Klebsiella pneumoniae Lipopolysaccharides Serotype O2afg Induce Poor Inflammatory Immune Responses Ex Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. K. pneumoniae Strains and Sequencing and General Reagents
2.2. Sequence Type and O-Antigen Serotype Determination
2.3. LPS ELISA and Immunoblot
2.4. Immunological Assays
2.5. Nuclear Translocation of NF-κB
2.6. Real-Time PCR
2.7. Statistical Analysis
2.8. Ethical Clearance
3. Results
3.1. Determination of O-Antigen Serotype of IRCCS-ISMETT Clinical Samples
3.2. Assessment of the Presence of Antibodies against LPS Serotypes O1, O2a and O2afg in Human Sera from Patients Who Suffered Either an Infection or a Colonization Case of K. pneumoniae
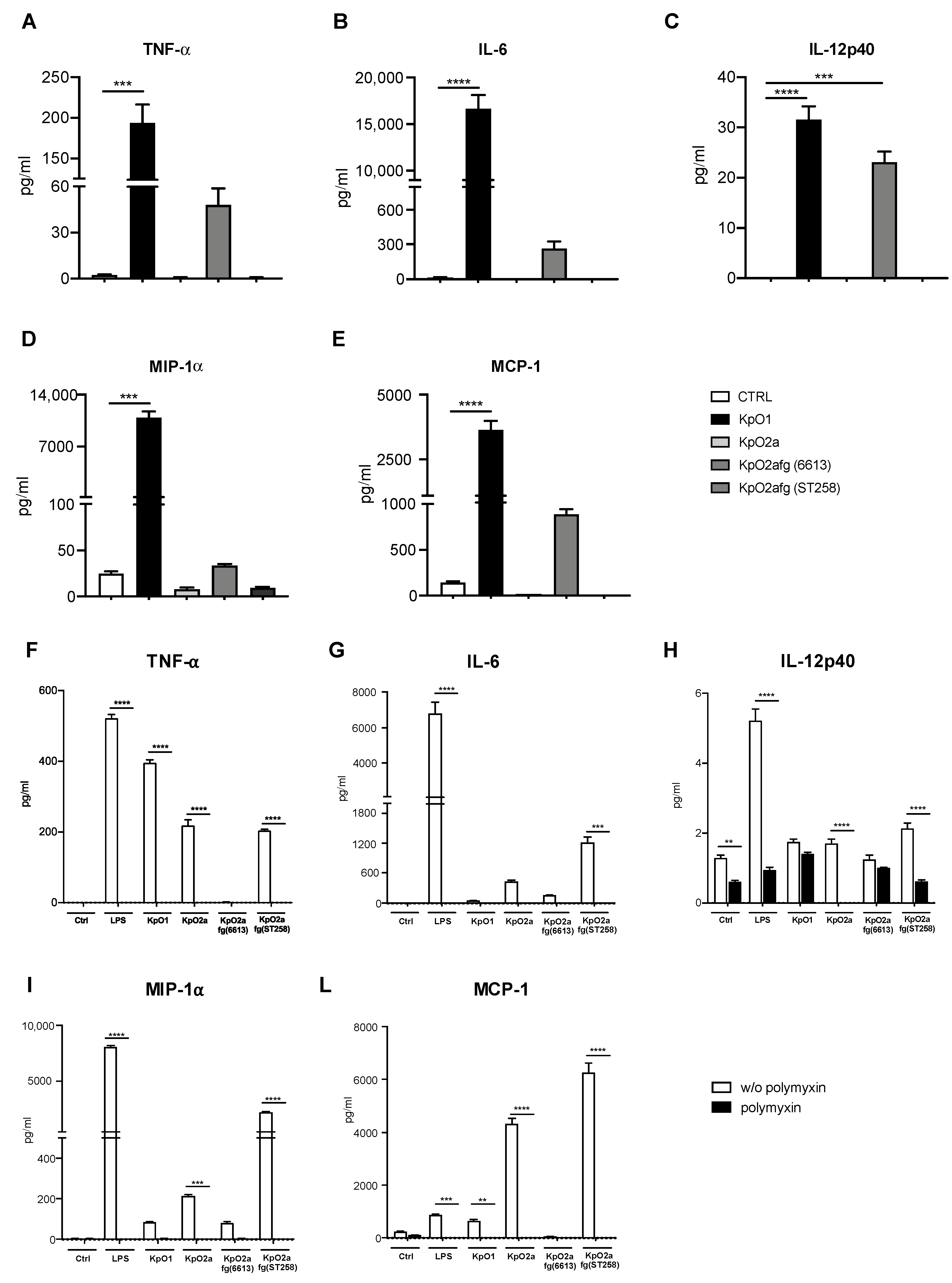
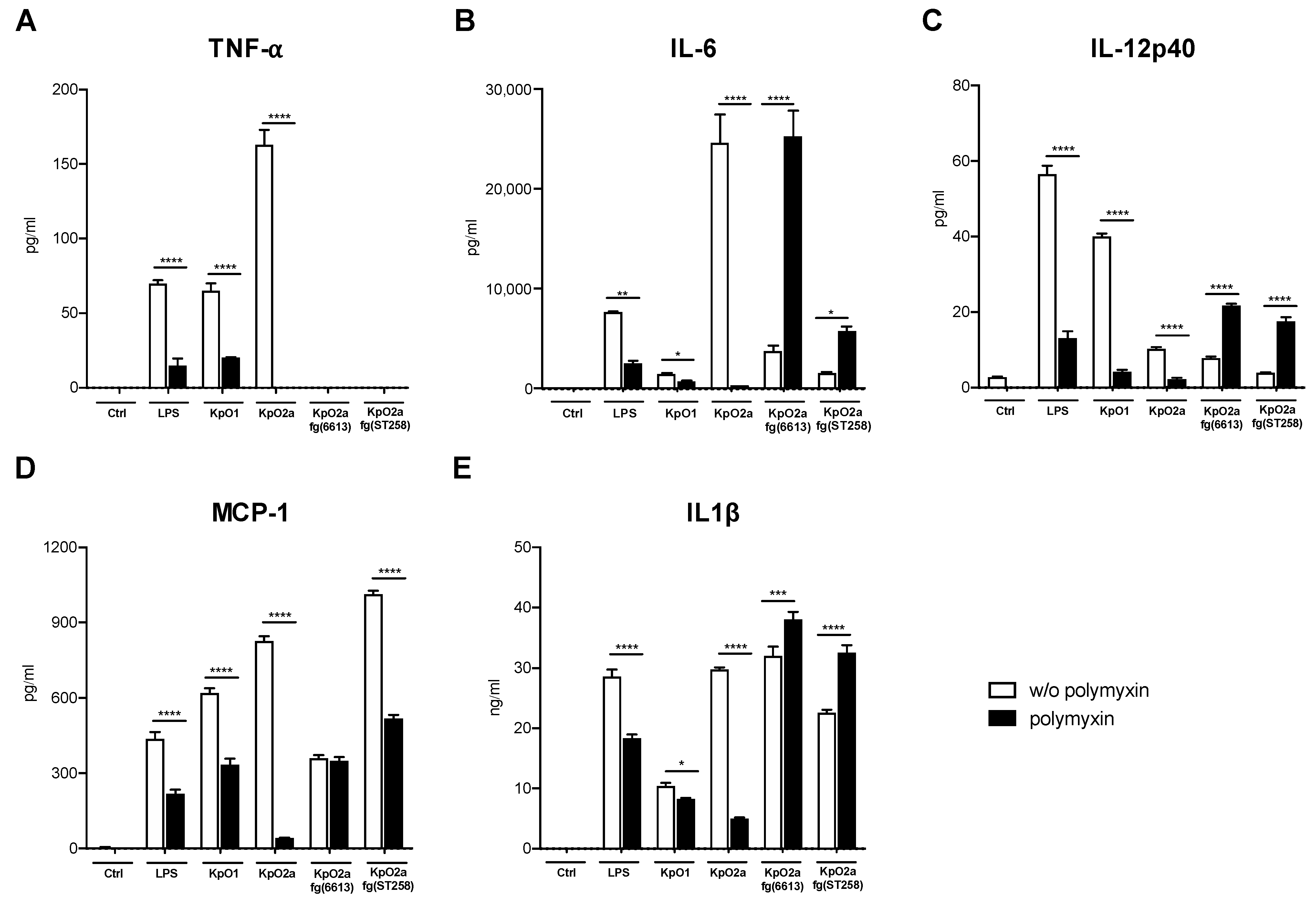
3.3. Quantification of Pro-Inflammatory Chemokines and Cytokines Induced by LPS O1, LPS O2a or LPS O2afg
3.4. Phagocytosis of K. pneumoniae Strains Expressing LPS O1, LPS O2a or LPS O2afg
3.5. Nuclear Translocation of NF-κB to the Nuclei of Human Monocytes Stimulated with K. pneumoniae LPS
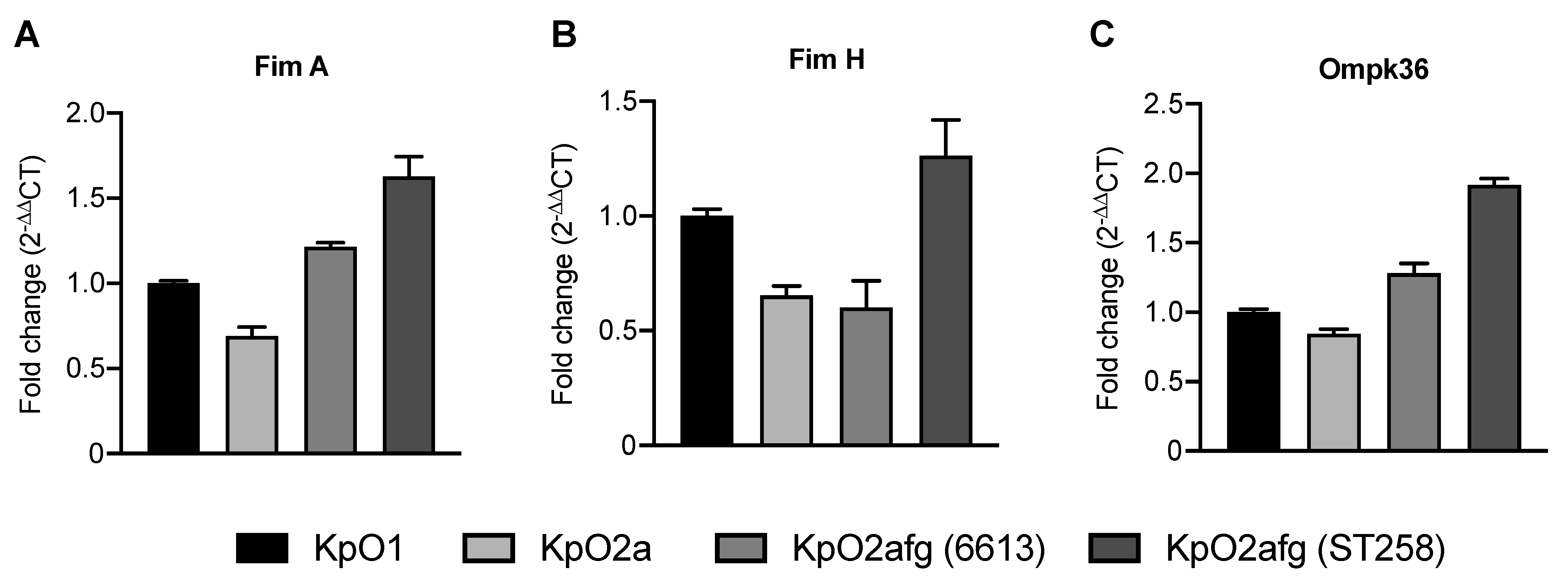
3.6. Transcriptional Levels of K. pneumoniae Genes Related with Phagocytosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 20 March 2017).
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Cerceo, E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Dalton, K.R.; Rock, C.; Carroll, K.C.; Davis, M.F. One Health in hospitals: How understanding the dynamics of people, animals, and the hospital built-environment can be used to better inform interventions for antimicrobial-resistant gram-positive infections. Antimicrob. Resist. Infect. Control 2020, 9, 78. [Google Scholar] [CrossRef]
- Temkin, E.; Fallach, N.; Almagor, J.; Gladstone, B.P.; Tacconelli, E.; Carmeli, Y. Estimating the number of infections caused by antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in 2014: A modelling study. Lancet Glob. Health 2018, 6, e969–e979. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Smith, L.M.; May, R.C. Mechanisms of microbial escape from phagocyte killing. Biochem. Soc. Trans. 2013, 41, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Cano, V.; March, C.; Insua, J.L.; Aguiló, N.; Llobet, E.; Moranta, D.; Regueiro, V.; Brennan, G.P.; Millán-Lou, M.I.; Martín, C.; et al. Klebsiella pneumoniae survives within macrophages by avoiding delivery to lysosomes. Cell. Microbiol. 2015, 17, 1537–1560. [Google Scholar] [CrossRef]
- Ares, M.A.; Sansabas, A.; Rodríguez-Valverde, D.; Siqueiros-Cendón, T.; Rascón-Cruz, Q.; Rosales-Reyes, R.; Jarillo-Quijada, M.D.; Alcántar-Curiel, M.D.; Cedillo, M.L.; Torres, J.; et al. The Interaction of Klebsiella pneumoniae With Lipid Rafts-Associated Cholesterol Increases Macrophage-Mediated Phagocytosis Due to Down Regulation of the Capsule Polysaccharide. Front. Cell. Infect. Microbiol. 2019, 9, 255. [Google Scholar] [CrossRef]
- Olonisakin, T.F.; Li, H.; Xiong, Z.; Kochman, E.J.K.; Yu, M.; Qu, Y.; Hulver, M.; Kolls, J.K.; St Croix, C.; Doi, Y.; et al. CD36 Provides Host Protection Against Klebsiella pneumoniae Intrapulmonary Infection by Enhancing Lipopolysaccharide Responsiveness and Macrophage Phagocytosis. J. Infect. Dis. 2016, 214, 1865–1875. [Google Scholar] [CrossRef]
- Patro, L.P.P.; Rathinavelan, T. Targeting the Sugary Armor of Klebsiella Species. Front. Cell. Infect. Microbiol. 2019, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, J.A.; Sa Pessoa, J. Klebsiella pneumoniae infection biology: Living to counteract host defences. FEMS Microbiol. Rev. 2019, 43, 123–144. [Google Scholar] [CrossRef]
- Codo, A.C.; Saraiva, A.C.; Dos Santos, L.L.; Visconde, M.F.; Gales, A.C.; Zamboni, D.S.; Medeiros, A.I. Inhibition of inflammasome activation by a clinical strain of Klebsiella pneumoniae impairs efferocytosis and leads to bacterial dissemination. Cell Death Dis. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Clarke, B.R.; Ovchinnikova, O.G.; Kelly, S.D.; Williamson, M.L.; Butler, J.E.; Liu, B.; Wang, L.; Gou, X.; Follador, R.; Lowary, T.L.; et al. Molecular basis for the structural diversity in serogroup O2-antigen polysaccharides in Klebsiella pneumoniae. J. Biol. Chem. 2018, 293, 4666–4679. [Google Scholar] [CrossRef]
- Trautmann, M.; Ruhnke, M.; Rukavina, T.; Held, T.K.; Cross, A.S.; Marre, R.; Whitfield, C. O-antigen seroepidemiology of Klebsiella clinical isolates and implications for immunoprophylaxis of Klebsiella infections. Clin. Diagn. Lab. Immunol. 1997, 4, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Pennini, M.E.; De Marco, A.; Pelletier, M.; Bonnell, J.; Cvitkovic, R.; Beltramello, M.; Cameroni, E.; Bianchi, S.; Zatta, F.; Zhao, W.; et al. Immune stealth-driven O2 serotype prevalence and potential for therapeutic antibodies against multidrug resistant Klebsiella pneumoniae. Nat. Commun. 2017, 8, 1991. [Google Scholar] [CrossRef] [PubMed]
- Szijártó, V.; Guachalla, L.M.; Hartl, K.; Varga, C.; Banerjee, P.; Stojkovic, K.; Kaszowska, M.; Nagy, E.; Lukasiewicz, J.; Nagy, G. Both clades of the epidemic KPC-producing Klebsiella pneumoniae clone ST258 share a modified galactan O-antigen type. Int. J. Med. Microbiol. 2016, 306, 89–98. [Google Scholar] [CrossRef]
- Fang, C.-T.; Shih, Y.-J.; Cheong, C.-M.; Yi, W.-C. Rapid and Accurate Determination of Lipopolysaccharide O-Antigen Types in Klebsiella pneumoniae with a Novel PCR-Based O-Genotyping Method. J. Clin. Microbiol. 2016, 54, 666–675. [Google Scholar] [CrossRef]
- Di Mento, G.; Cuscino, N.; Carcione, C.; Cardinale, F.; Conaldi, P.G.; Douradinha, B. Emergence of a Klebsiella pneumoniae ST392 clone harbouring KPC-3 in an Italian transplantation hospital. J. Hosp. Infect. 2017, 10–11. [Google Scholar] [CrossRef]
- Gona, F.; Caio, C.; Iannolo, G.; Monaco, F.; Di Mento, G.; Cuscino, N.; Fontana, I.; Panarello, G.; Maugeri, G.; Mezzatesta, M.L.; et al. Detection of the IncX3 plasmid carrying bla KPC-3 in a Serratia marcescens strain isolated from a kidney–liver transplanted patient. J. Med. Microbiol. 2017, 66, 1454–1456. [Google Scholar] [CrossRef] [PubMed]
- Monaco, F.; Di Mento, G.; Cuscino, N.; Conaldi, P.G.; Douradinha, B. Infant colonisation with Escherichia coli and Klebsiella pneumoniae strains co-harbouring blaOXA-48and blaNDM-1carbapenemases genes: A case report. Int. J. Antimicrob. Agents 2018, 52, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Di Mento, G.; Carreca, A.P.; Monaco, F.; Cuscino, N.; Cardinale, F.; Conaldi, P.G.; Douradinha, B. Mycobacterium saskatchewanense strain associated with a chronic kidney disease patient in an Italian transplantation hospital and almost misdiagnosed as Mycobacterium tuberculosis. Infect. Control Hosp. Epidemiol. 2019, 40, 496–497. [Google Scholar] [CrossRef]
- Gona, F.; Barbera, F.; Pasquariello, A.C.; Grossi, P.; Gridelli, B.; Mezzatesta, M.L.; Caio, C.; Stefani, S.; Conaldi, P.G. In vivo multiclonal transfer of blaKPC-3 from Klebsiella pneumoniae to Escherichia coli in surgery patients. Clin. Microbiol. Infect. 2014, 20, O633–O635. [Google Scholar] [CrossRef]
- Lundberg, U.; Senn, B.M.; Schuler, W.; Meinke, A.; Hanner, M. Identification and characterization of antigens as vaccine candidates against Klebsiella pneumoniae. Hum. Vaccin. Immunother. 2013, 9, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Fomsgaard, A.; Freudenberg, M.A.; Galanos, C. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J. Clin. Microbiol. 1990, 28, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Amirmozaffari, N.; Tabarraei, B.; Jeddi-Tehrani, M.; Zarei, O.; Alizadeh, R.; Masjedian, F.; Zarnani, A.H. Extraction, Purification and Characterization of Lipopolysaccharide from Escherichia coli and Salmonella typhi. Avicenna J. Med. Biotechnol. 2011, 3, 3–9. [Google Scholar]
- Cardoso, L.S.; Araujo, M.I.; Góes, A.M.; Pacífico, L.G.; Oliveira, R.R.; Oliveira, S.C. Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis. Microb. Cell Fact. 2007, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Ponte, C.G.G.; Hacker, M.A.; Antas, P.R.Z. A whole blood assay as a simple, broad assessment of cytokines and chemokines to evaluate human immune responses to Mycobacterium tuberculosis antigens. Acta Trop. 2013, 127, 75–81. [Google Scholar] [CrossRef]
- Iannolo, G.; Sciuto, M.R.; Cuscino, N.; Pallini, R.; Douradinha, B.; Ricci Vitiani, L.; De Maria, R.; Conaldi, P.G. Zika virus infection induces MiR34c expression in glioblastoma stem cells: New perspectives for brain tumor treatments. Cell Death Dis. 2019, 10, 263. [Google Scholar] [CrossRef]
- Bulati, M.; Miceli, V.; Gallo, A.; Amico, G.; Carcione, C.; Pampalone, M.; Conaldi, P.G. The Immunomodulatory Properties of the Human Amnion-Derived Mesenchymal Stromal/Stem Cells Are Induced by INF-γ Produced by Activated Lymphomonocytes and Are Mediated by Cell-To-Cell Contact and Soluble Factors. Front. Immunol. 2020, 11, 54. [Google Scholar] [CrossRef]
- Berger, S.B.; Romero, X.; Ma, C.; Wang, G.; Faubion, W.A.; Liao, G.; Compeer, E.; Keszei, M.; Rameh, L.; Wang, N.; et al. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat. Immunol. 2010, 11, 920–927. [Google Scholar] [CrossRef]
- D’Apolito, D.; Arena, F.; Conte, V.; De Angelis, L.H.; Di Mento, G.; Carreca, A.P.; Cuscino, N.; Russelli, G.; Iannolo, G.; Barbera, F.; et al. Phenotypical and molecular assessment of the virulence potential of KPC-3-producing Klebsiella pneumoniae ST392 clinical isolates. Microbiol. Res. 2020, 240, 126551. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.E.I.; Stuchi, L.P.; Siqueira, N.M.G.; Henrique, J.B.; Vicentini, R.; Ribeiro, M.L.; Darrieux, M.; Ferraz, L.F.C. Selection and validation of reference genes for gene expression studies in Klebsiella pneumoniae using Reverse Transcription Quantitative real-time PCR. Sci. Rep. 2018, 8, 9001. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Conte, V.; Monaco, M.; Giani, T.; D’Ancona, F.; Moro, M.L.; Arena, F.; D’Andrea, M.M.; Rossolini, G.M.; Pantosti, A. Molecular epidemiology of KPC-producing Klebsiella pneumoniae from invasive infections in Italy: Increasing diversity with predominance of the ST512 clade II sublineage. J. Antimicrob. Chemother. 2016, 71, 3386–3391. [Google Scholar] [CrossRef]
- Giani, T.; Pini, B.; Arena, F.; Conte, V.; Bracco, S.; Migliavacca, R.; Pantosti, A.; Pagani, L.; Luzzaro, F.; Rossolini, G.M. Epidemic diffusion Of KPC carbapenemase-producing Klebsiella pneumoniae in Italy: Results of the first countrywide survey, 15 may to 30 june 2011. EuroSurveill 2013, 18, 1–9. [Google Scholar] [CrossRef]
- Merino, S.; Camprubí, S.; Albertí, S.; Benedí, V.J.; Tomás, J.M. Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect. Immun. 1992, 60, 2529–2535. [Google Scholar] [CrossRef]
- Llobet, E.; Campos, M.A.; Giménez, P.; Moranta, D.; Bengoechea, J.A. Analysis of the networks controlling the antimicrobial-peptide-dependent induction of Klebsiella pneumoniae virulence factors. Infect. Immun. 2011, 79, 3718–3732. [Google Scholar] [CrossRef]
- Tomás, A.; Lery, L.; Regueiro, V.; Pérez-Gutiérrez, C.; Martínez, V.; Moranta, D.; Llobet, E.; González-Nicolau, M.; Insua, J.L.; Tomas, J.M.; et al. Functional Genomic Screen Identifies Klebsiella pneumoniae Factors Implicated in Blocking Nuclear Factor κB (NF-κB) Signaling. J. Biol. Chem. 2015, 290, 16678–16697. [Google Scholar] [CrossRef]
- Møller, A.-S.W.; Øvstebø, R.; Westvik, A.-B.; Joø, G.B.; Haug, K.-B.F.; Kierulf, P. Effects of bacterial cell wall components (PAMPs) on the expression of monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1alpha (MIP-1alpha) and the chemokine receptor CCR2 by purified human blood monocytes. J. Endotoxin Res. 2003, 9, 349–360. [Google Scholar] [CrossRef]
- Plevin, R.E.; Knoll, M.; McKay, M.; Arbabi, S.; Cuschieri, J. The Role of Lipopolysaccharide Structure in Monocyte Activation and Cytokine Secretion. Shock 2016, 45, 22–27. [Google Scholar] [CrossRef]
- Greten, F.R.; Arkan, M.C.; Bollrath, J.; Hsu, L.-C.; Goode, J.; Miething, C.; Göktuna, S.I.; Neuenhahn, M.; Fierer, J.; Paxian, S.; et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 2007, 130, 918–931. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, L.; Cao, H.; Wang, N.; Zheng, J.; Xiao, G. Polymyxin B antagonizing biological activity of lipopolysaccharide. Chin. J. Traumatol. 2007, 10, 180–183. [Google Scholar]
- Schenk, M.; Fabri, M.; Krutzik, S.R.; Lee, D.J.; Vu, D.M.; Sieling, P.A.; Montoya, D.; Liu, P.T.; Modlin, R.L. Interleukin-1β triggers the differentiation of macrophages with enhanced capacity to present mycobacterial antigen to T cells. Immunology 2014, 141, 174–180. [Google Scholar] [CrossRef]
- Bagaev, A.V.; Garaeva, A.Y.; Lebedeva, E.S.; Pichugin, A.V.; Ataullakhanov, R.I.; Ataullakhanov, F.I. Elevated pre-activation basal level of nuclear NF-κB in native macrophages accelerates LPS-induced translocation of cytosolic NF-κB into the cell nucleus. Sci. Rep. 2019, 9, 4563. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Høgåsen, A.K.; Abrahamsen, T.G. Polymyxin B stimulates production of complement components and cytokines in human monocytes. Antimicrob. Agents Chemother. 1995, 39, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Bauquier, J.R.; Tennent-Brown, B.S.; Tudor, E.; Bailey, S.R. Effects of polymyxin-B on TNF-α production in equine whole blood stimulated with three different bacterial toxins. J. Vet. Pharmacol. Ther. 2018, 41, e35–e39. [Google Scholar] [CrossRef]
- Shimomura, H.; Matsuura, M.; Saito, S.; Hirai, Y.; Isshiki, Y.; Kawahara, K. Unusual interaction of a lipopolysaccharide isolated from Burkholderia cepacia with polymyxin B. Infect. Immun. 2003, 71, 5225–5230. [Google Scholar] [CrossRef] [PubMed]
- LaRocque-de-Freitas, I.F.; Rocha, J.D.B.; Nunes, M.P.; Oliveira, P.A.V.; de Oliveira Nascimento, D.; Freire-de-Lima, L.; Takiya, C.M.; Morrot, A.; Decote-Ricardo, D.; Previato, J.O.; et al. Involvement of the capsular GalXM-induced IL-17 cytokine in the control of Cryptococcus neoformans infection. Sci. Rep. 2018, 8, 16378. [Google Scholar] [CrossRef]
- Chi, X.; Berglund, B.; Zou, H.; Zheng, B.; Börjesson, S.; Ji, X.; Ottoson, J.; Lundborg, C.S.; Li, X.; Nilsson, L.E. Characterization of Clinically Relevant Strains of Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae Occurring in Environmental Sources in a Rural Area of China by Using Whole-Genome Sequencing. Front. Microbiol. 2019, 10, 211. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulati, M.; Busà, R.; Carcione, C.; Iannolo, G.; Di Mento, G.; Cuscino, N.; Di Gesù, R.; Piccionello, A.P.; Buscemi, S.; Carreca, A.P.; et al. Klebsiella pneumoniae Lipopolysaccharides Serotype O2afg Induce Poor Inflammatory Immune Responses Ex Vivo. Microorganisms 2021, 9, 1317. https://doi.org/10.3390/microorganisms9061317
Bulati M, Busà R, Carcione C, Iannolo G, Di Mento G, Cuscino N, Di Gesù R, Piccionello AP, Buscemi S, Carreca AP, et al. Klebsiella pneumoniae Lipopolysaccharides Serotype O2afg Induce Poor Inflammatory Immune Responses Ex Vivo. Microorganisms. 2021; 9(6):1317. https://doi.org/10.3390/microorganisms9061317
Chicago/Turabian StyleBulati, Matteo, Rosalia Busà, Claudia Carcione, Gioacchin Iannolo, Giuseppina Di Mento, Nicola Cuscino, Roberto Di Gesù, Antonio Palumbo Piccionello, Silvestre Buscemi, Anna Paola Carreca, and et al. 2021. "Klebsiella pneumoniae Lipopolysaccharides Serotype O2afg Induce Poor Inflammatory Immune Responses Ex Vivo" Microorganisms 9, no. 6: 1317. https://doi.org/10.3390/microorganisms9061317
APA StyleBulati, M., Busà, R., Carcione, C., Iannolo, G., Di Mento, G., Cuscino, N., Di Gesù, R., Piccionello, A. P., Buscemi, S., Carreca, A. P., Barbera, F., Monaco, F., Cardinale, F., Conaldi, P. G., & Douradinha, B. (2021). Klebsiella pneumoniae Lipopolysaccharides Serotype O2afg Induce Poor Inflammatory Immune Responses Ex Vivo. Microorganisms, 9(6), 1317. https://doi.org/10.3390/microorganisms9061317








