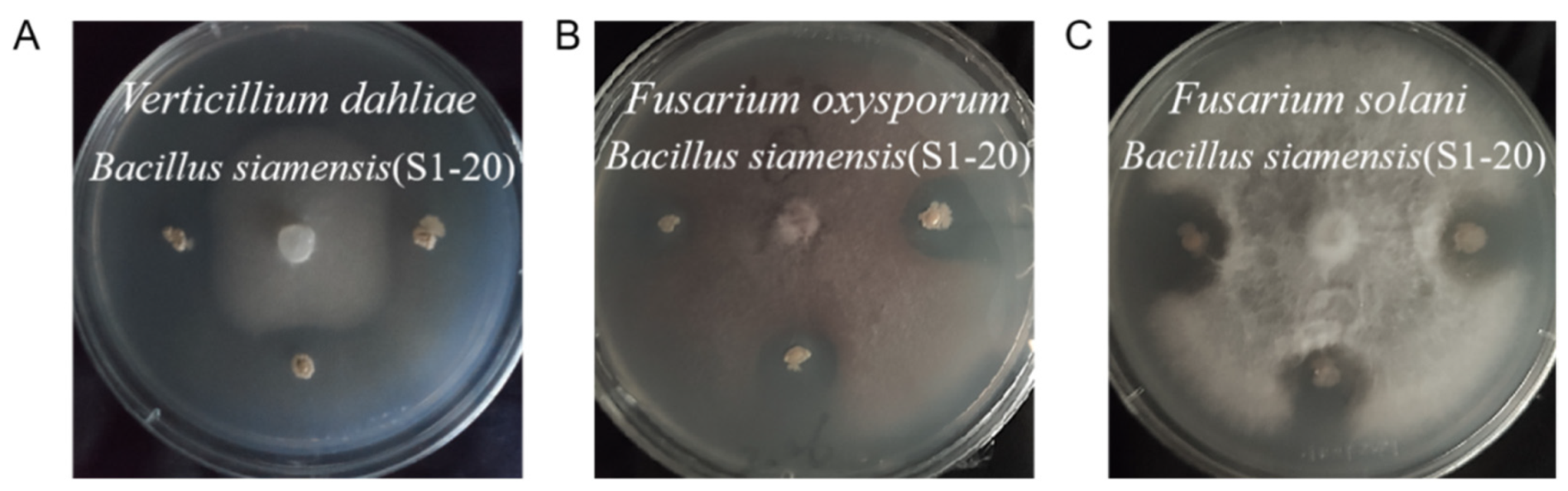
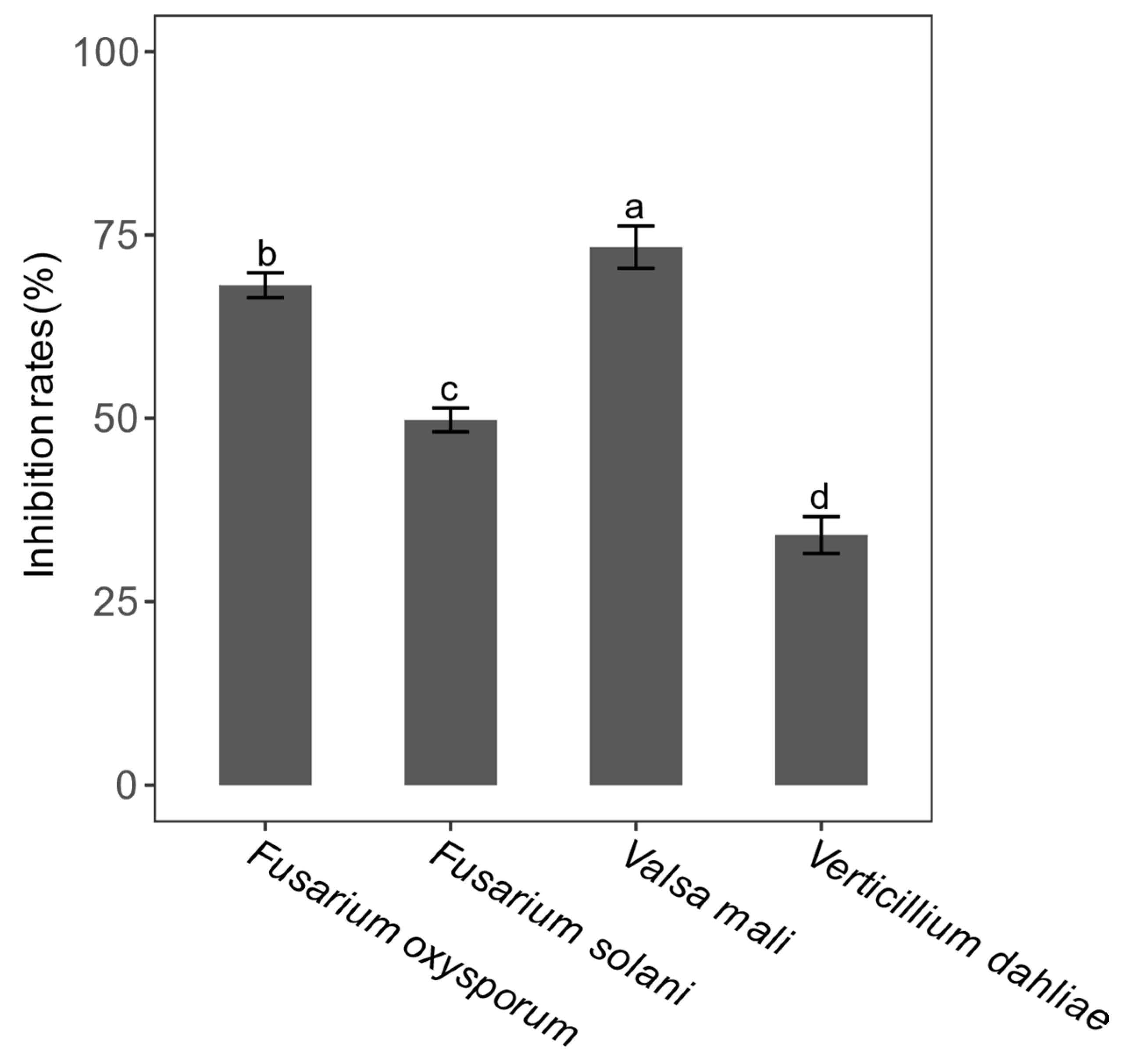
3.5. Antifungal Activity of Endophytic Bacterial Isolates
4. Discussion
4.1. Cultivable Endophytic Bacterial Community Composition Associated with the Halophytes Growing on the Shore in the Western Aral Sea Basin
4.2. The Exploration on the Best Isolation Protocol of Halophyte Endophytic Bacteria
4.3. The Screening of the Endophytic Bacterial Isolates Producing Enzyme and Inhibiting Pathogenic Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Austin, P.; Mackay, A.; Palagushkina, O.; Leng, M. A High-Resolution Diatom-Inferred Palaeoconductivity and Lake Level Record of the Aral Sea for the Last 1600 Yr. Quat. Res. 2007, 3, 383–393. [Google Scholar] [CrossRef]
- Herschy, R.W. Aral Seaaral Sea. In Encyclopedia of Hydrology and Lakes; Springer: Dordrecht, The Netherlands, 1998; p. 70. [Google Scholar]
- Varotsos, C.A.; Krapivin, V.F.; Mkrtchyan, F.A. On the Recovery of the Water Balance. Water Air Soil Pollut. 2020, 4, 170. [Google Scholar] [CrossRef]
- Shurigin, V.; Egamberdieva, D.; Li, L.; Davranov, K.; Panosyan, H.; Birkeland, N.; Wirth, S.; Bellingrath-Kimura, S.D. Endophytic Bacteria Associated with Halophyte Seidlitzia Rosmarinus Ehrenb. Ex Boiss. From Saline Soil of Uzbekistan and Their Plant Beneficial Traits. J. Arid Land 2020, 5, 730–740. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Diseases Caused by Fungi. In Plant Pathology, 2nd ed.; Agrios, G.N., Ed.; Academic Press: Cambridge, MA, USA, 1978; pp. 172–434. [Google Scholar]
- Widiantini, F.; Qadryani, M.R.; Hartati, F.; Yulia, E. Antifungal Potency of Secondary Metabolites Produced by Endophytic Bacteria against Pathogenic Fungi Pyricularia Oryzae Cav. J. Perlindungan Tanam. Indones. 2019, 2, 185. [Google Scholar] [CrossRef]
- Triky-Dotan, S.; Yermiyahu, U.; Katan, J.; Gamliel, A. Development of Crown and Root Rot Disease of Tomato under Irrigation with Saline Water. Phytopathology 2005, 12, 1438–1444. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Kucharova, Z.; Davranov, K.; Berg, G.; Makarova, N.; Azarova, T.; Chebotar, V.; Tikhonovich, I.; Kamilova, F.; Validov, S.Z.; et al. Bacteria Able to Control Foot and Root Rot and to Promote Growth of Cucumber in Salinated Soils. Biol. Fertil. Soils 2011, 2, 197–205. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Davranov, K.; Wirth, S.; Hashem, A.; Abd_Allah, E.F. Impact of Soil Salinity on the Plant-Growth—Promoting and Biological Control Abilities of Root Associated Bacteria. Saudi J. Biol. Sci. 2017, 7, 1601–1608. [Google Scholar] [CrossRef]
- Ludueña, L.M.; Taurian, T.; Tonelli, M.L.; Angelini, J.G.; Anzuay, M.S.; Valetti, L.; Muñoz, V.; Fabra, A.I. Biocontrol Bacterial Communities Associated with Diseased Peanut (Arachis Hypogaea L.) Plants. Eur. J. Soil Biol. 2012, 48–55. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.T.; Chang, H.H.; Egamberdieva, D.; Kamilova, F.; Lugtenberg, B.; Kuo, C.H. Genome Analysis of Pseudomonas Fluorescens Pcl1751: A Rhizobacterium That Controls Root Diseases and Alleviates Salt Stress for Its Plant Host. PLoS ONE 2015, 10, e0140231. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Beattie, G.A. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-Halophytic Crops. Front. Microbiol. 2018, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic Bacteria: Prospects and Applications for the Phytoremediation of Organic Pollutants. Chemosphere 2014, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Subramanian, S.; Smith, D.L. Plant Endophytes Promote Growth and Alleviate Salt Stress in Arabidopsis Thaliana. Sci. Rep. 2020, 1, 12740. [Google Scholar] [CrossRef]
- Martinez-Klimova, E.; Rodríguez-Peña, K.; Sánchez, S. Endophytes as Sources of Antibiotics. Biochem. Pharmacol. 2017, 1–17. [Google Scholar] [CrossRef]
- Zicca, S.; De-Bellis, P.; Masiello, M.; Saponari, M.; Saldarelli, P.; Boscia, D.; Sisto, A. Antagonistic Activity of Olive Endophytic Bacteria and of Bacillus Spp. Strains against Xylella Fastidiosa. Microbiol. Res. 2020, 126467. [Google Scholar] [CrossRef]
- Vurukonda, S.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. As Endophytes. Int. J. Mol. Sci. 2018, 4, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.M.; Xiong, K.; Mu, S.; Guo, J.; Guo, X.L.; Duan, Y.Y.; Li, J.; Cao, F.; Zou, Z.C.; Tan, H. Identification of Endophytic Bacteria Bzjn1 and Research on Biological Control of Root Rot of Atractylodes Macrocephala. Zhongguo Zhong Yao Za Zhi 2018, 3, 478–483. [Google Scholar] [CrossRef]
- Wang, S.S.; Liu, J.M.; Sun, J.; Sun, Y.F.; Liu, J.N.; Jia, N.; Fan, B.; Dai, X.F. Diversity of Culture-Independent Bacteria and Antimicrobial Activity of Culturable Endophytic Bacteria Isolated from Different Dendrobium Stems. Sci. Rep. 2019, 1, 10389. [Google Scholar] [CrossRef]
- Bibi, F.; Naseer, M.I.; Hassan, A.M.; Yasir, M.; Al-Ghamdi, A.A.K.; Azhar, E.I. Diversity and Antagonistic Potential of Bacteria Isolated from Marine Grass Halodule Uninervis. 3 Biotech 2018, 1, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.C.; Huang, J.R.; Li, L.; Huang, L.Q.; Manzoor, M.; Yang, J.; Wu, G.; Sun, X.X.; Wang, B.C.; Egamberdieva, D.; et al. Onshore Soil Microbes and Endophytes Respond Differently to Geochemical and Mineralogical Changes in the Aral Sea. Sci. Total Environ. 2021, 142675. [Google Scholar] [CrossRef]
- Liu, Y.H.; Guo, J.W.; Li, L.; Asem, M.; Zhang, Y.G.; OAMohamad Salam, N.; Li, W.J. Erratum To: Endophytic Bacteria Associated with Endangered Plant Ferula Sinkiangensis K. M. Shen in an Arid Land: Diversity and Plant Growth-Promoting Traits. J. Arid Land 2017, 4, 635. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wang, F.; Zhang, M.; Ou, T.; Wang, R.; Strobel, G.; Xiang, Z.; Zhou, Z.; Xie, J. Diversity of Cultivable Endophytic Bacteria in Mulberry and Their Potential for Antimicrobial and Plant Growth-Promoting Activities. Microbiol. Res. 2019, 126328. [Google Scholar] [CrossRef] [PubMed]
- Rohban RAMohammad, A.; Ventosa, A. Screening and Isolation of Halophilic Bacteria Producing Extracellular Hydrolyses from Howz Soltan Lake, Iran. J. Ind. Microbiol. Biotechnol. 2009, 3, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Jiang, J.; Zhou, Y.; Wu, Y.; Leung, F.C. Screening and Genomic Analysis of a Lignocellulose Degrading Bacterium. Acta Microbiol. Sin. 2016, 5, 765–777. [Google Scholar]
- Mesa, J.; Mateos-Naranjo, E.; Caviedes, M.A.; Redondo-Gómez, S.; Pajuelo, E.; Rodríguez-LlorenteIgnacio, D. Endophytic Cultivable Bacteria of the Metal Bioaccumulator Spartina Maritima Improve Plant Growth but Not Metal Uptake in Polluted Marshes Soils. Front. Microbiol. 2015, 1450. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Guo, J.W.; Salam, N.; Li, L.; Zhang, Y.G.; Han, J.; Mohamad, O.A.; Li, W.J. Culturable Endophytic Bacteria Associated with Medicinal Plant Ferula Songorica: Molecular Phylogeny, Distribution and Screening for Industrially Important Traits. 3 Biotech 2016, 2, 209. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, O.A.A.; Li, L.; Ma, J.B.; Hatab, S.; Xu, L.; Guo, J.W.; Rasulov, B.A.; Liu, Y.H.; Hedlund, B.P.; Li, W.J. Evaluation of the Antimicrobial Activity of Endophytic Bacterial Populations from Chinese Traditional Medicinal Plant Licorice and Characterization of the Bioactive Secondary Metabolites Produced by Bacillus Atrophaeus against Verticillium Dahliae. Front. Microbiol. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F. Study on Biodiversity of Endophytic Bacteria Isolated from Four Chenopodiaceae Halophytes in Xinjiang and Evaluation of Their Growth Promoting Function and Salt-Tolerance Ability. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2015. [Google Scholar]
- Pitzschke, A. Developmental Peculiarities and Seed-Borne Endophytes in Quinoa: Omnipresent, Robust Bacilli Contribute to Plant Fitness. Front. Microbiol. 2016, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Hui, N.; Liang, L.X.; Zhang, X.X.; Li, L.B.; Sun, Q.W. Sphingobacterium Haloxyli Sp. Nov., an Endophytic Bacterium Isolated from Haloxylon Ammodendron Stems in Kumtag Desert. Int. J. Syst. Evol. Microbiol. 2018, 10, 3279–3284. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, L.; Song, Z.; Wang, S.; Zhang, J.; Zhang, X.; Sun, Q. Parapedobacter Deserti Sp. Nov., an Endophytic Bacterium Isolated from Haloxylon Ammodendron Stems. Int. J. Syst. Evol. Microbiol. 2017, 7, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Li, Q.; Cui, R.; Wang, Z.; Wang, Y.; Zhang, Y.Z.; Ding, W.; Shen, X. Deciphering the Root Endosphere Microbiome of the Desert Plant Alhagi Sparsifolia for Drought Resistance-Promoting Bacteria. Appl. Environ. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhou, N.; Zhao, Z.; Zhang, K.; Wu, G.; Tian, C.Y. Estimation of Endophytic Bacterial Diversity in Root of Halophytes in Northern Xinjiang by High Throughput Sequencing. Acta Microbiol. Sin. 2016, 10, 1583–1594. [Google Scholar]
- Huang, C.M.; Chen, W.C.; Lin, S.H.; Wang, Y.N.; Shen, F.T. Exploration of Root-Associated Bacteria from the Medicinal Plant Platycodon Grandiflorum. Microbes Environ. 2019, 4, 413–420. [Google Scholar] [CrossRef]
- Cumagun, C.; Joseph, R. Chapter 39—Advances in Formulation of Trichoderma for Biocontrol. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 527–531. [Google Scholar]
- Saikkonen, K.; Wäli, P.; Helander, M.; Faeth, S.H. Evolution of Endophyte-Plant Symbioses. Trends Plant Sci. 2004, 6, 275–280. [Google Scholar] [CrossRef]
- Chen, H.; Xue, Y.F.; Meng, M.L.; Ma, Z.W.; Liu, Z.H.; Hu, J. Pathogen Identification and Biological Characteristics of Potato Wilt in Inner Mongolia. Chin. Potato J. 2016, 4, 226–234. [Google Scholar] [CrossRef]
- Wang, J.Y.; Cai, Y.; Gou, J.Y.; Mao, Y.B.; Xu, Y.H.; Jiang, W.H.; Chen, X.Y. Vdnep, an Elicitor from Verticillium Dahliae, Induces Cotton Plant Wilting. Appl. Environ. Microbiol. 2004, 8, 4989–4995. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Gao, X.; Chen, J.; Yin, Z.; Feng, H.; Huang, L. The Feruloyl Esterase Genes Are Required for Full Pathogenicity of the Apple Tree Canker Pathogen Valsa Mali. Mol. Plant Pathol. 2018, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafi, J.; Tian, H.; Ji, M.S. Bacillus Species as Versatile Weapons for Plant Pathogens: A Review. Biotechnol. Biotechnol. Equip. 2017, 3, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Sumpavapol, P.; Tongyonk, L.; Tanasupawat, S.; Chokesajjawatee, N.; Luxananil, P.; Visessanguan, W. Bacillus Siamensis Sp. Nov., Isolated from Salted Crab (Poo-Khem) in Thailand. Int. J. Syst. Evol. Microbiol. 2010, 10, 2364–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.H.; Ye, Z.W.; Zheng, Q.W.; Wei, T.; Lin, J.F.; Guo, L.Q. Isolation and Characterization of Cyclic Lipopeptides with Broad-Spectrum Antimicrobial Activity from Bacillus Siamensis Jfl15. 3 Biotech 2018, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Shurigin VAlaylar, B.; Wirth, S.; Kimura, S.K.B. Bacterial endophytes from horseradish (Armoracia rusticana) with antimicrobial efficacy against pathogens. Plant Soil Environ. 2020, 66, 309–316. [Google Scholar] [CrossRef]

| Sample Num. | Sample Name | Exposed Zone | The Distance to the Shoreline (m) | Location |
|---|---|---|---|---|
| S1 | Chenopodium album L. | E2009S | ~200 | 44°29′50.42″ N~44°29′50.4233″ N, 58°12′43.80″ E~58°13′42.31″ E |
| S2 | Chenopodium album L. | E2000S | ~300 | |
| S3 | Chenopodium album L. | E1990S | ~700 | |
| S4 | Salsola collina Pall. | E1990S | ~700 | |
| S5 | Haloxylon ammodendron (C. A. Mey.) Bunge | E1980S | ~1000 | |
| S6 | Haloxylon ammodendron (C. A. Mey.) Bunge | E1970S | ~1200 | |
| S7 | Alhagi sparsifolia Shap. | E1970S | ~1200 | |
| S8 | Anabasis eriopoda (Schrenk) Benth. ex Volkens | ODZ | >1500 | |
| S9 | Anabasis truncata (Schrenk) Bunge | ODZ | >1500 |
| Medium Num. | Medium Component (1 L), Agar 15 g, pH 7.2 |
|---|---|
| M1 | Yeast extract 0.25 g, K2HPO4 0.5 g, NaCl 30 g |
| M2 | Na2C2O4 2 g, Casamino acids 0.5 g, KH2PO4 0.3 g, Na2HPO4·12H2O 0.5 g, NaCl 30 g, ZnSO4·7H2O 0.02 g, CaCl2 0.5 g |
| M3 | Glycerol 10 g, L-Asparagine 1 g, NaCl 30 g, K2HPO4 1 g, FeSO4·7H2O 0.001 g, MnSO4·H2O 0.001 g, ZnSO4·7H2O 0.001 g, CuSO4·5H2O 0.001 g |
| M4 | Na2SeO3 1 g, L-Asparagine 1 g, MgSO4·7H2O 0.5 g, FeSO4·7H2O 0.05 g, NaCl 30 g, CaCl2 0.5 g, KH2PO4 0.2 g, |
| M5 | Sodium propionate 2 g, L-Asparagine 1 g, (NH4)2SO4 0.1 g, KCl 0.1 g, MgSO4·7H2O 30 g, FeSO4·7H2O 0.05 g |
| M6 | Chitin 2 g, NaCl 30 g, K2HPO4 0.7 g, KH2PO4 0.3 g, MgSO4·7H2O 0.5 g, MnCl2 0.001 g |
| M7 | Tryptone 15 g, Soybean peptone 5 g, NaCl 30 g |
| M8 | Citric Acid 0.12 g, Ferric citrate 0.12 g, NaCl 30 g, NaNO3 0.5 g, K2HPO4 0.4 g, MgSO4·7H2O 0.2 g, CaCl2 0.5 g |
| M9 | Cellulose microcrystalline 2.5 g, Proline 1 g, NaCl 30 g, KNO3 0.25 g, MgSO4·7H2O 0.2 g, K2HPO4 0.2 g, CaCl2 0.5 g, FeSO4·7H2O 0.01 g |
| M10 | Sodium propionate 2 g, Arginine 1 g, NaCl 30 g, MgSO4·7H2O 1 g, KH2PO4 0.1 g, FeSO4·7H2O 0.05 g |
| M11 | Starch 20 g, KNO3 1 g, NaCl 0.5 g, MgSO4·7H2O 0.5 g, K2HPO4 0.5 g, FeSO4·7H2O 0.01 g |
| M12 | Sodium succinic hexahydrate 1 g, NaCl 30 g, L-Asparagine 1 g, KH2PO4 0.9 g, K2HPO4 0.5 g, KCl 0.3 g, CaCl2 0.2 g, MgSO4·7H2O 0.1 g, FeSO4·7H2O 0.001 g |
| Plant | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
|---|---|---|---|---|---|---|---|---|---|
| Phylum | 2 | 2 | 3 | 2 | 3 | 2 | 3 | 3 | 3 |
| Class | 2 | 2 | 3 | 2 | 3 | 2 | 4 | 5 | 3 |
| Order | 4 | 2 | 3 | 4 | 3 | 3 | 7 | 9 | 5 |
| Family | 5 | 3 | 4 | 5 | 3 | 4 | 7 | 9 | 5 |
| Genus | 5 | 3 | 4 | 5 | 4 | 4 | 7 | 10 | 5 |
| Isolates | 26 | 30 | 46 | 49 | 16 | 16 | 42 | 46 | 18 |
| Richness | 13.00 | 7.00 | 7.00 | 5.00 | 5.00 | 5.00 | 8.00 | 16.00 | 7.00 |
| Shannon | 2.08 | 1.44 | 1.13 | 1.40 | 1.24 | 1.19 | 0.85 | 2.52 | 1.61 |
| Simpson | 0.79 | 0.68 | 0.59 | 0.73 | 0.63 | 0.61 | 0.34 | 0.90 | 0.75 |
| Pielou | 0.81 | 0.74 | 0.58 | 0.87 | 0.77 | 0.74 | 0.41 | 0.91 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Ma, J.; Liu, Y.; Huang, Y.; Mohamad, O.A.A.; Jiang, H.; Egamberdieva, D.; Li, W.; Li, L. Diversity and Biocontrol Potential of Cultivable Endophytic Bacteria Associated with Halophytes from the West Aral Sea Basin. Microorganisms 2021, 9, 1448. https://doi.org/10.3390/microorganisms9071448
Gao L, Ma J, Liu Y, Huang Y, Mohamad OAA, Jiang H, Egamberdieva D, Li W, Li L. Diversity and Biocontrol Potential of Cultivable Endophytic Bacteria Associated with Halophytes from the West Aral Sea Basin. Microorganisms. 2021; 9(7):1448. https://doi.org/10.3390/microorganisms9071448
Chicago/Turabian StyleGao, Lei, Jinbiao Ma, Yonghong Liu, Yin Huang, Osama Abdalla Abdelshafy Mohamad, Hongchen Jiang, Dilfuza Egamberdieva, Wenjun Li, and Li Li. 2021. "Diversity and Biocontrol Potential of Cultivable Endophytic Bacteria Associated with Halophytes from the West Aral Sea Basin" Microorganisms 9, no. 7: 1448. https://doi.org/10.3390/microorganisms9071448
APA StyleGao, L., Ma, J., Liu, Y., Huang, Y., Mohamad, O. A. A., Jiang, H., Egamberdieva, D., Li, W., & Li, L. (2021). Diversity and Biocontrol Potential of Cultivable Endophytic Bacteria Associated with Halophytes from the West Aral Sea Basin. Microorganisms, 9(7), 1448. https://doi.org/10.3390/microorganisms9071448









