Biocontrol Ability and Production of Volatile Organic Compounds as a Potential Mechanism of Action of Olive Endophytes against Colletotrichum acutatum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Isolates and Inocula Production
2.2. Antagonist Effect of Endophytes against C. acutatum
2.2.1. In Vitro Assays
2.2.2. In Vivo Assays
2.3. Characterization of Volatile Compounds Emitted during Fungal Interaction
2.4. Data analysis
3. Results
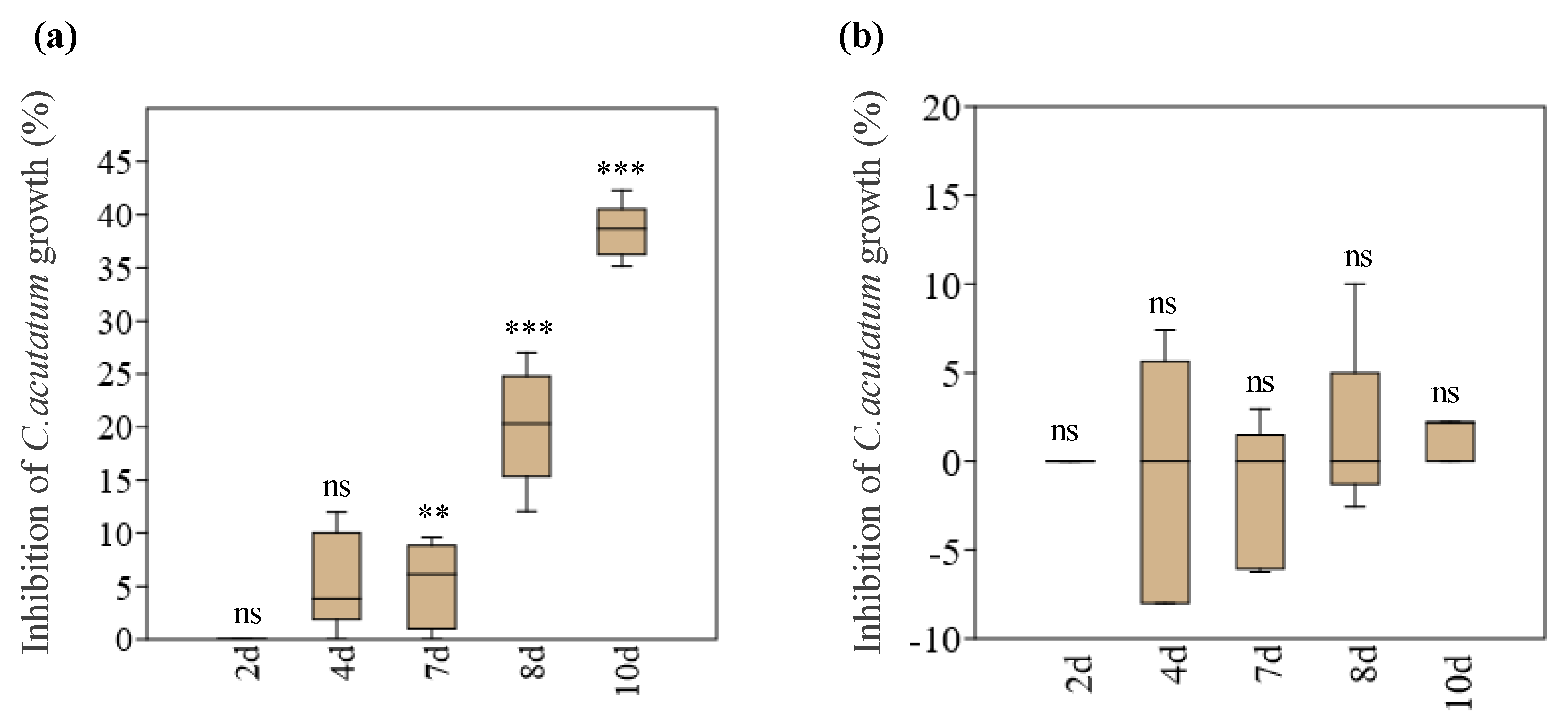
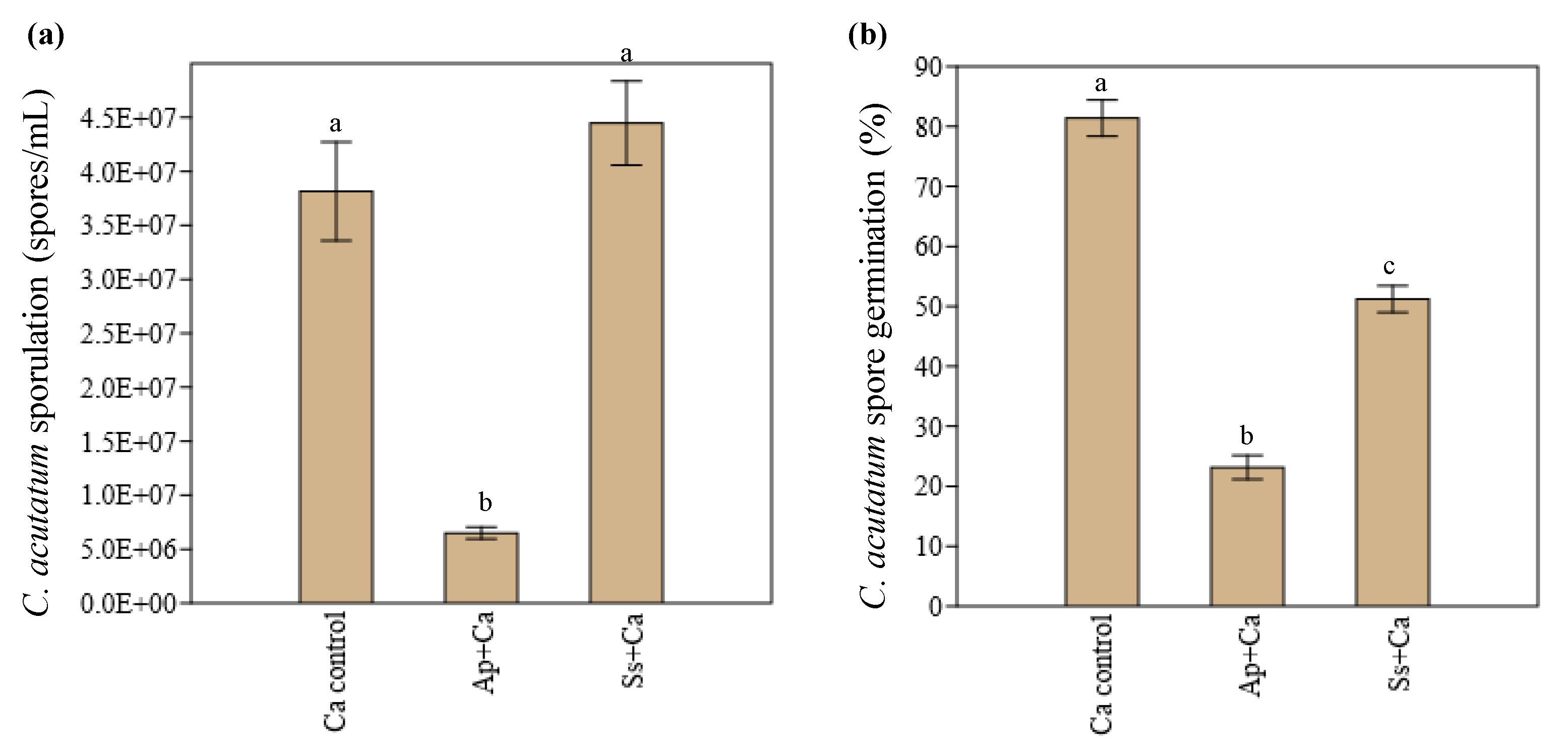
3.1. In Vitro Interaction between Endophytes and C. acutatum
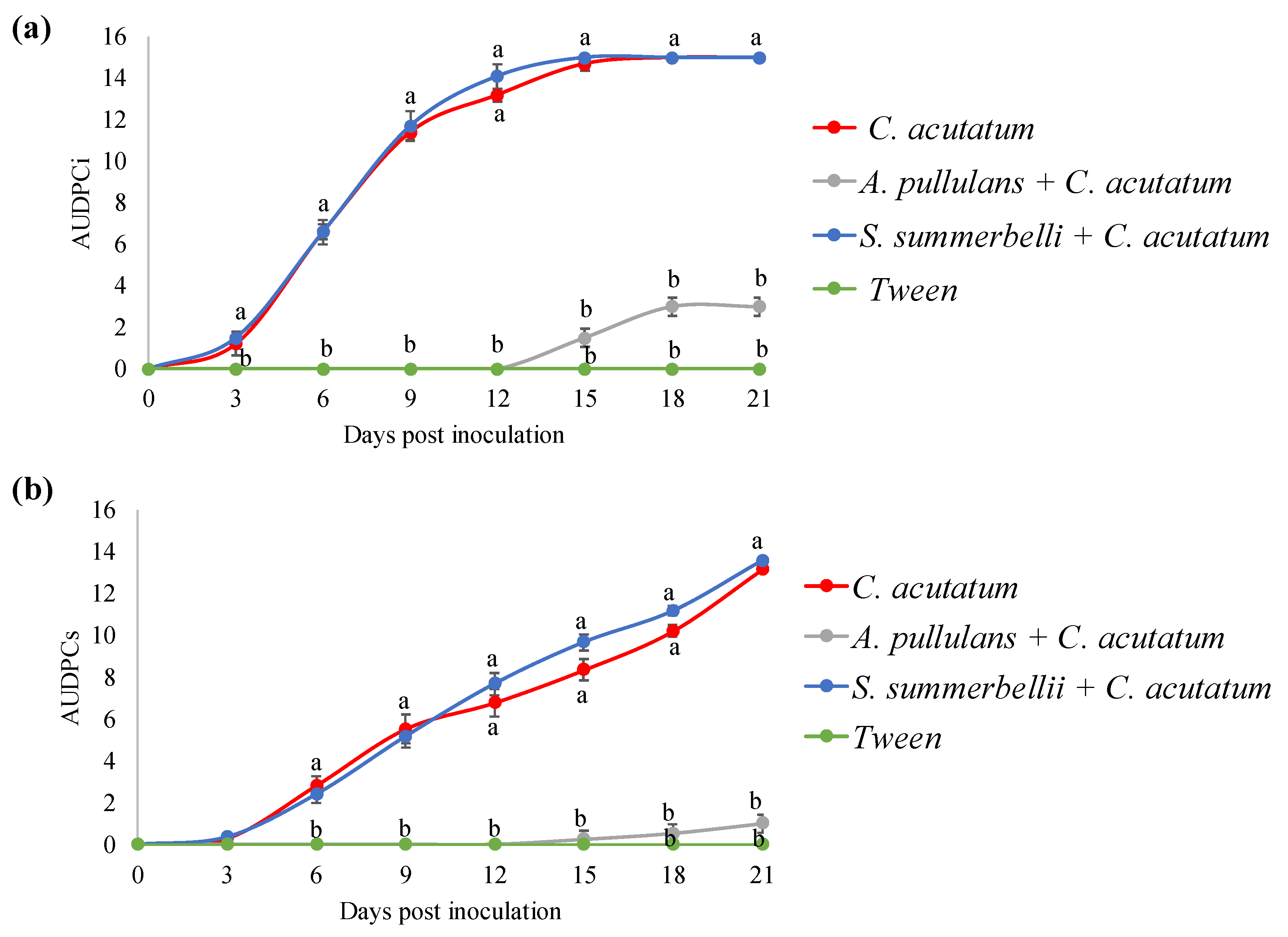
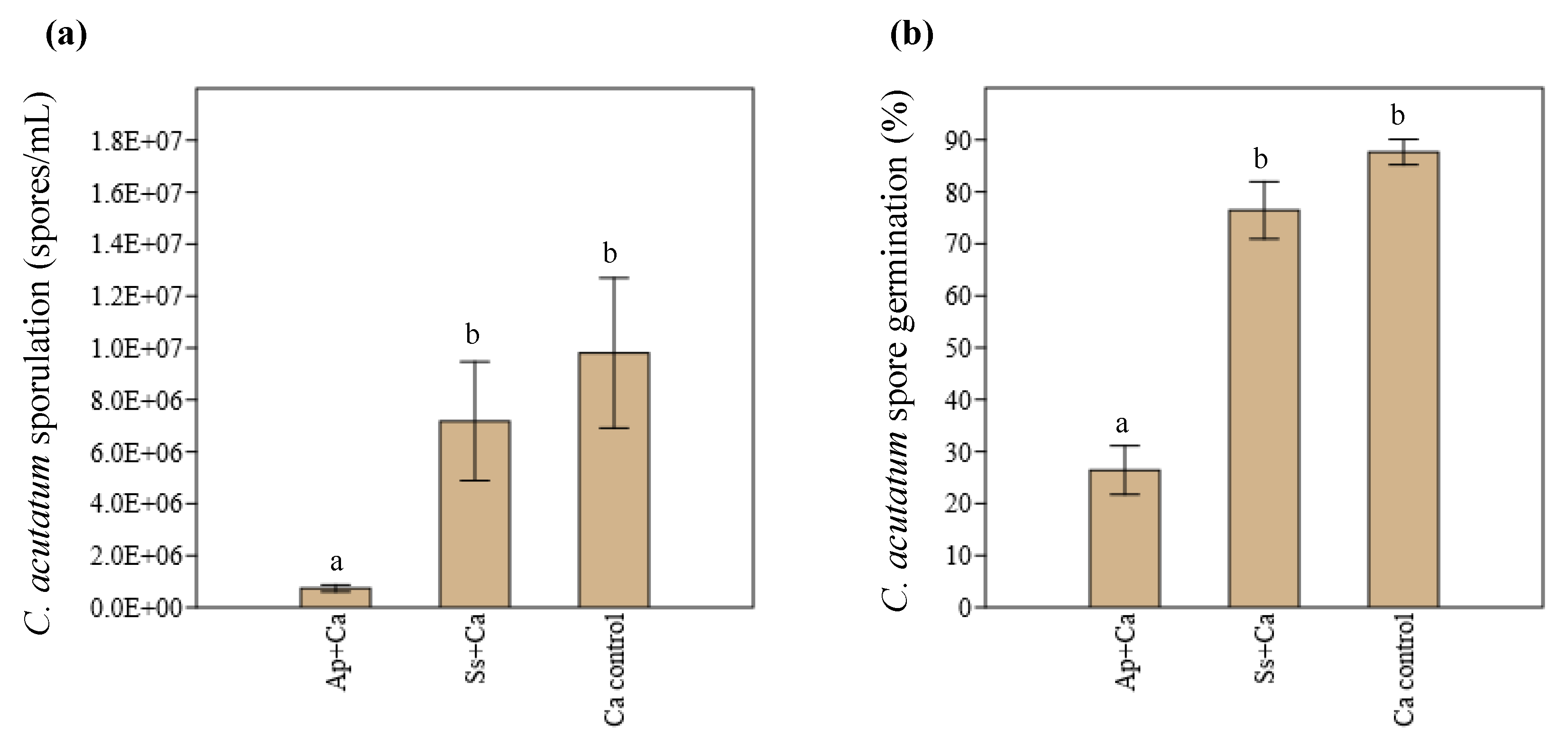
3.2. In Vivo Interaction between Endophytes and C. acutatum
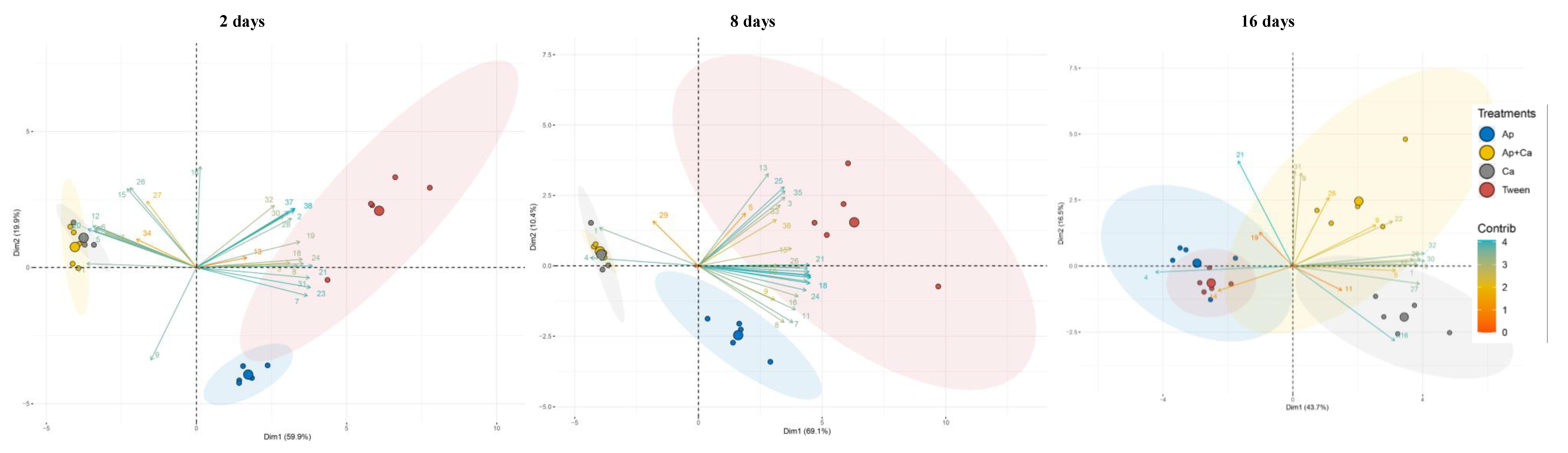
3.3. Volatile Compounds Produced during the Interaction of A. pullulans–C. acutatum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IOC. International Olive Council—Statistical Series. 2021. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 10 January 2022).
- Cacciola, S.A.; Faedda, R.; Sinatra, F.; Agosteo, G.E.; Schena, L.; Frisullo, S.; di San Lio, M.G. Olive anthracnose. J. Plant Pathol. 2012, 94, 29–44. [Google Scholar]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar]
- Latz, M.A.C.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J.L. Endophytic fungi as biocontrol agents: Elucidating mechanisms in disease suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Tilocca, B.; Cao, A.; Migheli, Q. Scent of a killer: Microbial volatilome and its role in the biological control of plant pathogens. Front. Microbiol. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumlinson, J.H. The importance of volatile organic compounds in ecosystem functioning. J. Chem. Ecol. 2014, 40, 212–213. [Google Scholar] [CrossRef]
- Fuchs, B.; Krauss, J. Can Epichloë endophytes enhance direct and indirect plant defence? Fungal Ecol. 2019, 38, 98–103. [Google Scholar] [CrossRef]
- Preto, G.; Martins, F.; Pereira, J.A.; Baptista, P. Fungal community in olive fruits of cultivars with different susceptibilities to anthracnose and selection of isolates to be used as biocontrol agents. Biol. Control 2017, 110, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gomes, T.; Pereira, J.A.; Benhadi, J.; Lino-Neto, T.; Baptista, P. Endophytic and epiphytic phyllosphere fungal communities are shaped by different environmental factors in a Mediterranean ecosystem. Microb. Ecol. 2018, 76, 668–679. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.; Mina, D.; Pereira, J.A.; Baptista, P. Endophytic fungal community structure in olive orchards with high and low incidence of olive anthracnose. Sci. Rep. 2021, 11, 689. [Google Scholar] [CrossRef]
- Martins, F.; Cameirão, C.; Mina, D.; Benhadi-Marín, J.; Pereira, J.A.; Baptista, P. Endophytic fungal community succession in reproductive organs of two olive tree cultivars with contrasting anthracnose susceptibilities. Fungal Ecol. 2021, 49, 101003. [Google Scholar] [CrossRef]
- Costa, D.; Fernandes, T.; Martins, F.; Pereira, J.A.; Tavares, R.M.; Santos, P.M.; Baptista, P.; Lino-Neto, T. Illuminating Olea europaea L. endophyte fungal community. Microbiol. Res. 2021, 245, 126693. [Google Scholar] [CrossRef] [PubMed]
- Giampetruzzi, A.; Baptista, P.; Morelli, M.; Cameirão, C.; Neto, T.L.; Costa, D.; D’attoma, G.; Kubaa, R.A.; Altamura, G.; Saponari, M.; et al. Differences in the endophytic microbiome of olive cultivars infected by Xylella fastidiosa across seasons. Pathogens 2020, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Hartati, S.; Wiyono, S.; Hidayat, S.H.; Sinaga, M.S. Mode of action of yeast-like fungus Aureobasidium pullulans in controlling anthracnose of postharvest chilli. Int. J. Sci. 2015, 4531, 253–263. [Google Scholar]
- Liu, X.B.; Guo, Z.K.; Huang, G.X. Sarocladium brachiariae sp. nov., an endophytic fungus isolated from Brachiaria brizantha. Mycosphere 2017, 8, 827–834. [Google Scholar] [CrossRef]
- Nigro, F.; Antelmi, I.; Labarile, R.; Sion, V.; Pentimone, I. Biological control of olive anthracnose. Acta Hort. 2018, 1199, 439–444. [Google Scholar] [CrossRef]
- Di Francesco, A.; Zajc, J.; Gunde-Cimerman, N.; Aprea, E.; Gasperi, F.; Placì, N.; Caruso, F.; Baraldi, E. Bioactivity of volatile organic compounds by Aureobasidium species against gray mold of tomato and table grape. World J. Microbiol. Biotechnol. 2020, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yalage Don, S.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci. Rep. 2020, 10, 4498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, N.D.; Vaughan, M.M.; McCormick, S.P.; Brown, J.A.; Bakker, M.G. Sarocladium zeae is a systemic endophyte of wheat and an effective biocontrol agent against Fusarium head blight. Biol. Control 2020, 149, 104329. [Google Scholar] [CrossRef]
- Bahri, H.; Ramos, V.; Mina, D.; Pereira, J.A.; Baptista, P. Characterization of olive associated fungi of cultivars with different levels of resistance to anthracnose. Biol. Life Sci. Forum 2021, 4, 60. [Google Scholar] [CrossRef]
- Costa, H.; Ramos, V.; Pereira, J.A.; Baptista, P. Understanding fungal communities of olive tree leaves for application to climate change adaptation. Biol. Life Sci. Forum 2021, 4, 13. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Mosca, S.; Destri Nicosia, M.G.; Cacciola, S.O.; Schena, L. Molecular analysis of Colletotrichum species in the carposphere and phyllosphere of olive. PLoS ONE 2014, 9, e114031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IOC. International Olive Council, Guide for the Determination of the Characteristics of Oil-Olives; COI/OH/Doc. No 1; IOC: Madrid, Spain, 2011; 39p. [Google Scholar]
- Moral, J.; Bouhmidi, K.; Trapero, A. Influence of fruit maturity, cultivar susceptibility, and inoculation method on infection of olive fruit by Colletotrichum acutatum. Plant Dis. 2008, 92, 1421–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malheiro, R.; Casal, S.; Rodrigues, N.; Renard, C.M.G.C.; Pereira, J.A. Volatile changes in cv. Verdeal Transmontana olive oil: From the drupe to the table, including storage. Food Res. Int. 2018, 106, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.5–6 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 16 January 2022).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Package Version 1.0.7. 2020. Available online: https://CRAN.R614project.org/package=factoextra (accessed on 16 January 2022).
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Besset-Manzoni, Y.; Joly, P.; Brutel, A.; Gerin, F.; Soudière, O.; Langin, T.; Prigent-Combaret, C. Does in vitro selection of biocontrol agents guarantee success in planta? A study case of wheat protection against Fusarium seedling blight by soil bacteria. PLoS ONE 2019, 14, e0225655. [Google Scholar] [CrossRef] [Green Version]
- Piechulla, B.; Degenhardt, J. The emerging importance of microbial volatile organic compounds. Plant Cell Environ. 2014, 37, 811–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenhagen, U.; Baumgartner, R.; Bailly, A.; Gardiner, A.; Eberl, L.; Schulz, S.; Weisskopf, L. Production of bioactive volatiles by different Burkholderia ambifaria strains. J. Chem. Ecol. 2013, 39, 892–906. [Google Scholar] [CrossRef]
- Stergiopoulos, I.; Collemare, J.; Mehrabi, R.; De Wit, P.J. Phytotoxic secondary metabolites and peptides produced by plant pathogenic Dothideomycete fungi. FEMS Microbiol. Rev. 2013, 37, 67–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbeva, P.; Weisskopf, L. Airborne medicine: Bacterial volatiles and their influence on plant health. New Phytol. 2020, 226, 32–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, X.; Shi, X.; Wang, B.; Li, M.; Wang, Q.; Zhang, S. Antifungal effect of volatile organic compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes 2020, 8, 1674. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Hildebrand, P.D.; Fan, L.; Forney, C.F.; Renderos, W.E.; Campbell-Palmer, L.; Doucette, C. Effect of hexanal vapor on the growth of postharvest pathogens and fruit decay. J. Food Sci. 2007, 72, M108–M112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, M.; Zhai, H.; Ma, P.; Lyu, Y.; Hu, Y.; Cai, J. Effects of hexanal fumigation on fungal spoilage and grain quality of stored wheat. GOST 2021, 4, 10–17. [Google Scholar] [CrossRef]
- Gutiérrez-Santa Ana, A.; Carrillo-Cerda, H.A.; Rodriguez-Campos, J.; Kirchmayr, M.R.; Contreras-Ramos, S.M.; Velázquez-Fernández, J.B. Volatile emission compounds from plant growth-promoting bacteria are responsible for the antifungal activity against F. solani. 3 Biotech 2020, 10, 292. [Google Scholar] [CrossRef]
- Ahuchaogu, A.A.; Ogbuehi, G.I.; Obike, A.I.; Egedeuzu, C.S.; Chukwu, O.J.; Echeme, J.B.O. GC-MS Analysis of bioactive compounds from whole plant chloroform extract of Ageratum conyzoides. IJMPNP 2018, 4, 13–24. [Google Scholar]
- D’Auria, J.C.; Pichersky, E.; Schaub, A.; Hansel, A.; Gershenzon, J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2007, 49, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Kang, L. Roles of (Z)-3-hexenol in plant-insect interactions. Plant Signal. Behav. 2011, 6, 369–371. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Qin, X.; Wu, Y.; Yu, W.; Liu, J.; Xi, Y.; Dou, G.; Wang, L.; Xiao, H. Biocontrol of gray mold of cherry tomatoes with the volatile organic monomer from Hanseniaspora uvarum, trans-cinnamaldehyde. Food Bioprocess Technol. 2019, 12, 1809–1820. [Google Scholar] [CrossRef]
- Lemos Junior, W.J.F.; Binati, R.L.; Felis, G.E.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Volatile organic compounds from Starmerella bacillaris to control gray mold on apples and modulate cider aroma profile. Food Microbiol. 2020, 89, 103446. [Google Scholar] [CrossRef] [PubMed]
- Landum, M.C.; Félix, M.R.; Alho, J.; Garcia, R.; Cabrita, M.J.; Rei, F.; Varanda, C.M.R. Antagonistic activity of fungi of Olea europaea L. against Colletotrichum acutatum. Microbiol. Res. 2016, 183, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choińska, R.; Piasecka-Jóźwiak, K.; Chabłowska, B.; Dumka, J.; Łukaszewicz, A. Biocontrol ability and volatile organic compounds production as a putative mode of action of yeast strains isolated from organic grapes and rye grains. Antonie Leeuwenhoek 2020, 113, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sun, H.L.; Chen, S.Y.; Zeng, L.; Wang, T.T. Anti-fungal activity, mechanism studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neri, F.; Cappellin, L.; Aprea, E.; Biasioli, F.; Gasperi, F.; Spadoni, A.; Cameldi, I.; Folchi, A.; Baraldi, E. Interplay of apple volatile organic compounds with Neofabraea vagabunda and other post-harvest pathogens. Plant Pathol. 2019, 68, 1508–1524. [Google Scholar] [CrossRef]
- Reese, K.L.; Rasley, A.; Avila, J.R.; Jones, A.D.; Frank, M. Metabolic profiling of volatile organic compounds (VOCs) emitted by the pathogens Francisella tularensis and Bacillus anthracis in liquid culture. Sci. Rep. 2020, 10, 9333. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Cheng, S.; Lin, C.; Chang, S. Profiling of volatile compounds with characteristic odors in Bambusa oldhamii shoots from Taiwan. BioResources 2021, 16, 5901–5914. [Google Scholar]
| Nº | Compound | A. pullulans | A. pullulans + C. acutatum | C. acutatum | Tween | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 Days | 8 Days | 16 Days | 2 Days | 8 Days | 16 Days | 2 Days | 8 Days | 16 Days | 2 Days | 8 Days | 16 Days | ||
| 1 | 2-Methyl-1-propanol | - | - | 12.2 ± 4.02 | 5.8 ± 0.90 | 7.6 ± 1.98 | 21.7 ± 1.37 | 3.4 ± 0.72 | 7.1 ± 1.60 | 21.9 ± 0.90 | - | - | 16.1 ± 2.32 |
| 2 | 3-Methyl-butanal | - | - | - | - | - | - | - | - | - | 1.4 ± 0.51 | - | - |
| 3 | 2-Methyl-butanal | 3.1 ± 1.31 | - | - | - | - | - | 2.4 ± 0.49 | - | - | 4.6 ± 1.51 | 1.8 ± 0.62 | - |
| 4 | 2,2,4-Trimethyl-pentane | - | 15.6 ± 6.81 | 30.8 ± 9.10 | - | 57.3 ± 4.68 | 6.1 ± 1.26 | - | 58.4 ± 6.90 | 4.7 ± 1.67 | - | - | 27.9 ± 4.12 |
| 5 | 2-Methyl-1-butanol | - | 19.5 ± 5.74 | 30.1 ± 3.86 | 17.6 ± 2.99 | 18.4 ± 2.95 | 39.5 ± 2.93 | 11.4 ± 4.87 | 18.2 ± 6.26 | 40.2 ± 3.81 | - | 25.7 ± 10.07 | 33.8 ± 3.54 |
| 6 | 2-Hexanone | - | - | - | 1.2 ± 0.62 | - | - | 1.2 ± 0.09 | - | - | - | - | - |
| 7 | Hexanal | 23.9 ± 3.53 | 4.3 ± 2.32 | - | 3.4 ± 1.70 | - | - | 8.7 ± 1.92 | - | - | 27.0 ± 4.20 | 4.0 ± 2.51 | - |
| 8 | 2-Hexenal | 14.5 ± 4.44 | 2.0 ± 1.72 | - | 2.2 ± 1.10 | - | - | 4.5 ± 1.41 | - | - | 22.4 ± 12.28 | 1.9 ± 1.43 | - |
| 9 | Z-3-hexen-1-ol | 25.2 ± 2.71 | 12.2 ± 3.14 | 4.3 ± 1.43 | 13.7 ± 2.50 | 3.2 ± 0.90 | 5.6 ± 0.98 | 12.4 ± 1.43 | 2.9 ± 0.63 | 5.2 ± 0.72 | - | 9.7 ± 4.24 | 2.3 ± 0.94 |
| 10 | Z-2-hexen-1-ol | - | 2.5 ± 0.84 | - | - | - | - | - | - | - | - | 4.1 ± 3.84 | - |
| 11 | 1-Hexanol | 8.9 ± 5.96 | 29.4 ± 6.69 | 15.0 ± 3.93 | 23.0 ± 3.71 | 8.5 ± 2.95 | 13.9 ± 2.58 | 20.3 ± 3.76 | 8.5 ± 2.83 | 16.9 ± 4.09 | - | 28.4 ± 2.20 | 11.2 ± 3.88 |
| 12 | 2-Heptanone | - | - | - | 3.2 ± 0.94 | - | - | 3.9 ± 0.91 | - | - | - | - | - |
| 13 | Methoxy-phenyl-oxime | - | - | - | - | - | - | - | 0.2 ± 0.03 | - | 1.7 ± 2.21 | 0.5 ± 0.21 | - |
| 14 | Z-hept-4-enol | - | - | - | - | - | - | - | - | - | - | - | 0.3 ± 0.03 |
| 15 | 1-Heptanol | - | 0.4 ± 0.06 | - | 1.7 ± 0.56 | 0.3 ± 0.11 | 1.0 ± 0.40 | 1.9 ± 0.30 | 0.2 ± 0.02 | 1.2 ± 0.52 | 0.9 ± 0.41 | 0.6 ± 0.22 | 0.6 ± 0.22 |
| 16 | 6-Methyl-5-hepten-2-one | - | 0.3 ± 0.06 | - | 0.7 ± 0.21 | - | - | 0.5 ± 0.06 | 0.2 ± 0.06 | 0.5 ± 0.12 | 0.8 ± 0.16 | 0.4 ± 0.09 | - |
| 17 | 2-Octanone | - | - | - | 2.4 ± 1.33 | - | - | 4.8 ± 1.10 | - | - | - | - | - |
| 18 | Z-3-hexen-1-ol acetate | 1.4 ± 0.27 | 0.6 ± 0.06 | - | 0.9 ± 0.24 | - | - | 0.8 ± 0.06 | - | - | 2.2 ± 0.57 | 0.8 ± 0.18 | - |
| 19 | Hexyl ester acetic acid | 1.4 ± 0.27 | 2.4 ± 0.38 | 0.5 ± 0.11 | 4.2 ± 1.03 | 0.4 ± 0.14 | 0.4 ± 0.12 | 3.6 ± 0.27 | 0.3 ± 0.09 | 0.4 ± 0.20 | 8.3 ± 1.90 | 3.5 ± 0.71 | 0.5 ± 0.06 |
| 20 | Limonene | - | - | - | 4.2 ± 0.71 | - | - | 3.7 ± 0.16 | - | - | - | - | - |
| 21 | Not identified | 1.7 ± 0.35 | 1.4 ± 0.18 | 0.7 ± 0.11 | - | 0.5 ± 0.08 | 1.0 ± 0.18 | - | 0.4 ± 0.04 | - | 3.0 ± 0.76 | 2.0 ± 0.39 | 0.7 ± 0.09 |
| 22 | Benzyl alcohol | - | - | - | - | - | 0.5 ± 0.16 | - | - | 0.3 ± 0.19 | - | - | 0.3 ± 0.07 |
| 23 | Benzeneacetaldehyde | 0.9 ± 0.22 | - | - | - | - | - | - | - | - | 1.2 ± 0.16 | - | - |
| 24 | E-2-octenal | 1.1 ± 0.21 | 0.4 ± 0.09 | - | - | - | - | 0.7 ± 0.04 | - | - | 1.7 ± 0.35 | 0.5 ± 0.15 | - |
| 25 | E-2-octen-1-ol | - | - | - | - | - | - | - | - | - | - | 0.3 ± 0.10 | - |
| 26 | 1-Octanol | - | 0.4 ± 0.13 | - | 1.0 ± 0.40 | 0.2 ± 0.06 | 0.7 ± 0.22 | 1.2 ± 0.14 | 0.2 ± 0.03 | 0.8 ± 0.16 | 0.6 ± 0.23 | 0.6 ± 0.21 | 0.4 ± 0.06 |
| 27 | 2-Nonanone | 0.8 ± 0.17 | 0.5 ± 0.13 | 0.2 ± 0.06 | 1.4 ± 0.27 | 0.2 ± 0.04 | 0.3 ± 0.07 | 1.7 ± 0.26 | 0.2 ± 0.06 | 0.4 ± 0.11 | 1.3 ± 0.33 | 0.8 ± 0.19 | 0.2 ± 0.03 |
| 28 | Nonanal | 1.4 ± 0.26 | 0.7 ± 0.10 | 0.3 ± 0.16 | 1.0 ± 0.16 | 0.2 ± 0.10 | 0.4 ± 0.21 | 1.4 ± 0.13 | 0.2 ± 0.04 | 0.3 ± 0.05 | 2.5 ± 0.65 | 1.0 ± 0.36 | 0.3 ± 0.05 |
| 29 | Phenylethyl alcohol | - | 0.3 ± 0.07 | 2.4 ± 1.55 | - | 0.6 ± 0.21 | 3.5 ± 0.86 | - | 0.5 ± 0.41 | 1.9 ± 0.77 | - | 0.4 ± 0.10 | 2.4 ± 0.68 |
| 30 | (1S)-1,7,7-trimethyl-bicyclo [2.2.1]heptan-2-one | 2.8 ± 0.50 | 1.6 ± 0.16 | 0.5 ± 0.08 | 2.9 ± 0.61 | 0.5 ± 0.07 | 0.7 ± 0.11 | 1.4 ± 0.13 | 0.5 ± 0.09 | 0.7 ± 0.03 | 4.6 ± 1.04 | 2.6 ± 0.72 | 0.5 ± 0.08 |
| 31 | [1S-(1α,2α.,5β)]-Cyclohexanol, 5-methyl-2-(1-methylethyl) | 0.5 ± 0.10 | 0.3 ± 0.05 | 0.1 ± 0.04 | - | 0.1 ± 0.01 | 0.2 ± 0.04 | - | 0.1 ± 0.02 | - | 0.8 ± 0.22 | 0.5 ± 0.15 | - |
| 32 | Levomenthol | 8.2 ± 1.73 | 5.5 ± 0.54 | 2.7 ± 0.86 | 9.0 ± 1.67 | 1.9 ± 0.28 | 4.1 ± 0.75 | 8.4 ± 0.25 | 1.9 ± 0.39 | 4.3 ± 0.41 | 13.7 ± 3.71 | 9.1 ± 2.80 | 2.6 ± 0.34 |
| 33 | Decanal | - | - | - | - | 0.08 ± 0.03 | - | - | - | - | - | 0.3 ± 0.07 | - |
| 34 | 2-Undecanone | - | - | - | - | - | - | 0.6 ± 0.14 | - | - | - | - | - |
| 35 | α-Cubebene | - | - | - | - | - | - | - | - | - | - | 0.2 ± 0.07 | - |
| 36 | Copaene | - | - | - | - | - | - | - | - | - | 0.5 ± 0.25 | - | - |
| 37 | Tetradecane | - | 0.2 ± 0.03 | 0.2 ± 0.04 | - | 0.08 ± 0.01 | 0.2 ± 0.04 | - | 0.08 ± 0.02 | 0.3 ± 0.06 | 0.3 ± 0.09 | 0.3 ± 0.09 | 0.2 ± 0.03 |
| 38 | Longifolene | - | 0.2 ± 0.04 | - | - | - | - | - | 0.1 ± 0.03 | - | 0.4 ± 0.13 | 0.4 ± 0.28 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sdiri, Y.; Lopes, T.; Rodrigues, N.; Silva, K.; Rodrigues, I.; Pereira, J.A.; Baptista, P. Biocontrol Ability and Production of Volatile Organic Compounds as a Potential Mechanism of Action of Olive Endophytes against Colletotrichum acutatum. Microorganisms 2022, 10, 571. https://doi.org/10.3390/microorganisms10030571
Sdiri Y, Lopes T, Rodrigues N, Silva K, Rodrigues I, Pereira JA, Baptista P. Biocontrol Ability and Production of Volatile Organic Compounds as a Potential Mechanism of Action of Olive Endophytes against Colletotrichum acutatum. Microorganisms. 2022; 10(3):571. https://doi.org/10.3390/microorganisms10030571
Chicago/Turabian StyleSdiri, Yosra, Teresa Lopes, Nuno Rodrigues, Kevin Silva, Isabel Rodrigues, José Alberto Pereira, and Paula Baptista. 2022. "Biocontrol Ability and Production of Volatile Organic Compounds as a Potential Mechanism of Action of Olive Endophytes against Colletotrichum acutatum" Microorganisms 10, no. 3: 571. https://doi.org/10.3390/microorganisms10030571
APA StyleSdiri, Y., Lopes, T., Rodrigues, N., Silva, K., Rodrigues, I., Pereira, J. A., & Baptista, P. (2022). Biocontrol Ability and Production of Volatile Organic Compounds as a Potential Mechanism of Action of Olive Endophytes against Colletotrichum acutatum. Microorganisms, 10(3), 571. https://doi.org/10.3390/microorganisms10030571









