Leishmania-Induced Dendritic Cell Migration and Its Potential Contribution to Parasite Dissemination
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Leishmania Parasites
2.3. Dendritic Cell Cultures
2.4. hDC Infection
2.5. Migration Assay
2.6. Zymosan Phagocytosis
2.7. Immunofluorescence Assay
2.8. Actin Polymerization Assay
2.9. CCR7 Analysis
2.10. Statistical Analysis
3. Results
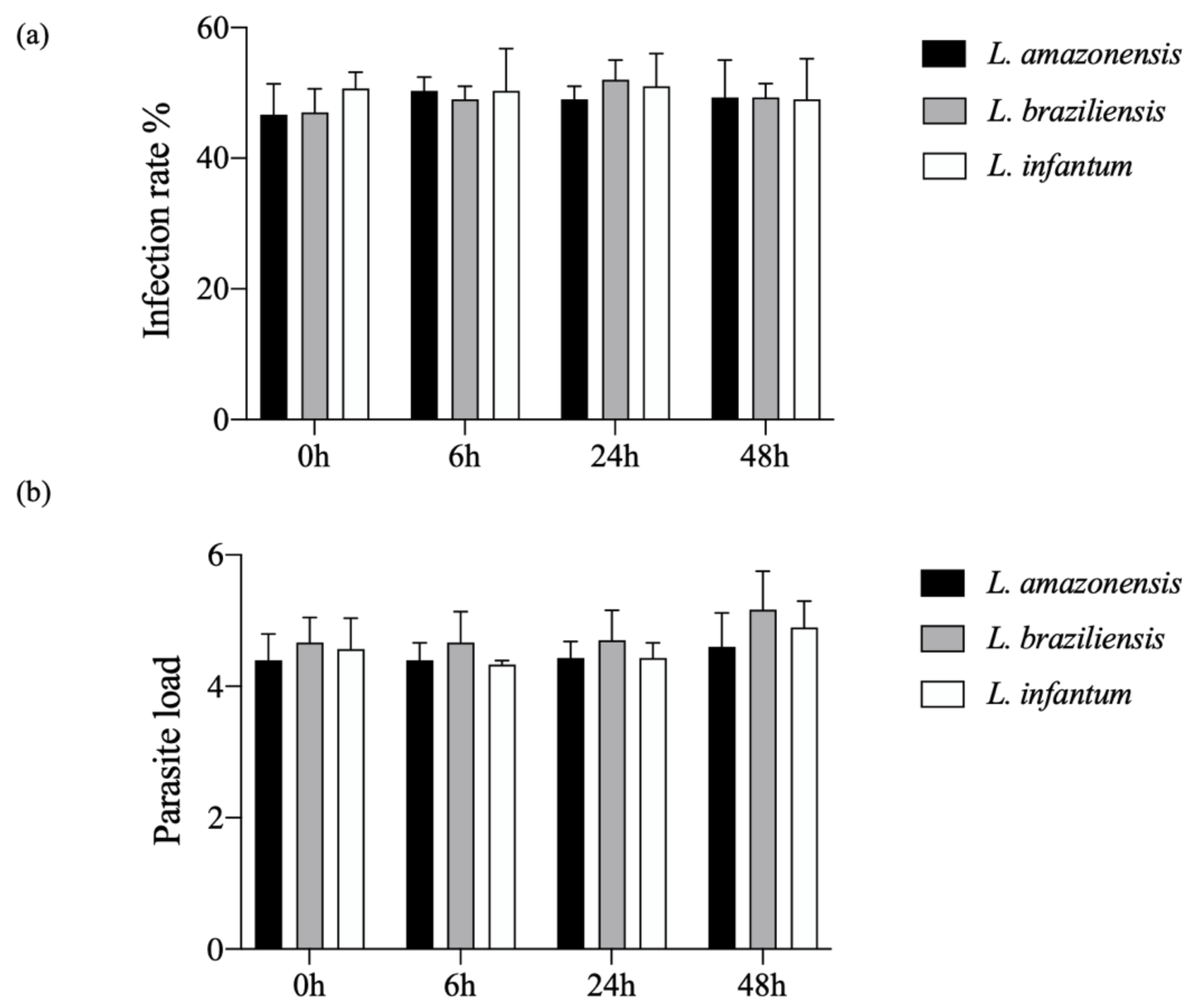
3.1. Kinetics of hDC Infection with L. amazonensis, L. braziliensis or L. infantum
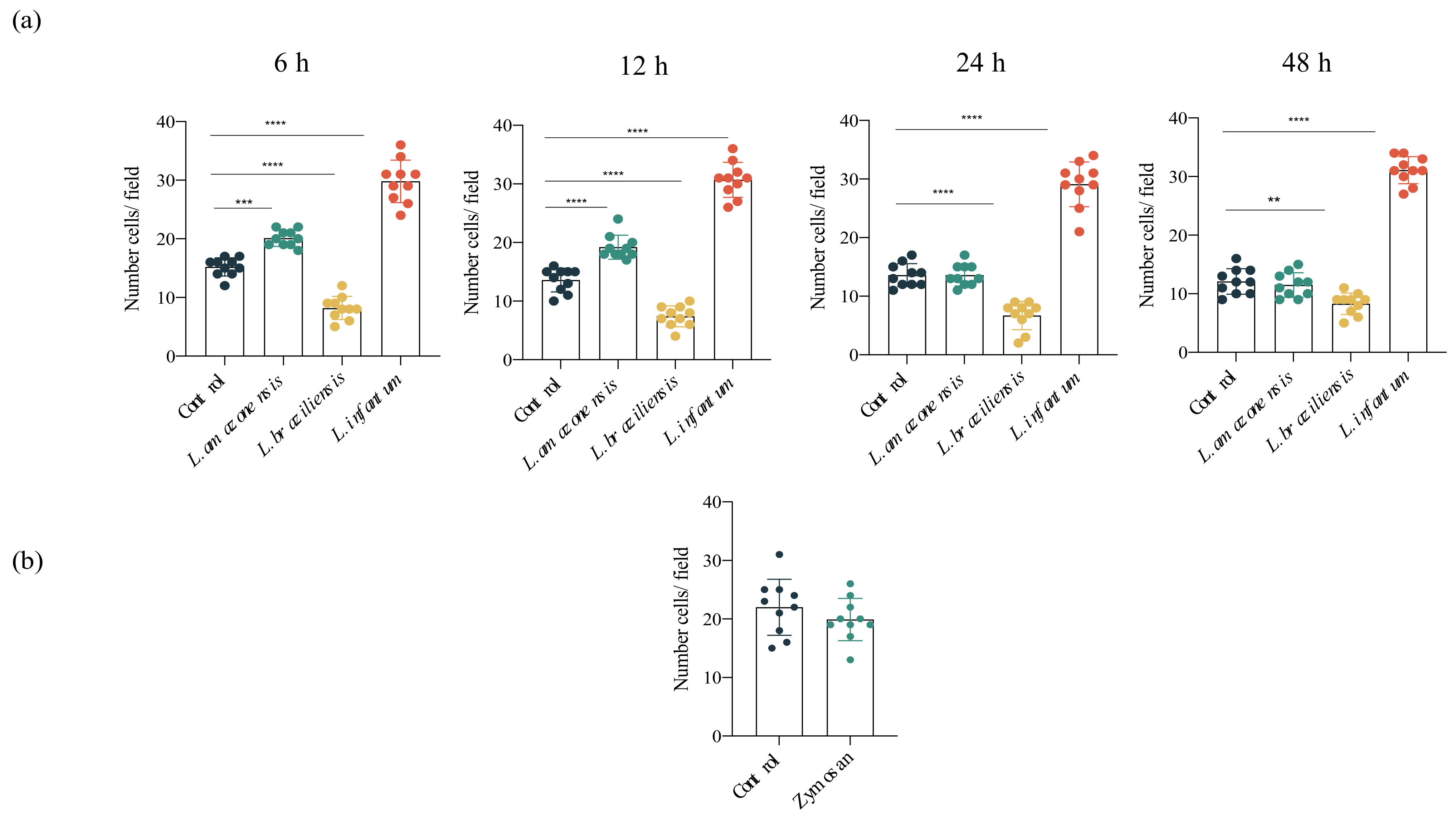
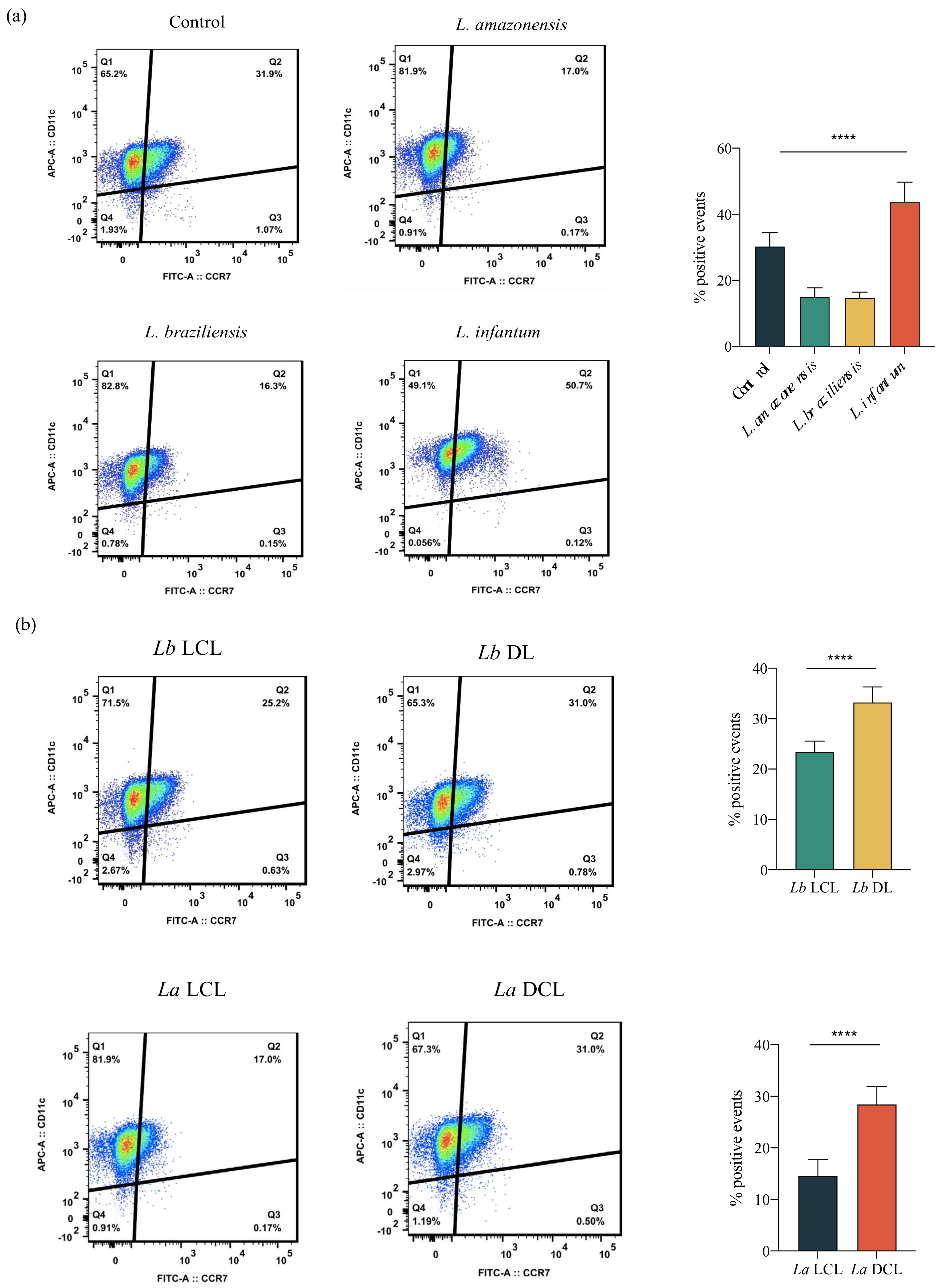
3.2. Increased hDC Migration in L. infantum Infection
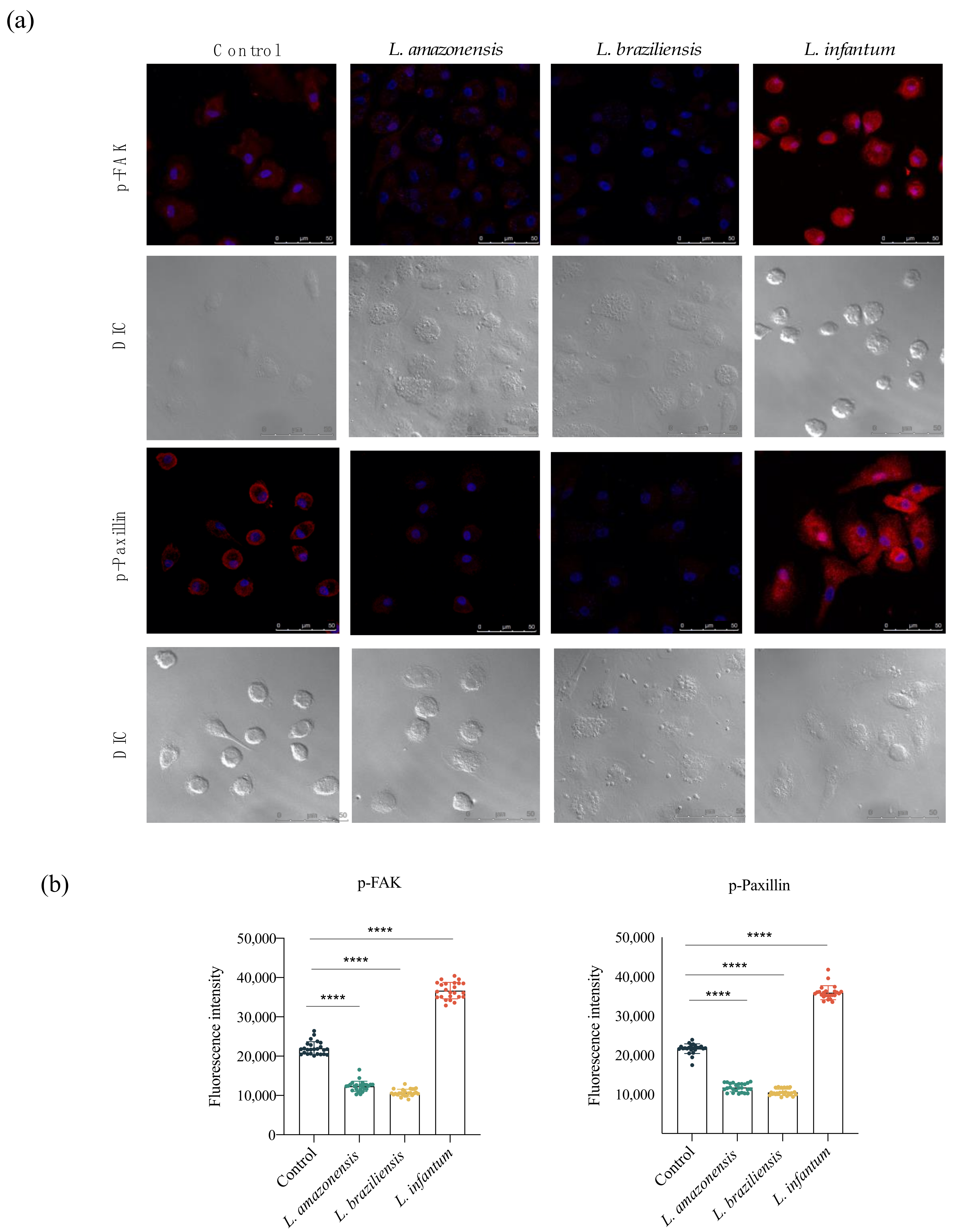
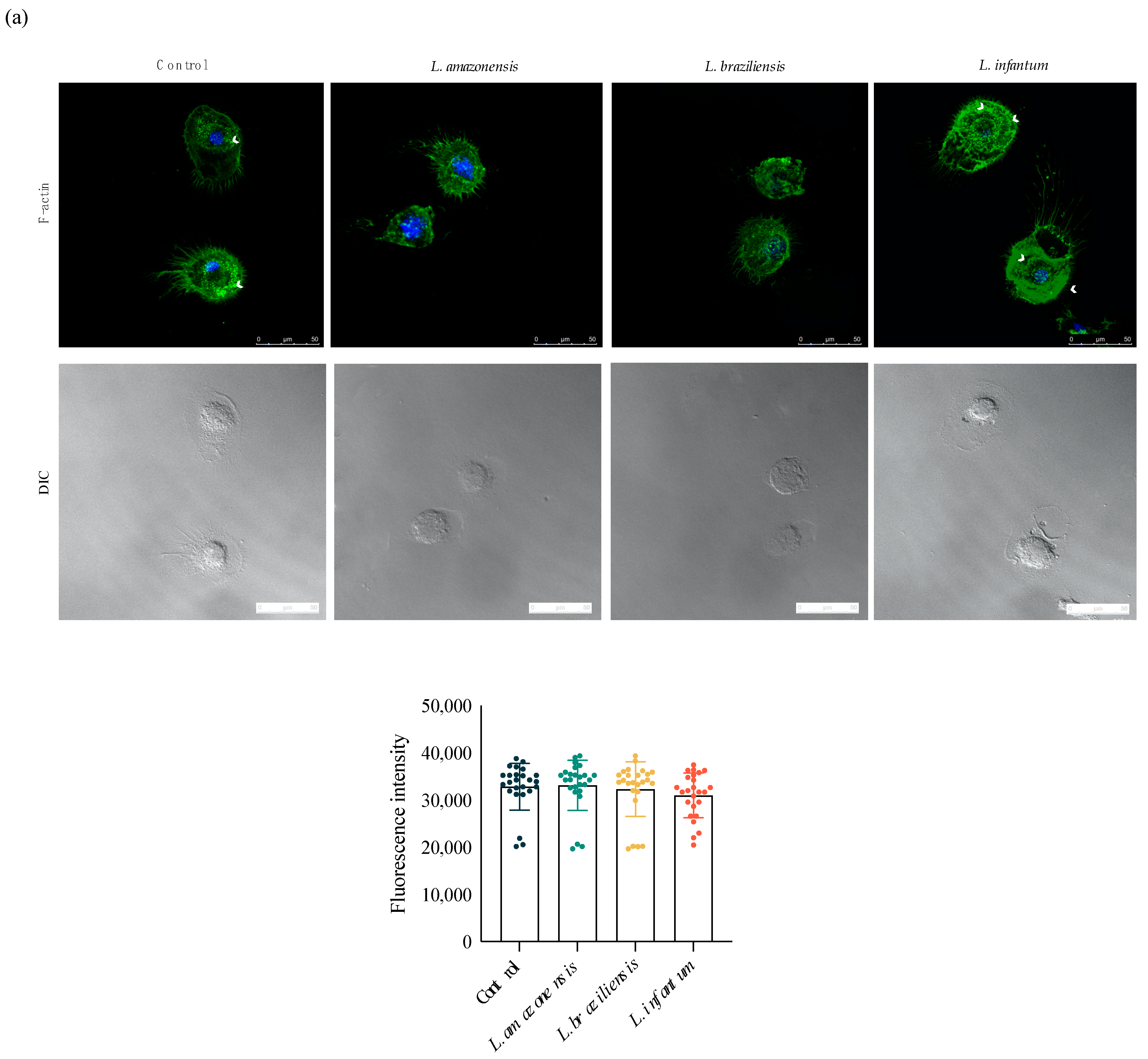
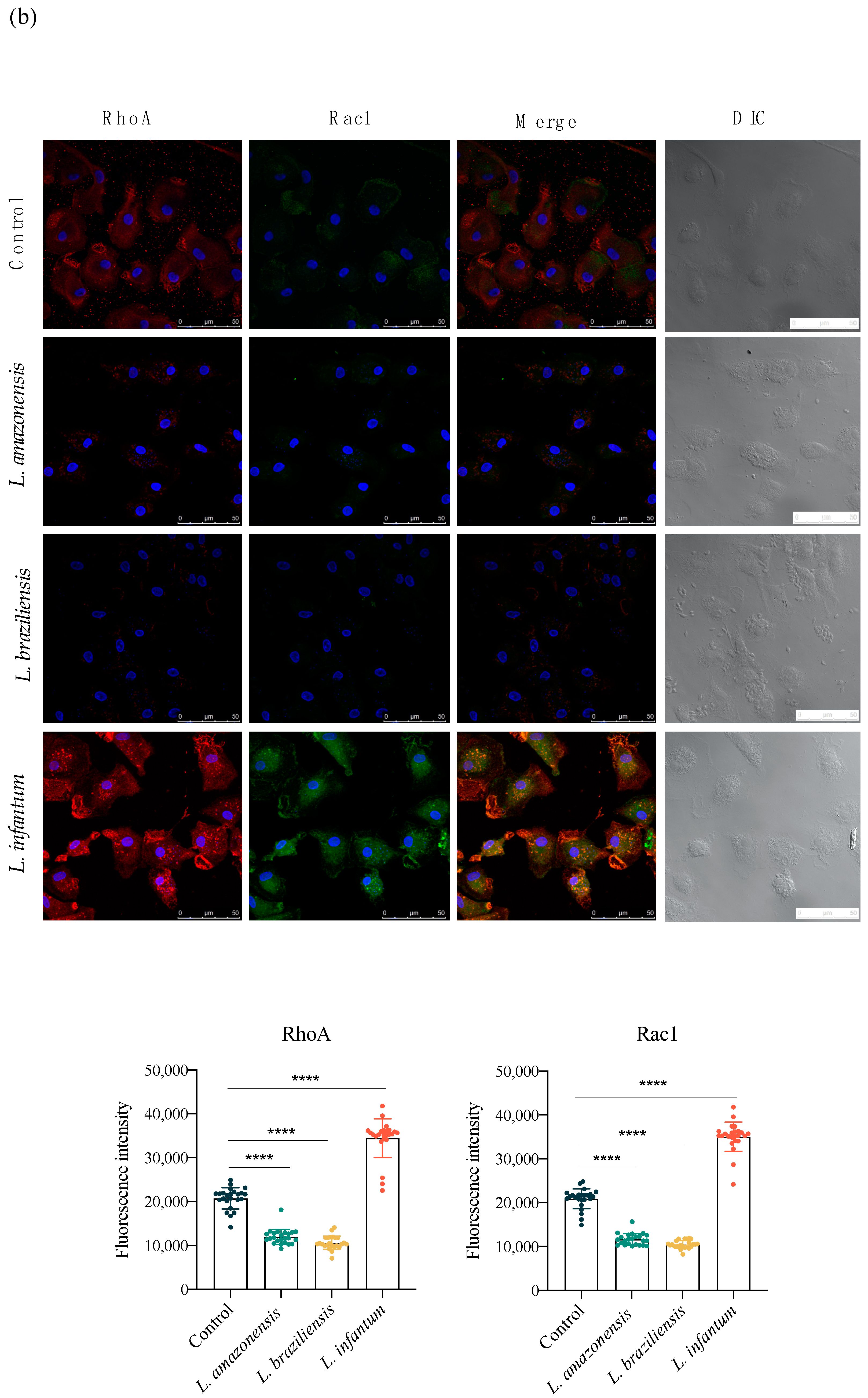
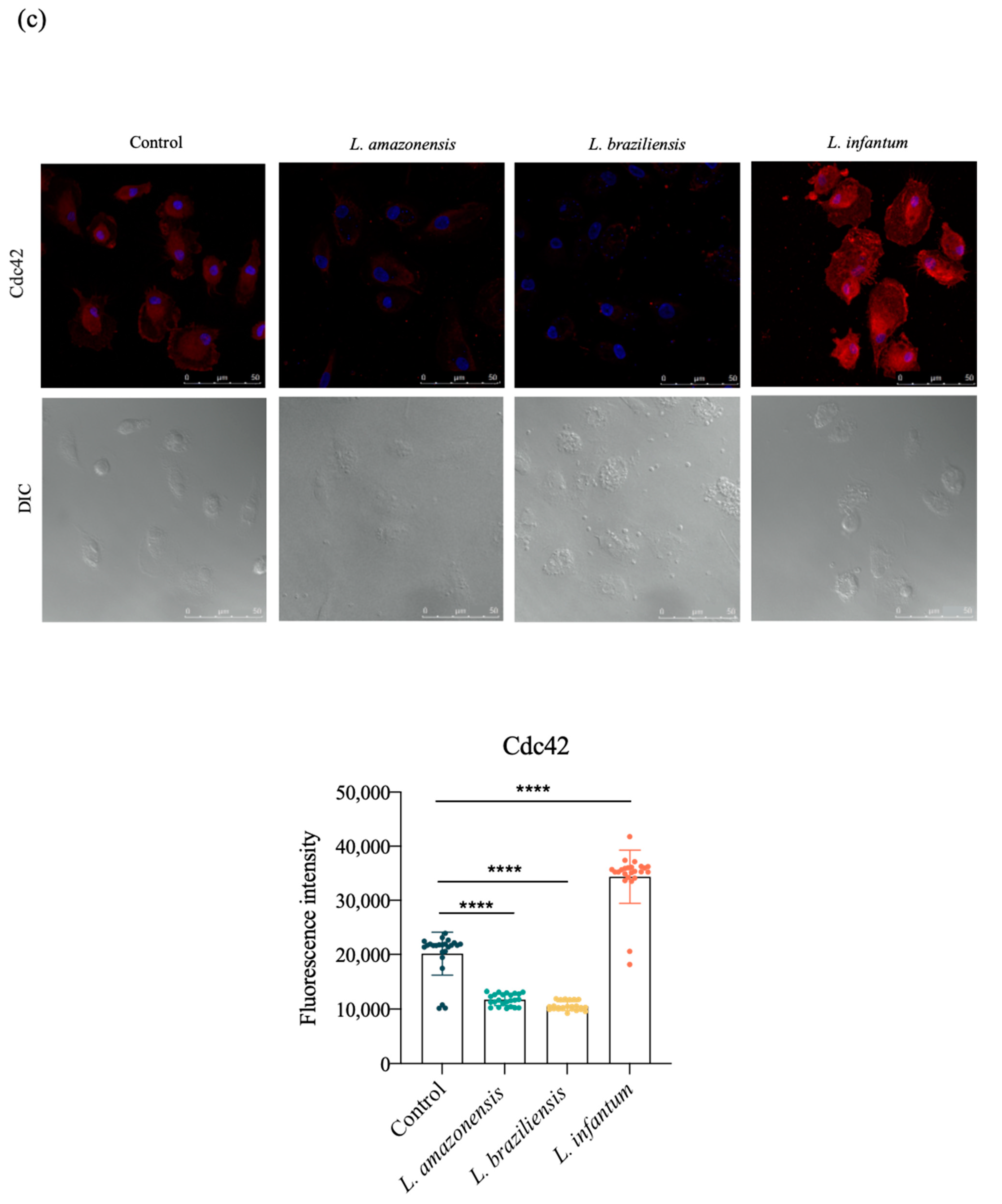
3.3. L. infantum Induces hDC Expression of Adhesion Complex Proteins and Actin Polymerization
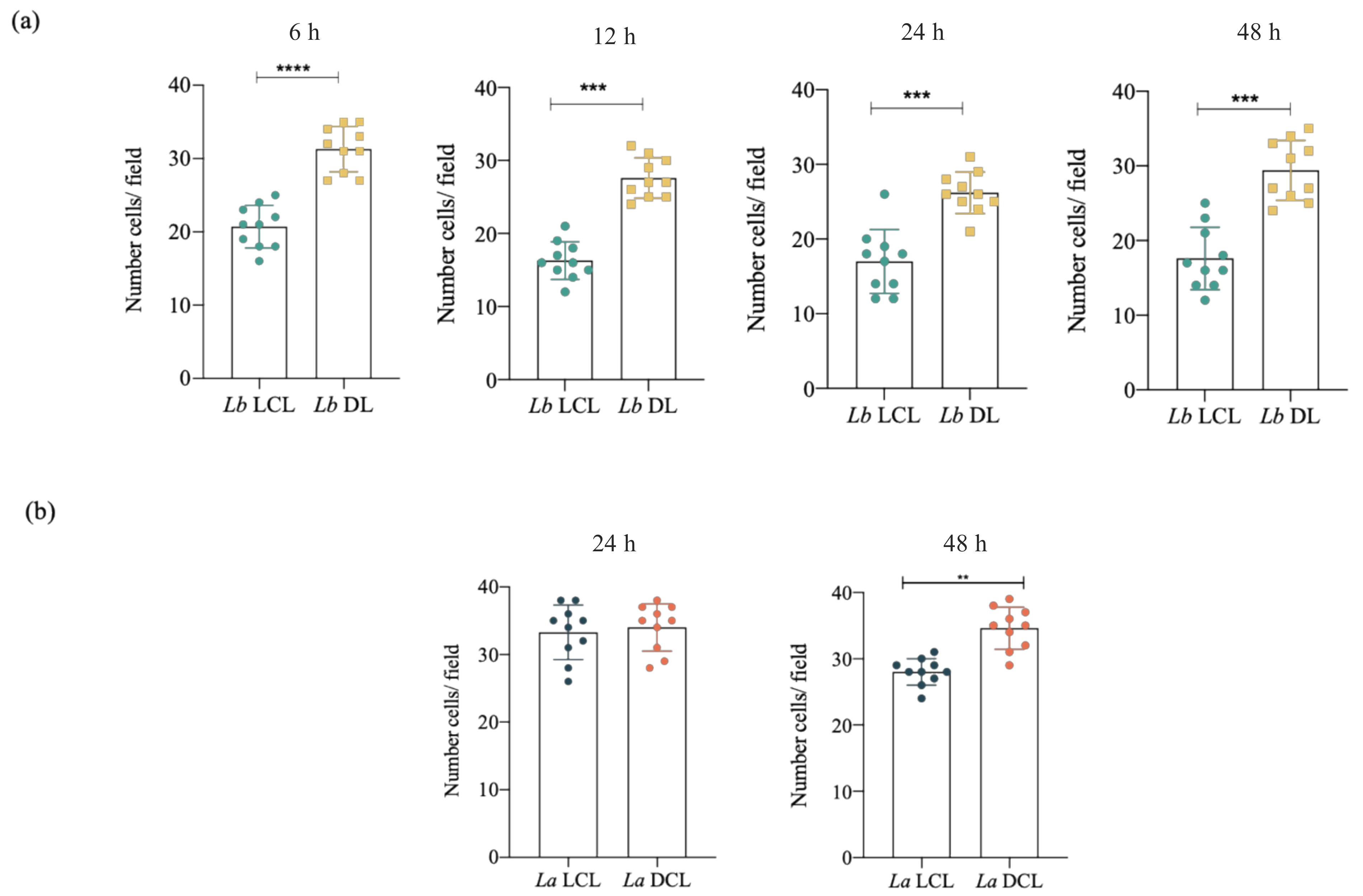
3.4. Infection with L. braziliensis (DL) and L. amazonensis (DCL) Isolates Increases hDC Migration
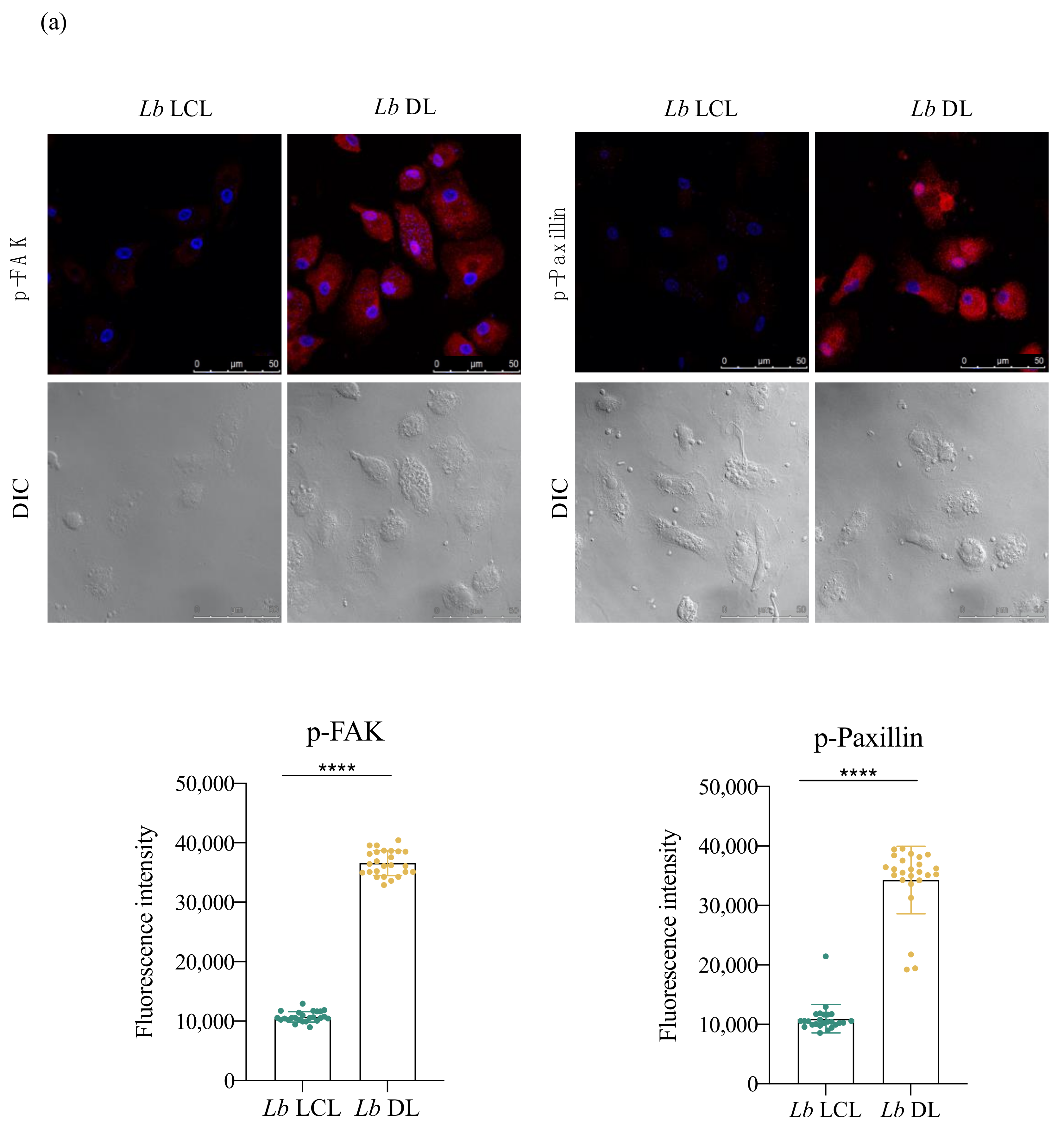
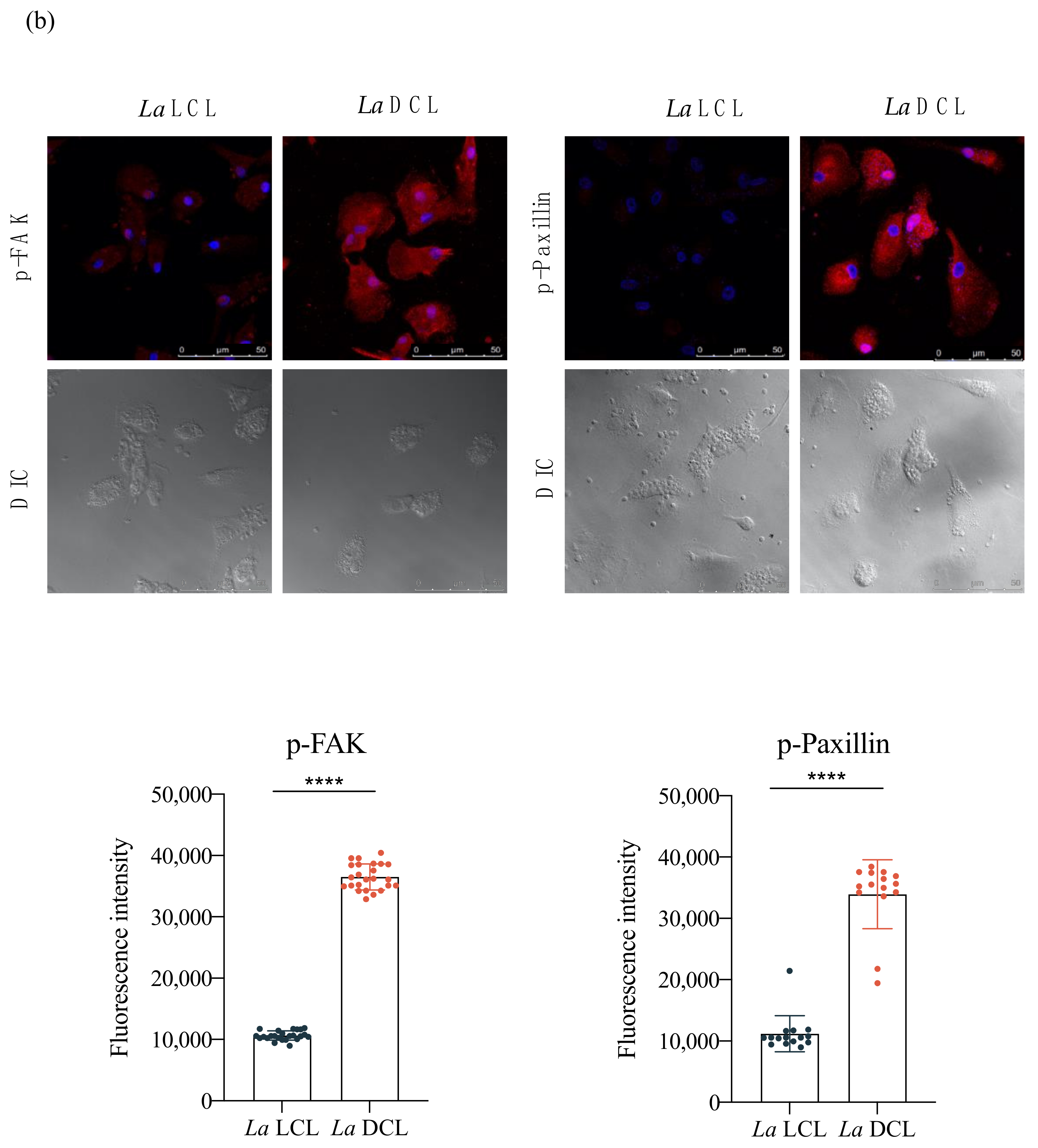
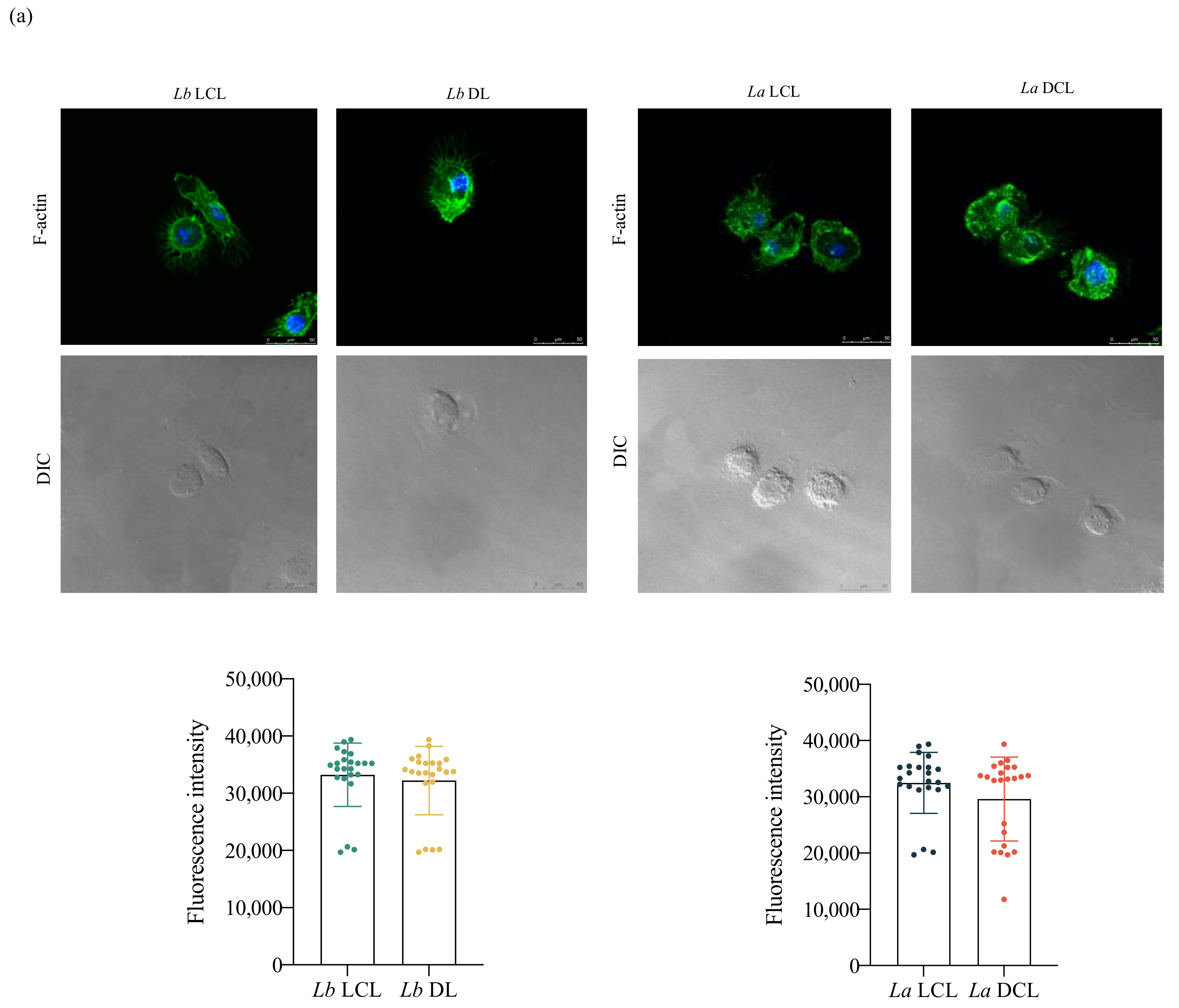
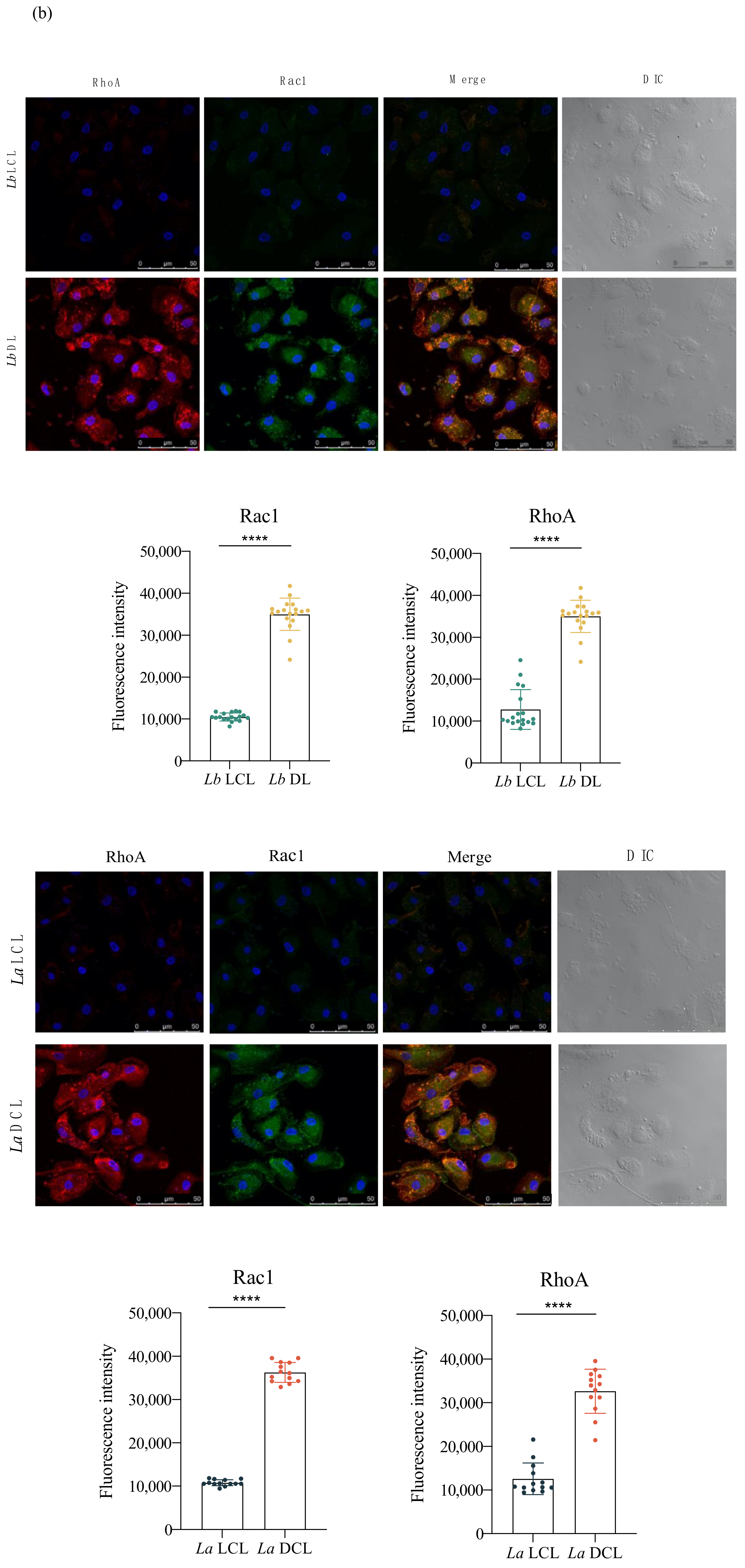
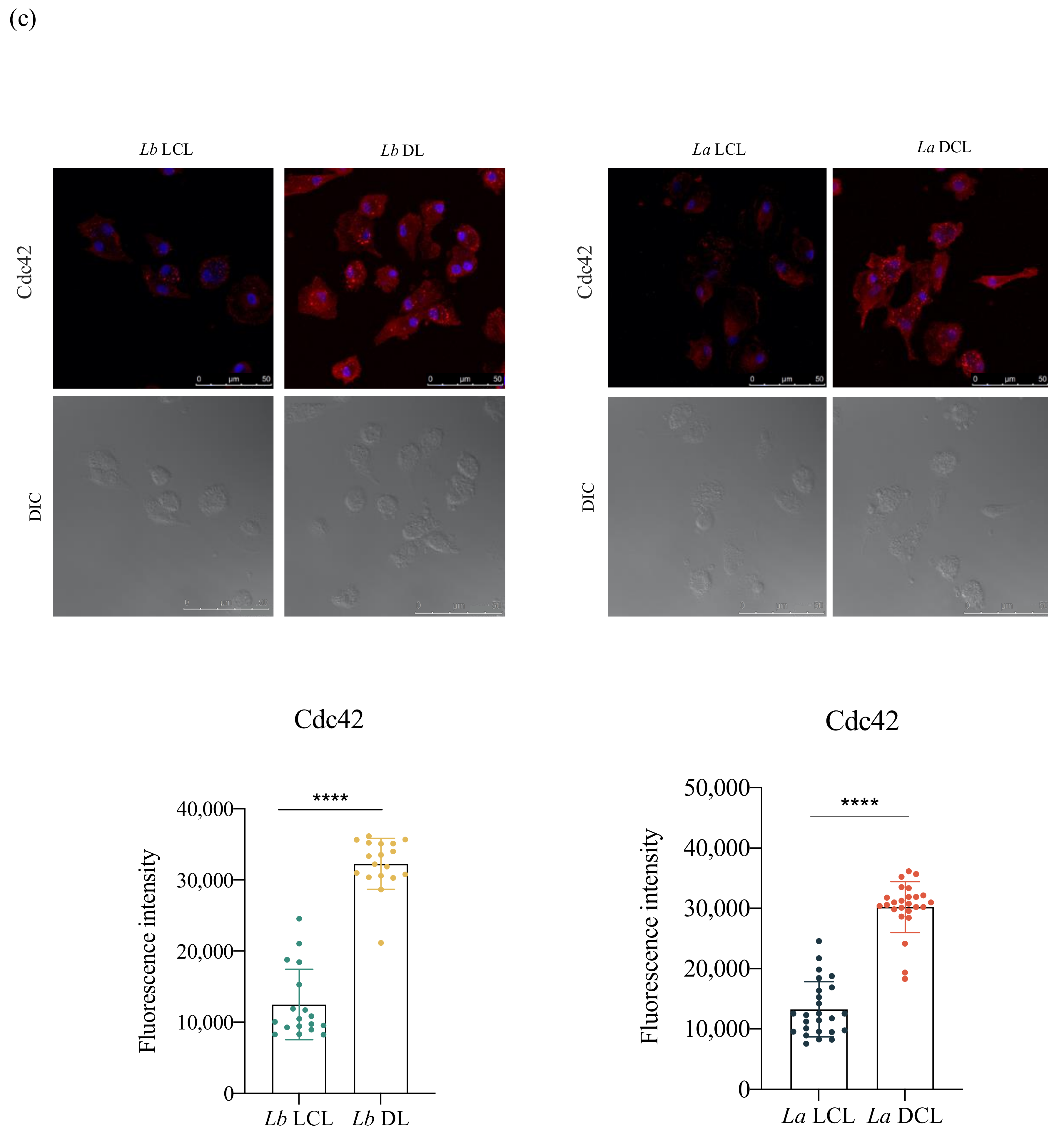
3.5. Lb DL and La DCL Isolates Induce hDC Expression of Adhesion Complex Proteins and Actin Polymerization
3.6. Infection with L. infantum, Lb DL and La DCL Isolates Induces CCR7 Expression in hDCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Control of the Leishmaniases; WHO: Geneva, Switzerland, 2010; Volume xii–xiii, pp. 1–186. [Google Scholar]
- Podinovskaia, M.; Descoteaux, A. Leishmania and the macrophage: A multifaceted interaction. Future Microbiol. 2015, 10, 111–129. [Google Scholar] [CrossRef]
- Silva, J.; Queiroz, A.; Moura, I.; Sousa, R.S.; Guimarães, L.H.; Machado, P.R.; Lessa, M.; Lago, E.; Wilson, M.E.; Schriefer, A. Dynamics of American tegumentary leishmaniasis in a highly endemic region for Leishmania (Viannia) braziliensis infection in northeast Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0006015. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Leishmania survival in the macrophage: Where the ends justify the means. Curr. Opin. Microbiol. 2015, 26, 32–40. [Google Scholar] [CrossRef]
- Bray, R.S.; Heikal, B.; Kaye, P.M.; Bray, M.A. The effect of parasitization by Leishmania mexicana mexicana on macrophage function in vitro. Acta Trop. 1983, 40, 29–38. [Google Scholar] [PubMed]
- Pinheiro, N.F., Jr.; Hermida, M.D.; Macedo, M.P.; Mengel, J.; Bafica, A.; dos-Santos, W.L. Leishmania infection impairs beta 1-integrin function and chemokine receptor expression in mononuclear phagocytes. Infect. Immun. 2006, 74, 3912–3921. [Google Scholar] [CrossRef]
- Hermida, M.D.; Doria, P.G.; Taguchi, A.M.; Mengel, J.O.; dos-Santos, W. Leishmania amazonensis infection impairs dendritic cell migration from the inflammatory site to the draining lymph node. BMC Infect. Dis. 2014, 14, 450. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, M.C.; Vilhena, V.; Barral, A.; Barral-Netto, M. Leishmanial infection: Analysis of its first steps. A review. Mem. Inst. Oswaldo Cruz 2003, 98, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Carvalhal, D.G.; Barbosa, A., Jr.; D’El-Rei Hermida, M.; Clarencio, J.; Pinheiro, N.F., Jr.; Veras, P.S.; dos-Santos, W.L. The modelling of mononuclear phagocyte-connective tissue adhesion in vitro: Application to disclose a specific inhibitory effect of Leishmania infection. Exp. Parasitol. 2004, 107, 189–199. [Google Scholar] [CrossRef]
- Ballet, R.; Emre, Y.; Jemelin, S.; Charmoy, M.; Tacchini-Cottier, F.; Imhof, B.A. Blocking junctional adhesion molecule C enhances dendritic cell migration and boosts the immune responses against Leishmania major. PLoS Pathog. 2014, 10, e1004550. [Google Scholar] [CrossRef]
- Steigerwald, M.; Moll, H. Leishmania major modulates chemokine and chemokine receptor expression by dendritic cells and affects their migratory capacity. Infect. Immun. 2005, 73, 2564–2567. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [CrossRef] [PubMed]
- Sheetz, M.P.; Felsenfeld, D.; Galbraith, C.G.; Choquet, D. Cell migration as a five-step cycle. Biochem. Soc. Symp. 1999, 65, 233–243. [Google Scholar] [PubMed]
- Gupton, S.L.; Waterman-Storer, C.M. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 2006, 125, 1361–1374. [Google Scholar] [CrossRef]
- De Fougerolles, A.R.; Koteliansky, V.E. Regulation of monocyte gene expression by the extracellular matrix and its functional implications. Immunol. Rev. 2002, 186, 208–220. [Google Scholar] [CrossRef]
- Cortesio, C.L.; Boateng, L.R.; Piazza, T.M.; Bennin, D.A.; Huttenlocher, A. Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration. J. Biol. Chem. 2011, 286, 9998–10006. [Google Scholar] [CrossRef]
- Huveneers, S.; Danen, E.H. Adhesion signaling—Crosstalk between integrins, Src and Rho. J. Cell Sci. 2009, 122, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000, 2, E231–E236. [Google Scholar] [CrossRef]
- Ren, X.D.; Kiosses, W.B.; Schwartz, M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999, 18, 578–585. [Google Scholar] [CrossRef]
- Zegers, M.M.; Friedl, P. Rho GTPases in collective cell migration. Small GTPases 2014, 5, e28997. [Google Scholar] [CrossRef]
- Ng, L.G.; Hsu, A.; Mandell, M.A.; Roediger, B.; Hoeller, C.; Mrass, P.; Iparraguirre, A.; Cavanagh, L.L.; Triccas, J.A.; Beverley, S.M.; et al. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 2008, 4, e1000222. [Google Scholar] [CrossRef]
- Leon, B.; Lopez-Bravo, M.; Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Ato, M.; Stager, S.; Engwerda, C.R.; Kaye, P.M. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat. Immunol. 2002, 3, 1185–1191. [Google Scholar] [CrossRef]
- Yano, H.; Mazaki, Y.; Kurokawa, K.; Hanks, S.K.; Matsuda, M.; Sabe, H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J. Cell Biol. 2004, 166, 283–295. [Google Scholar] [CrossRef]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef]
- Playford, M.P.; Schaller, M.D. The interplay between Src and integrins in normal and tumor biology. Oncogene 2004, 23, 7928–7946. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, A.; Horwitz, A.R. Integrins in cell migration. Cold Spring Harb. Perspect. Biol. 2011, 3, a005074. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, J.P.; Koushik, A.; Das, S.; Guven, C.; Siegel, A.; Laranjeira-Silva, M.F.; Losert, W.; Andrews, N.W. Leishmania infection inhibits macrophage motility by altering F-actin dynamics and the expression of adhesion complex proteins. Cell Microbiol. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.D.; Gerlach, B.D. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir. Res. 2017, 18, 54. [Google Scholar] [CrossRef]
- Rossier, O.M.; Gauthier, N.; Biais, N.; Vonnegut, W.; Fardin, M.A.; Avigan, P.; Heller, E.R.; Mathur, A.; Ghassemi, S.; Koeckert, M.S.; et al. Force generated by actomyosin contraction builds bridges between adhesive contacts. EMBO J. 2010, 29, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Sander, E.E.; ten Klooster, J.P.; van Delft, S.; van der Kammen, R.A.; Collard, J.G. Rac downregulates Rho activity: Reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 1999, 147, 1009–1022. [Google Scholar] [CrossRef]
- Sanchez-Barinas, C.D.; Ocampo, M.; Vanegas, M.; Castaneda-Ramirez, J.J.; Patarroyo, M.A.; Patarroyo, M.E. Mycobacterium tuberculosis H37Rv LpqG Protein Peptides Can Inhibit Mycobacterial Entry through Specific Interactions. Molecules 2018, 23, 526. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.P.; Wells, C.M.; Smith, S.D.; Vega, F.M.; Henderson, R.B.; Tybulewicz, V.L.; Ridley, A.J. Rac1 and Rac2 regulate macrophage morphology but are not essential for migration. J. Cell Sci. 2006, 119, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Lavina, B.; Castro, M.; Niaudet, C.; Cruys, B.; Alvarez-Aznar, A.; Carmeliet, P.; Bentley, K.; Brakebusch, C.; Betsholtz, C.; Gaengel, K. Defective endothelial cell migration in the absence of Cdc42 leads to capillary-venous malformations. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Franca-Costa, J.; Wanderley, J.L.; Deolindo, P.; Zarattini, J.B.; Costa, J.; Soong, L.; Barcinski, M.A.; Barral, A.; Borges, V.M. Exposure of phosphatidylserine on Leishmania amazonensis isolates is associated with diffuse cutaneous leishmaniasis and parasite infectivity. PLoS ONE 2012, 7, e36595. [Google Scholar] [CrossRef][Green Version]
- Tiberio, L.; Del Prete, A.; Schioppa, T.; Sozio, F.; Bosisio, D.; Sozzani, S. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol. Immunol. 2018, 15, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Ohl, L.; Mohaupt, M.; Czeloth, N.; Hintzen, G.; Kiafard, Z.; Zwirner, J.; Blankenstein, T.; Henning, G.; Forster, R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 2004, 21, 279–288. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebouças, A.; Silva, T.S.; Medina, L.S.; Paredes, B.D.; Aragão, L.S.; Souza, B.S.F.; Borges, V.M.; Schriefer, A.; Veras, P.S.T.; Brodskyn, C.I.; et al. Leishmania-Induced Dendritic Cell Migration and Its Potential Contribution to Parasite Dissemination. Microorganisms 2021, 9, 1268. https://doi.org/10.3390/microorganisms9061268
Rebouças A, Silva TS, Medina LS, Paredes BD, Aragão LS, Souza BSF, Borges VM, Schriefer A, Veras PST, Brodskyn CI, et al. Leishmania-Induced Dendritic Cell Migration and Its Potential Contribution to Parasite Dissemination. Microorganisms. 2021; 9(6):1268. https://doi.org/10.3390/microorganisms9061268
Chicago/Turabian StyleRebouças, Amanda, Thaílla S. Silva, Lilian S. Medina, Bruno D. Paredes, Luciana S. Aragão, Bruno S. F. Souza, Valéria M. Borges, Albert Schriefer, Patricia S. T. Veras, Claudia I. Brodskyn, and et al. 2021. "Leishmania-Induced Dendritic Cell Migration and Its Potential Contribution to Parasite Dissemination" Microorganisms 9, no. 6: 1268. https://doi.org/10.3390/microorganisms9061268
APA StyleRebouças, A., Silva, T. S., Medina, L. S., Paredes, B. D., Aragão, L. S., Souza, B. S. F., Borges, V. M., Schriefer, A., Veras, P. S. T., Brodskyn, C. I., & de Menezes, J. P. B. (2021). Leishmania-Induced Dendritic Cell Migration and Its Potential Contribution to Parasite Dissemination. Microorganisms, 9(6), 1268. https://doi.org/10.3390/microorganisms9061268





