Exogenous Polyamines Influence In Vitro Microbial Adhesion to Human Mucus According to the Age of Mucus Donor
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Isolation of Human Intestinal Mucus
2.3. Polyamine Preparations
2.4. In Vitro Adhesion Assay
2.5. Statistical Analysis
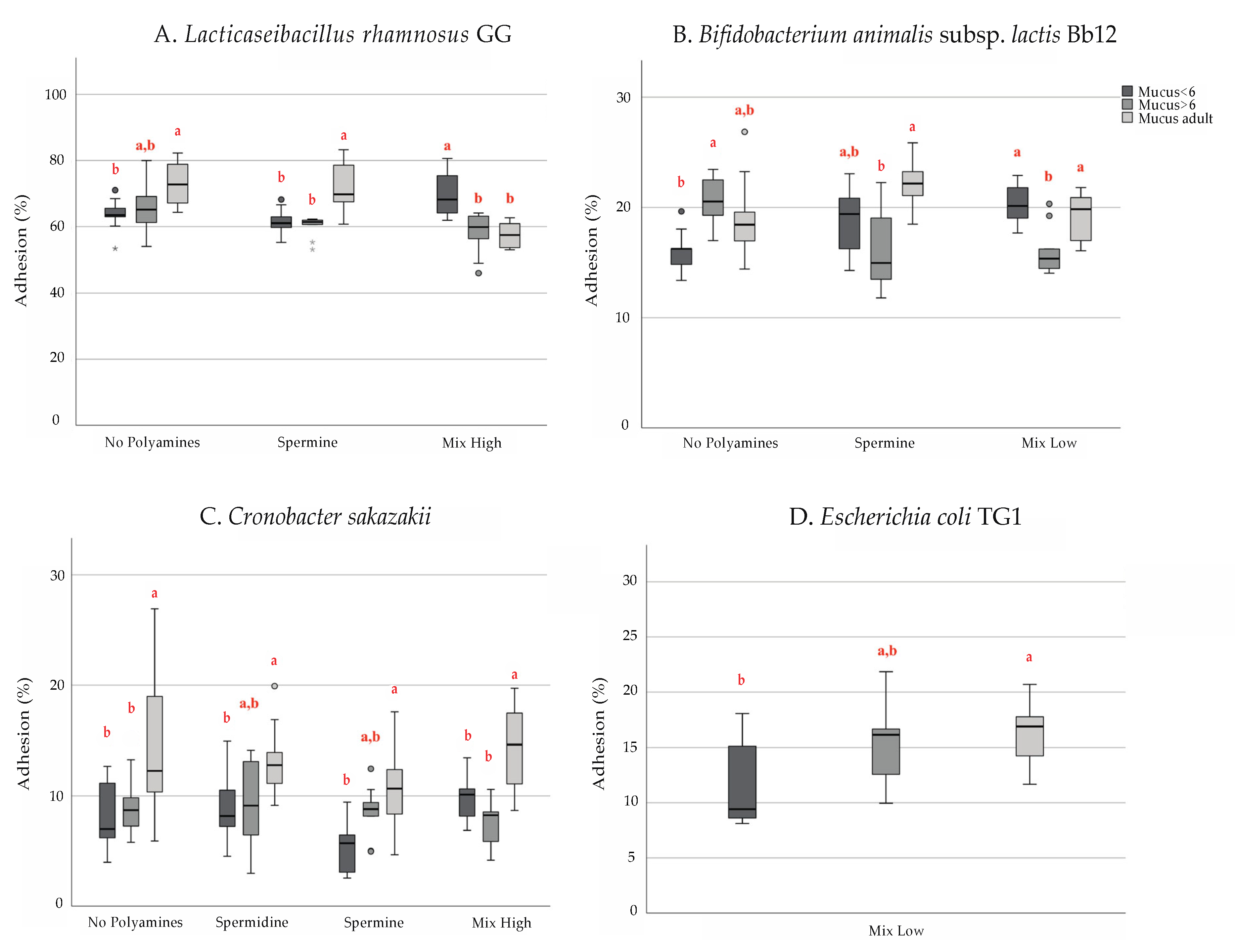
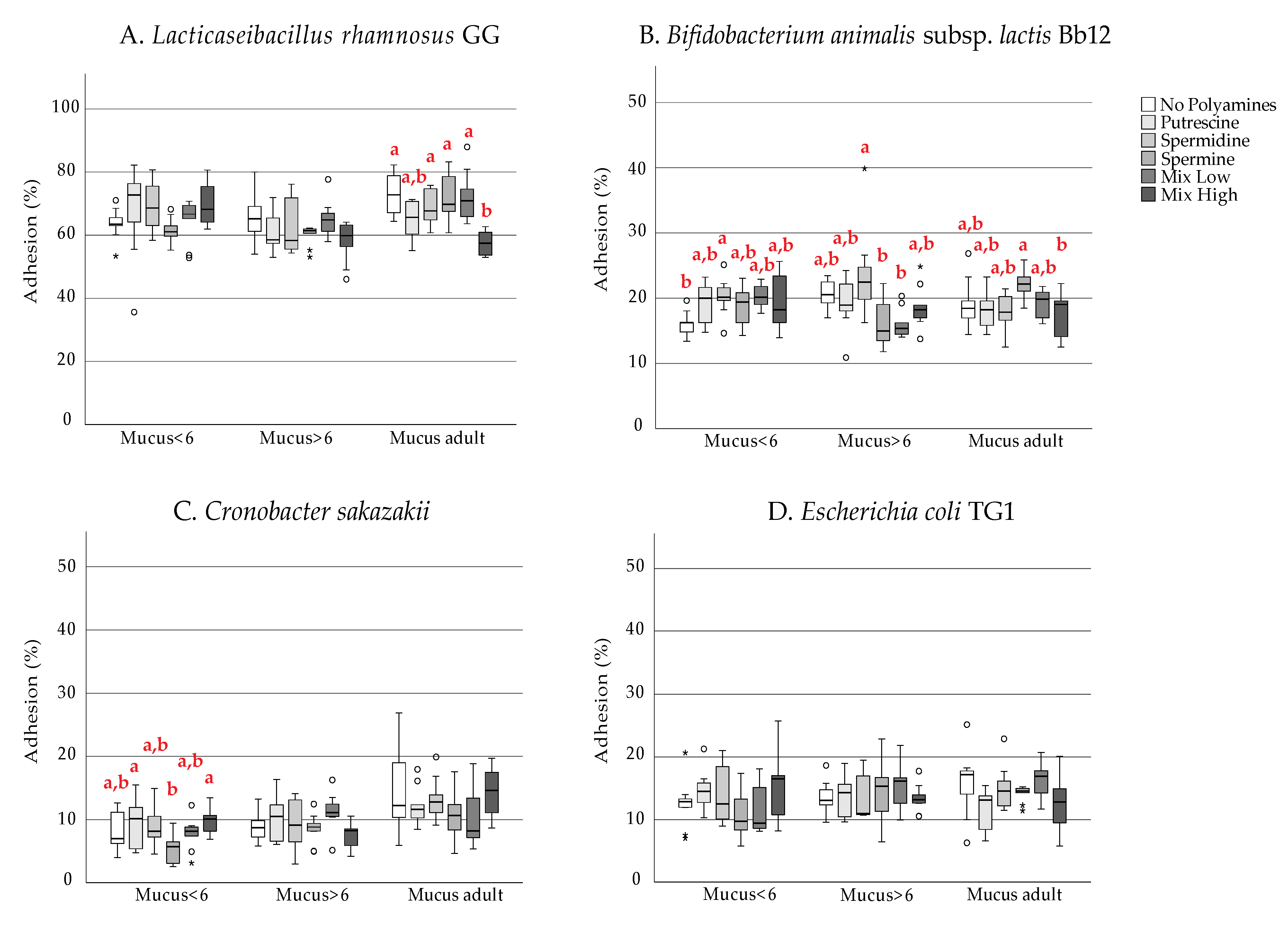
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Mammalian Polyamine Metabolism and Function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef]
- Timmons, J. Polyamines and Gut Mucosal Homeostasis. J. Gastrointest. Dig. Syst. 2013, 3. [Google Scholar] [CrossRef]
- Fang, T.; Liu, G.; Cao, W.; Wu, X.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Wang, J. Spermine: New Insights into the Intestinal Development and Serum Antioxidant Status of Suckling Piglets. RSC Adv. 2016, 6, 31323–31335. [Google Scholar] [CrossRef]
- Van Wettere, W.H.E.J.; Willson, N.-L.; Pain, S.J.; Forder, R.E.A. Effect of Oral Polyamine Supplementation Pre-Weaning on Piglet growth and Intestinal Characteristics. Animal 2016, 10, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Löser, C.; Eisel, A.; Harms, D.; Fölsch, U.R. Dietary Polyamines Are Essential Luminal Growth Factors for Small Intestinal and Colonic Mucosal Growth and Development. Gut 1999, 44, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Rogers, S.; Lausten-Thomsen, U.; Björkbom, A.; Laursen, S.S.; Courraud, J.; Børglum, A.; Nordentoft, M.; Werge, T.; Mortensen, P.B.; et al. Gestational-Age-Dependent Development of the Neonatal Metabolome. medRxiv 2020. [Google Scholar] [CrossRef]
- Plaza-Zamora, J.; Sabater-Molina, M.; Rodríguez-Palmero, M.; Rivero, M.; Bosch, V.; Nadal, J.M.; Zamora, S.; Larqué, E. Polyamines in Human Breast Milk for Preterm and Term Infants. Br. J. Nutr. 2013, 110, 524–528. [Google Scholar] [CrossRef]
- Taylor, S.N.; Basile, L.A.; Ebeling, M.; Wagner, C.L. Intestinal Permeability in Preterm Infants by Feeding Type: Mother’s Milk Versus Formula. Breastfeed. Med. 2009, 4, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, R.S.; Cleveland, J.L.; Epling-Burnette, P.K. Role of Polyamines in Immune Cell Functions. Med. Sci. 2018, 6, 22. [Google Scholar] [CrossRef]
- Schuster, I.; Bernhardt, R. Interactions of Natural Polyamines with Mammalian Proteins. Biomol. Concepts 2011, 2, 79–94. [Google Scholar] [CrossRef]
- Bekebrede, A.F.; Keijer, J.; Gerrits, W.J.J.; de Boer, V.C.J. The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine. Nutrients 2020, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Hanfrey, C.C.; Pearson, B.M.; Hazeldine, S.; Lee, J.; Gaskin, D.J.; Woster, P.M.; Phillips, M.A.; Michael, A.J. Alternative Spermidine Biosynthetic Route Is Critical for Growth of Campylobacter Jejuni and Is the Dominant Polyamine Pathway in Human Gut Microbiota *. J. Biol. Chem. 2011, 286, 43301–43312. [Google Scholar] [CrossRef]
- Kitada, Y.; Muramatsu, K.; Toju, H.; Kibe, R.; Benno, Y.; Kurihara, S.; Matsumoto, M. Bioactive Polyamine Production by a Novel Hybrid System Comprising Multiple Indigenous Gut Bacterial Strategies. Sci. Adv. 2018, 4, eaat0062. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kurihara, S.; Kibe, R.; Ashida, H.; Benno, Y. Longevity in Mice Is Promoted by Probiotic-Induced Suppression of Colonic Senescence Dependent on Upregulation of Gut Bacterial Polyamine Production. PLoS ONE 2011, 6, e23652. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. The Functional Role of Polyamines in Eukaryotic Cells. Int. J. Biochem. Cell Biol. 2019, 107, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and Gut Microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef]
- Di Martino, M.L.; Campilongo, R.; Casalino, M.; Micheli, G.; Colonna, B.; Prosseda, G. Polyamines: Emerging Players in Bacteria-Host Interactions. Int. J. Med. Microbiol. 2013, 303, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Banerji, R.; Kanojiya, P.; Patil, A.; Saroj, S.D. Polyamines in the Virulence of Bacterial Pathogens of Respiratory Tract. Mol. Oral Microbiol. 2021, 36, 1–11. [Google Scholar] [CrossRef]
- McCurtain, J.L.; Gilbertsen, A.J.; Evert, C.; Williams, B.J.; Hunter, R.C. Agmatine Accumulation by Pseudomonas Aeruginosa Clinical Isolates Confers Antibiotic Tolerance and Dampens Host Inflammation. J. Med. Microbiol. 2019, 68, 446–455. [Google Scholar] [CrossRef]
- Johnson, L.; Mulcahy, H.; Kanevets, U.; Shi, Y.; Lewenza, S. Surface-Localized Spermidine Protects the Pseudomonas Aeruginosa Outer Membrane from Antibiotic Treatment and Oxidative Stress. J. Bacteriol. 2012, 194, 813–826. [Google Scholar] [CrossRef]
- Nanduri, B.; Swiatlo, E. The Expansive Effects of Polyamines on the Metabolism and Virulence of Streptococcus Pneumoniae. Pneumonia 2021, 13, 4. [Google Scholar] [CrossRef]
- Shah, P.; Nanduri, B.; Swiatlo, E.; Ma, Y.; Pendarvis, K. Polyamine Biosynthesis and Transport Mechanisms Are Crucial for Fitness and Pathogenesis of Streptococcus Pneumoniae. Microbiology 2011, 157, 504–515. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Nara, M.; Sakanaka, M.; Kitakata, A.; Okuda, S.; Kurihara, S. Analysis of Polyamine Biosynthetic- and Transport Ability of Human Indigenous Bifidobacterium. Biosci. Biotechnol. Biochem. 2018, 82, 1606–1614. [Google Scholar] [CrossRef]
- Mikelsaar, M.; Stsepetova, J.; Mikelsaar, R.-H.; Truusalu, K.; Smidt, I.; Hütt, P.; Rätsep, M.; Songisepp, E. Polyamines of Human Strain Lactobacillus Plantarum Inducia Induce Modulation of Innate Immune Markers. J. Funct. Foods 2020, 72, 104064. [Google Scholar] [CrossRef]
- Garcia, A.F.; Benchimol, M.; Alderete, J.F. Trichomonas Vaginalis Polyamine Metabolism Is Linked to Host Cell Adherence and Cytotoxicity. Infect. Immun. 2005, 73, 2602–2610. [Google Scholar] [CrossRef]
- Harata, G.; Yoda, K.; Wang, R.; Miyazawa, K.; Sato, M.; He, F.; Endo, A. Species- and Age/Generation-Dependent Adherence of Bifidobacterium Bifidum to Human Intestinal Mucus In Vitro. Microorganisms 2021, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gallego, C.; Recio, I.; Gómez-Gómez, V.; Ortuño, I.; Bernal, M.J.; Ros, G.; Periago, M.J. Effect of Processing on Polyamine Content and Bioactive Peptides Released after in Vitro Gastrointestinal Digestion of Infant Formulas. J. Dairy Sci. 2016, 99, 924–932. [Google Scholar] [CrossRef]
- Gómez-Gallego, C.; Kumar, H.; García-Mantrana, I.; du Toit, E.; Suomela, J.-P.; Linderborg, K.M.; Zhang, Y.; Isolauri, E.; Yang, B.; Salminen, S.; et al. Breast Milk Polyamines and Microbiota Interactions: Impact of Mode of Delivery and Geographical Location. Ann. Nutr. Metab. 2017, 70, 184–190. [Google Scholar] [CrossRef]
- Mantziari, A.; Tölkkö, S.; Ouwehand, A.C.; Löyttyniemi, E.; Isolauri, E.; Salminen, S.; Rautava, S. The Effect of Donor Human Milk Fortification on the Adhesion of Probiotics in Vitro. Nutrients 2020, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Specian, R.D.; Neutra, M.R. Regulation of Intestinal Goblet Cell Secretion. I. Role of Parasympathetic Stimulation. Am. J. Physiol.-Gastrointest. Liver Physiol. 1982, 242, G370–G379. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Niemi, P.; Salminen, S.J. The Normal Faecal Microflora Does Not Affect the Adhesion of Probiotic Bacteria in Vitro. FEMS Microbiol. Lett. 1999, 177, 35–38. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Isolauri, E.; Kirjavainen, P.V.; Tölkkö, S.; Salminen, S.J. The Mucus Binding of Bifidobacterium Lactis Bb12 Is Enhanced in the Presence of Lactobacillus GG and Lact. Delbrueckii Subsp. Bulgaricus. Lett. Appl. Microbiol. 2000, 30, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Benno, Y. The Relationship between Microbiota and Polyamine Concentration in the Human Intestine: A Pilot Study. Microbiol. Immunol. 2007, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Gueimonde, M.; Sanz, Y.; Salminen, S. Adhesion Properties and Competitive Pathogen Exclusion Ability of Bifidobacteria with Acquired Acid Resistance. J. Food Prot. 2006, 69, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Gueimonde, M.; Hernández, M.; Sanz, Y.; Salminen, S. Adhesion of Selected Bifidobacterium Strains to Human Intestinal Mucus and the Role of Adhesion in Enteropathogen Exclusion. J. Food Prot. 2005, 68, 2672–2678. [Google Scholar] [CrossRef]
- Gréco, S.; Niepceron, E.; Hugueny, I.; George, P.; Louisot, P.; Biol, M.-C. Dietary Spermidine and Spermine Participate in the Maturation of Galactosyltransferase Activity and Glycoprotein Galactosylation in Rat Small Intestine. J. Nutr. 2001, 131, 1890–1897. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shub, M.D.; Pang, K.Y.; Swann, D.A.; Walker, W.A. Age-Related Changes in Chemical Composition and Physical Properties of Mucus Glycoproteins from Rat Small Intestine. Biochem. J. 1983, 215, 405–411. [Google Scholar] [CrossRef]
- Midtvedt, A.-C.; Carlstedt-Duke, B.; Midtvedt, T. Establishment of a Mucin-Degrading Intestinal Microflora During the First Two Years of Human Life. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 321. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. The Biology of Mucus: Composition, Synthesis and Organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef]
- Ioannidis, N.E.; Kotzabasis, K. Effects of Polyamines on the Functionality of Photosynthetic Membrane in Vivo and in Vitro. Biochim. Biophys. Acta BBA-Bioenerg. 2007, 1767, 1372–1382. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Salminen, S. Specific Probiotic Strains and Their Combinations Counteract Adhesion of Enterobacter Sakazakii to Intestinal Mucus. FEMS Microbiol. Lett. 2008, 285, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Limenitakis, J.P.; Fuhrer, T.; Geuking, M.B.; Lawson, M.A.; Wyss, M.; Brugiroux, S.; Keller, I.; Macpherson, J.A.; Rupp, S.; et al. The Outer Mucus Layer Hosts a Distinct Intestinal Microbial Niche. Nat. Commun. 2015, 6, 8292. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.L.; Avelino, F.; Xicohtencatl-Cortes, J.; Girón, J.A. Host Protein Binding and Adhesive Properties of H6 and H7 Flagella of Attaching and Effacing Escherichia Coli. J. Bacteriol. 2007, 189, 7426–7435. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G.; Vazquez-Juarez, R.C.; Tutt, C.B.; Garcia-Gallegos, J.G. Pathoadaptive Mutation That Mediates Adherence of Shiga Toxin-Producing Escherichia Coli O111. Infect. Immun. 2005, 73, 4766–4776. [Google Scholar] [CrossRef] [PubMed]
- Taddei, C.R.; Oliveira, F.F.; Duarte, R.T.D.; Talarico, S.T.; Takagi, E.H.; Ramos Carvalho, I.I.; Gomes, F.M.S.; Brandt, K.; Martinez, M.B. High Abundance of Escherichia During the Establishment of Fecal Microbiota in Brazilian Children. Microb. Ecol. 2014, 67, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzian, F.; Hesselmar, B.; Saalman, R.; Strannegård, I.-L.; Åberg, N.; Wold, A.E.; Adlerberth, I. Escherichia Coli in Infants’ Intestinal Microflora: Colonization Rate, Strain Turnover, and Virulence Gene Carriage. Pediatr. Res. 2003, 54, 8–14. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; van den Brandt, P.A.; Kummeling, I.; Snijders, B.; Stelma, F.; Adams, H.; van Ree, R.; Stobberingh, E.E. Gut Microbiota Composition and Development of Atopic Manifestations in Infancy: The KOALA Birth Cohort Study. Gut 2007, 56, 661–667. [Google Scholar] [CrossRef]
- Penders, J.; Stobberingh, E.E.; Thijs, C.; Adams, H.; Vink, C.; Ree, R.V.; Brandt, P.A.V.D. Molecular Fingerprinting of the Intestinal Microbiota of Infants in Whom Atopic Eczema Was or Was Not Developing. Clin. Exp. Allergy 2006, 36, 1602–1608. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantziari, A.; Mannila, E.; Collado, M.C.; Salminen, S.; Gómez-Gallego, C. Exogenous Polyamines Influence In Vitro Microbial Adhesion to Human Mucus According to the Age of Mucus Donor. Microorganisms 2021, 9, 1239. https://doi.org/10.3390/microorganisms9061239
Mantziari A, Mannila E, Collado MC, Salminen S, Gómez-Gallego C. Exogenous Polyamines Influence In Vitro Microbial Adhesion to Human Mucus According to the Age of Mucus Donor. Microorganisms. 2021; 9(6):1239. https://doi.org/10.3390/microorganisms9061239
Chicago/Turabian StyleMantziari, Anastasia, Enni Mannila, Maria Carmen Collado, Seppo Salminen, and Carlos Gómez-Gallego. 2021. "Exogenous Polyamines Influence In Vitro Microbial Adhesion to Human Mucus According to the Age of Mucus Donor" Microorganisms 9, no. 6: 1239. https://doi.org/10.3390/microorganisms9061239
APA StyleMantziari, A., Mannila, E., Collado, M. C., Salminen, S., & Gómez-Gallego, C. (2021). Exogenous Polyamines Influence In Vitro Microbial Adhesion to Human Mucus According to the Age of Mucus Donor. Microorganisms, 9(6), 1239. https://doi.org/10.3390/microorganisms9061239





