Reliable and Sensitive Nested PCR for the Detection of Chlamydia in Sputum
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. DNA Analysis
2.3. PCR
2.4. Analysis of PCR Products and Their DNA Sequences
3. Results
3.1. Pros and Cons of Published Nested PCR Assays
3.2. Primer Design, Optimization
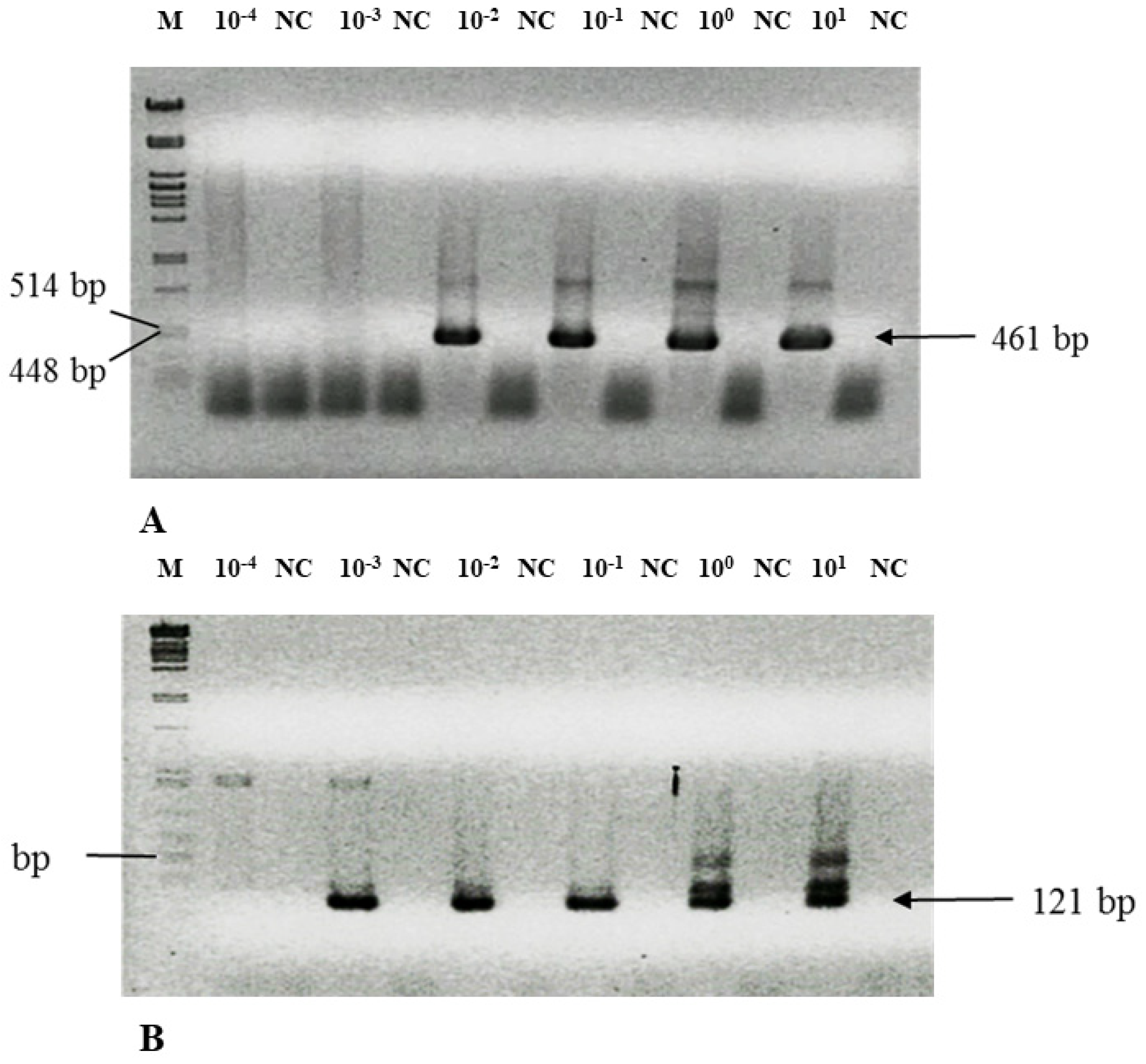
3.3. Nested PCR Is Highly Sensitive—Detection Limit
3.4. Solving Technical Pitfalls—Scale of DNA Degradation
3.5. Detection of Chlamydial DNA in Sputum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roulis, E.; Polkinghorne, A.; Timms, P. Chlamydia pneumoniae: Modern insights into an ancient pathogen. Trends Microbiol. 2013, 21, 120–128. [Google Scholar] [CrossRef]
- Loens, K.; Ieven, M. Using Nucleic Acid Amplification Techniques in a Syndrome-Oriented Approach: Detection of Respiratory Agents. In Molecular Microbiology: Diagnostic Principles and Practise, 3th ed.; Persing, D., Tenover, F., Hayden, R., Ieven, M., Miller, M., Nolte, F., Tang, Y., van Belkum, A., Eds.; ASM Press: Washington, DC, USA, 2016; Chapter 25; pp. 306–335. [Google Scholar]
- Benitez, A.J.; Thurman, K.A.; Diaz, M.H.; Conklin, L.; Kendig, N.E.; Winchell, J.M. Comparison of real-time PCR and a microimmunofluorescence serological assay for detection of Chlamydophila pneumoniae infection in an outbreak investigation. J. Clin. Microbiol. 2012, 50, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, M.R. Chlamydia pneumoniae. In Clinical Infectious Disease, 2nd ed.; Schlossberg, D., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 1860–1863. [Google Scholar]
- Paldanius, M.; Bloigu, A.; Alho, M.; Leinonen, M.; Saikku, P. Prevalence and persistence of Chlamydia pneumoniae antibodies in healthy laboratory personnel in Finland. Clin. Diagn. Lab. Immun. 2005, 12, 654–659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blasi, F.; Tarsia, P.; Aliberti, S. Chlamydophila pneumoniae. Clin. Microbiol. Infect. 2009, 15, 29–35. [Google Scholar] [CrossRef]
- Puolakkainen, M. Laboratory diagnosis of persistent human chlamydial infection. Front. Cell Infect. Microbiol. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dowell, S.F.; Peeling, R.W.; Boman, J.; Carlone, G.M.; Fields, B.S.; Guarner, J.; Hammerschlag, M.R.; Jackson, L.A.; Kuo, C.C.; Maass, M.; et al. Standardizing Chlamydia pneumoniae assays: Recommendations from the centers for disease control and prevention (USA) and the laboratory centre for disease control (Canada). Clin. Infect. Dis. 2001, 33, 492–503. [Google Scholar] [CrossRef]
- Kumar, S.; Hammerschlag, M.R. Acute respiratory infection due to Chlamydia pneumoniae: Current status of diagnostic methods. Clin. Infect. Dis. 2007, 44, 568–576. [Google Scholar] [CrossRef]
- Hvidsten, D.; Halvorsen, D.S.; Berdal, B.P.; Gutteberg, T.J. Chlamydophila pneumoniae diagnostics: Importance of methodology in relation to timing of sampling. Clin. Microbiol. Infect. 2009, 15, 42–49. [Google Scholar] [CrossRef][Green Version]
- Padalko, E.; Boel, A.; Lagrou, K.; Reynders, M.; China, B.; Vernelen, K.; Expert Committee on Infectious Serology. Low yield by molecular detection of Chlamydophila pneumoniae in respiratory samples in Belgium questioning its etiological role in respiratory tract infections. Acta Clin. Belg. 2013, 68, 166–168. [Google Scholar] [CrossRef]
- Miyashita, N.; Kawai, Y.; Tanaka, T.; Akaike, H.; Teranishi, H.; Wakabayashi, T.; Nakano, T.; Ouchi, K.; Okimoto, N. Antibody responses of Chlamydophila pneumoniae pneumonia: Why is the diagnosis of C. pneumoniae pneumonia difficult? J. Infect. Chemother. 2015, 21, 497–501. [Google Scholar] [CrossRef]
- Noguchi, S.; Yatera, K.; Kawanami, T.; Fuguda, K.; Yamasaki, K.; Naito, K.; Akata, K.; Ishimoto, H.; Mukae, H. Frequency of detection of Chlamydophila pneumoniae using bronchoalveolar lavage fluid in patients with community-onset pneumonia. Respir. Investig. 2017, 55, 357–364. [Google Scholar] [CrossRef]
- Pignanelli, S.; Shurdhi, A.; Delucca, F.; Donati, M. Simultaneous use of direct and indirect diagnostic techniques in atypical respiratory infections from Chlamydophila pneumoniae and Mycoplasma pneumoniae. J. Clin. Lab. Anal. 2009, 23, 206–209. [Google Scholar] [CrossRef]
- Herrera, M.; Aguilar, Y.A.; Rueda, Z.V.; Muskus, C.; Vélez, L.A. Comparison of serological methods with PCR-based methods for the diagnosis of community-acquired pneumonia caused by atypical bacteria. J. Negat. Results Biomed. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Yu, G.; Fadrosh, D.; Goedert, J.J.; Ravel, J.; Goldstein, A.M. Nested PCR biases in interpreting microbial community structure in 16S rRNA gene sequence datasets. PLoS ONE 2015, 10, e0132253. [Google Scholar] [CrossRef] [PubMed]
- Strom, C.M.; Rechitsky, S. Use of nested PCR to identify charred human remains and minute amounts of blood. J. Forensic. Sci. 1998, 43, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Ripa, T.; Nilsson, P.A.A. Chlamydia trachomatis strain with a 377-bp deletion in the cryptic plasmid causing false-negative nucleic acid amplification tests. Sex. Transm. Dis. 2007, 34, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Rossello-Mora, R. Towards a taxonomy of Bacteria and Archaea based on interactive and 435 cumulative data repositories. Environ. Microbiol. 2012, 14, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; Wiley: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Chakravorty, S.; Helb, D.; Burday, M.; Connell, N.; Alland, D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J. Microbiol. Methods 2007, 69, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Van de Peer, Y.; Chapelle, S.; De Wachter, R. A quantitative map of nucleotide substitution rates in bacterial rRNA. Nucleic Acids Res. 1996, 24, 3381–3391. [Google Scholar] [CrossRef]
- Šeligová, B.; Lukáč, Ľ.; Bábelová, M.; Vávrová, S.; Sulo, P. Diagnostic reliability of nested PCR depends on the primer design and threshold abundance of Helicobacter pylori in biopsy, stool, and saliva samples. Helicobacter 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.J.; Marti, H.; Didelot, X.; Castillo-Ramirez, S.; Read, T.D.; Dean, D. Chlamydiaceae genomics reveals interspecies admixture and the recent evolution of Chlamydia abortus infecting lower mammalian species and humans. Genome Biol. Evol. 2015, 7, 3070–3084. [Google Scholar] [CrossRef]
- Freise, J.; Bernau, I.; Meier, S.; Zeidler, H.; Kuipers, J.G. Detection of Chlamydia trachomatis-DNA in synovial fluid: Evaluation of the sensitivity of different DNA extraction methods and amplification systems. Arthritis Res. Ther. 2009, 11, 1–10. [Google Scholar] [CrossRef]
- Freise, J.; Bernau, I.; Meier, S.; Zeidler, H.; Kuipers, J.G. Optimized testing for C. trachomatis DNA in synovial fluid samples in clinical practice. Z. Rheumatol. 2015, 74, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Fukano, H. Comparison of five PCR assays for detecting Chlamydophila pneumoniae DNA. Microbiol Immunol. 2004, 48, 441–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Aydie, S.N.; Obeidat, N.M.; Al-Younes, H.M. Role of Chlamydia pneumoniae in community-acquired pneumonia in hospitalized Jordanian adults. J. Infect. Dev. Ctries. 2016, 10, 227–236. [Google Scholar] [CrossRef]
- LaBiche, R.; Koziol, D.; Quinn, T.C.; Gaydos, C.; Azhar, S.; Ketron, G.; Sood, S.; DeGraba, T.J. Presence of Chlamydia pneumoniae in human symptomatic and asymptomatic carotid atherosclerotic plaque. Stroke 2001, 32, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Nadareishvili, Z.G.; Koziol, D.E.; Szekely, B.; Ruetzler, C.; LaBiche, R.; McCarron, R.; DeGraba, T.J. Increased CD8(+) T cells associated with Chlamydia pneumoniae in symptomatic carotid plaque. Stroke 2001, 32, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Reszka, E.; Jegier, B.; Wasowicz, W.; Lelonek, M. Detection of infectious agents by polymerase chain reaction in human aortic wall. Cardiovasc. Pathol. 2008, 17, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Assar, O.; Nejatizadeh, A.; Dehghan, F.; Kargar, M.; Zolghadri, N. Association of Chlamydia pneumoniae infection with atherosclerotic plaque formation. Glob. J. Health Sci. 2015, 8, 260–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maass, M.; Krause, E.; Engel, P.M.; Kruger, S. Endovascular presence of Chlamydia pneumoniae in patients with hemodynamically effective carotid artery stenosis. Angiology 1997, 48, 699–706. [Google Scholar] [CrossRef]
- Yazouli, L.E.; Hejaji, H.; Elmdaghri, N.; Alami, A.A.; Dakka, N.; Radouani, F. Investigation of Chlamydia pneumoniae infection in Moroccan patients suffering from cardiovascular diseases. J. Infect. Public Health 2018, 11, 246–249. [Google Scholar] [CrossRef]
- Messmer, T.O.; Skelton, S.K.; Moroney, J.F.; Daugharty, H.; Fields, B.S. Application of a nested, multiplex PCR to psittacosis outbreaks. J. Clin. Microbiol. 1997, 35, 2043–2046. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.A.; Phipps, J.; Samuel, D.; Saunders, N.A. Development of a simplified polymerase chain reaction–enzyme immunoassay for the detection of Chlamydia pneumoniae. J. Appl. Bacteriol. 1996, 80, 431–438. [Google Scholar] [CrossRef]
- Black, C.M.; Fields, P.I.; Messmer, T.O.; Berdal, B.P. Detection of Chlamydia pneumoniae in clinical specimens by polymerase chain reaction using nested primers. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Denti, F.; Erba, M.; Cosentini, R.; Raccanelli, R.; Rinaldi, A.; Fagetti, L.; Esposito, G.; Ruberti, U.; Allegra, L. Detection of Chlamydia pneumoniae but not Helicobacter pylori in atherosclerotic plaques of aortic aneurysms. J. Clin. Microbiol. 1996, 34, 2766–2769. [Google Scholar] [CrossRef]
- Tong, C.; Sillis, M. Detection of Chlamydia pneumoniae and Chlamydia psittaci in sputum samples by PCR. J. Clin. Pathol. 1993, 46, 313–317. [Google Scholar] [CrossRef]
- Sessa, R.; Di Pietro, M.; Schiavoni, G.; Santino, I.; Cipriani, P.; Romano, S.; Penco, M.; Dagianti, A.; del Piano, M. Prevalence of Chlamydia pneumoniae in peripheral blood mononuclear cells in Italian patients with acute ischaemic heart disease. Atherosclerosis 2001, 159, 521–525. [Google Scholar] [CrossRef]
- Apfalter, P.; Assadian, O.; Blasi, F.; Boman, J.; Gaydos, C.A.; Kundi, M.; Makristathis, A.; Nehr, M.; Rotter, M.L.; Hirschl, A.M. Reliability of nested PCR for detection of Chlamydia pneumoniae DNA in atheromas: Results from a multicenter study applying standardized protocols. J. Clin. Microbiol. 2002, 40, 4428–4434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, S.; Saigal, S.R.; Sethi, G.R.; Kumar, S. Application of serology and nested polymerase chain reaction for identifying Chlamydophila pneumoniae in community-acquired lower respiratory tract infections in children. Indian J. Pathol. Microbiol. 2016, 59, 499–503. [Google Scholar] [CrossRef]
- Nystrom-Rosander, C.; Thelin, S.; Hjelm, E.; Lindquist, O.; Pahlson, C.; Friman, G. High incidence of Chlamydia pneumoniae in sclerotic heart valves of patients undergoing aortic valve replacement. Scand. J. Infect. Dis. 1997, 29, 361–365. [Google Scholar] [CrossRef]
- Kaltenbock, B.; Schmeer, N.; Schneider, R. Evidence for numerous omp1 alleles of porcine Chlamydia trachomatis and novel chlamydial species obtained by PCR. J. Clin. Microbiol. 1997, 35, 1835–1841. [Google Scholar] [CrossRef]
- Sachse, K.; Hotzel, H. Detection and Differentiation of Chlamydiae by Nested PCR. In PCR Detection of Microbial Pathogens. Methods in Molecular Biology™; Sachse, K., Frey, J., Eds.; Humana Press: Totowa, NJ, USA, 2003; Volume 216, pp. 123–136. [Google Scholar]
- Lindholt, J.S.; Ostergard, L.; Henneberg, E.W.; Fasting, H.; Andersen, P. Failure to demonstrate Chlamydia pneumoniae in symptomatic abdominal aortic aneurysms by a nested polymerase chain reaction (PCR). Eur. J. Vasc. Endovasc. Surg. 1998, 15, 161–164. [Google Scholar] [CrossRef][Green Version]
- Contini, C.; Rotondo, J.C.; Magagnoli, F.; Maritati, M.; Seraceni, S.; Graziano, A.; Poggi, A.; Capucci, R.; Vesce, F.; Tognon, M. Investigation on silent bacterial infections in specimens from pregnant women afected by spontaneous miscarriage. J. Cell. Physiol. 2018, 234, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Mahony, J.B.; Chong, S.; Coombes, B.K.; Smieja, M.; Petrich, A. Analytical sensitivity, reproducibility of results, and clinical performance of five PCR assays for detecting Chlamydia pneumoniae DNA in peripheral blood mononuclear cells. J. Clin. Microbiol. 2000, 38, 2622–2627. [Google Scholar] [CrossRef]
- Lienard, J.; Croxatto, A.; Aeby, S.; Jaton, K.; Posfay-Barbe, K.; Gervaix, A.; Greub, G. Development of a new Chlamydiales-specific real-time PCR and its application to respiratory clinical samples. J. Clin. Microbiol. 2011, 49, 2637–2642. [Google Scholar] [CrossRef]
- Brunelle, B.W.; Sensabaugh, G.F. The ompA gene in Chlamydia trachomatis differs in phylogeny and rate of evolution from other regions of the genome. Infect. Immun. 2006, 74, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, N.; Kingsley, G.; Jones, H.; Sieper, J.; Braun, J.; Ward, M. Detection of DNA from a range of bacterial species in the joints of patients with a variety of arthitides using a nested broad- range polymerase chain reaction. Rheumatology 1999, 38, 260–266. [Google Scholar] [CrossRef]
- Bobo, L.; Coutlee, F.; Yolken, R.; Quinn, T.; Viscidi, R. Diagnosis of Chlamydia trachomatis cervical infection by detection of amplified DNA with an enzyme immunoassay. J. Clin. Microbiol. 1990, 28, 1968–1973. [Google Scholar] [CrossRef]
- Rosón, B.; Carratalà, J.; Verdaguer, R.; Dorca, J.; Manresa, F.; Gudiol, F. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin. Infect. Dis. 2000, 31, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.N.; Huffine, E.F.; Ryan, J.H.; Holland, M.M.; Parsons, T.J.J. A mini-primer set covering the mtDNA hypervariable regions for the genetic typing of old skeletal remains. Forensic. Sci. 2001, 46, 247–253. [Google Scholar] [CrossRef]
- Foran, D.R.; Fischer, A.B.; Stoloff, M.E. A comparison of mitochondrial DNA amplification strategies for species identification. J. Forensic. Investig. 2015, 3, 7. [Google Scholar]
- Stackebrandt, E. Taxonomic parameters revisited: Tarnished gold standards. Microbiol. Today 2006, 33, 152–155. [Google Scholar]
- Sutcliffe, I.C. Challenging the anthropocentric emphasis on phenotypic testing in prokaryotic species descriptions: Rip it up and start again. Front. Genet. 2015, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Brown, A.; Polkinghorne, A. New and emerging chlamydial infections of creatures great and small. New Microbes New Infect. 2017, 18, 28–33. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenter. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Takeshita, T.; Kageyama, S.; Furuta, M.; Tsuboi, H.; Takeuchi, K.; Shibata, Y.; Shimazaki, Y.; Akifusa, S.; Ninomiya, T.; Kiyohara, Y.; et al. Bacterial diversity in saliva and oral health-related conditions: The Hisayama Study. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Cheong, H.C.; Lee, C.Y.Q.; Cheok, Y.Y.; Tan, G.M.Y.; Looi, C.Y.; Wong, W.F. Chlamydiaceae: Diseases in primary hosts and soonosis. Microorganisms 2019, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Venditti, D. Detection of novel Chlamydiae and Legionellales from human nasal samples of healthy volunteers. Folia Microbiol. 2015, 60, 325–334. [Google Scholar] [CrossRef]
- Reid, F.; Oakeshott, P.; Kerry, S.R.; Hay, P.E.; Jensen, J.S. Chlamydia related bacteria Chlamydiales in early pregnancy: Community-based cohort study. Clin. Microbiol. Infect. 2017, 23, 9–14. [Google Scholar] [CrossRef]
- Polkinghorne, A.; Borel, N.; Heijne, M.; Pannekoek, Y. New evidence for domesticated animals as reservoirs of Chlamydia-associated community-acquired pneumonia. Clin. Microbiol. Infect. 2019, 25, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Hokynar, K.; Kurkela, S.; Nieminen, T.; Saxen, H.; Vesterinen, E.J.; Mannonen, L.; Pietikäinen, R.; Puolakkainen, M. Parachlamydia acanthamoebae Detected during a pneumonia outbreak in southeastern Finland, in 2017–2018. Microorganisms 2019, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Work, T.M. Candidatus Renichlamydia lutjani, a Gram-negative bacterium in internal organs of blue-striped snapper Lutjanus kasmira from Hawaii. Dis. Aquat. Org. 2012, 98, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Borel, N.; Polkinghorne, A.; Pospischil, A. A review on chlamydial diseases in animals: Still a challenge for pathologists? Vet. Pathol. 2018, 55, 374–390. [Google Scholar] [CrossRef]
- Kostanjsek, R.; Strus, J.; Drobne, D.; Avguštin, G. ‘Candidatus Rhabdochlamydia porcellionis’, an intracellular bacterium from the hepatopancreas of the terrestrial isopod Porcellio scaber (Crustacea: Isopoda). Int. J. Syst. Evol. Microbiol. 2004, 54, 543–549. [Google Scholar] [CrossRef] [PubMed]

| Primer | Sequence 5′→3′ | Target/Size | Tm (°C) |
|---|---|---|---|
| Chp up | CATACTTGATGTGGATGGTCTCAACC | 16S rDNA 461 bp | 54.2 |
| Chp down | GATTTGCTCCATCTCACGATCTT | 54.0 | |
| PNEU S | TGGGGAAAAGGGAATTCCAC | 16S rDNA 635 bp | 55.1 |
| PNEU Nok | GGGGCTAGCTTTTAGGATTTGC | 55.3 | |
| PNEU S | TGGGGAAAAGGGAATTCCAC | 16S rDNA 208 bp | 55.1 |
| Short down | CACGTTAGCTCCGACACGGAT | 56.5 | |
| Short up | GCGAAGGCGCTTTTCTAATTTA | 16S rDNA 121 bp | 54.9 |
| Chtinok | GTTGAGACCATCCACATCAAGTATG | 56.8 | |
| MT for | CACCATTAGCACCCAAAGCT | mtDNA 1023 bp | 51.9 |
| MT rev | CTGTTAAAAGTGCATACCGCCA | 54.6 | |
| 16S1 F | CCCGCCTGTTTACCAAAAACAT | mtDNA 250 bp | 56.8 |
| 16S1 R | AAGCTCCATAGGGTCTTCTCGTC | 54.7 |
| Primers | Program |
|---|---|
| PNEU S/PNEU Nok PNEU S/Short down Chp up/Chp down | 94 °C—3 min, 35 × (94 °C—45 s, 50 °C—1 min, 72 °C—1 min), 72 °C—5 min, 14 °C |
| Short up/Chtinok | 94 °C—3 min, 30 × (94 °C—45 s, 50 °C—1 min, 72 °C—1 min), 72 °C—5 min, 14 °C |
| MT for/MT rev | 94 °C—5 min, 30 × (94 °C—1 min, 54 °C—1 min, 72 °C—1 min), 72 °C—3 min, 14 °C |
| 16S1 F/16S1 R | 94 °C—5 min, 30 × (94 °C—1 min, 55 °C—1 min, 72 °C—1 min), 72 °C—3 min, 14 °C |
| Reference | Source | Primers/Tm (°C) | PCR Product Size (bp) | Comments | Gene |
|---|---|---|---|---|---|
| Fukano, 2004 b [28] Al-Aydie et al., 2016 b [29] | EB, B, NPS | 53K-1 ATGATCGCGGTTTCTGTTGCCA (61.1 °C) 53K-2 GAGCGACGTTTTGTTGCATCTC (56.3 °C) 53K-3 TGTCCAAGCGGTGAAACAAG (53.5 °C) 53K-4 CAACCGTGACCCATTTACTG (50.3 °C) | 499 | Primers specific to C. pneumoniae, but only 80% DNA sequence identity to the counterparts from other Chlamydia infecting humans. | CopB |
| 239 | |||||
| LaBiche et al., 2001 b [30] Nadareishvili et al., 2001 b [31] Reszka et al., 2008 b [32] Assar et al., 2016 b [33] | AP, AW | TTATTCACCGTCCTACAGCAGAAA (55.6 °C) GGGGGTTCAGGGATCATTTGT (52.7 °C) TTACGAAACGGCATTACAACGGCTAGAAATCAAT (67.4 °C) TATGGCATATCCGCTTCGGGAACGAT (65.7 °C) | 404 | Primers specific to C. pneumoniae, but only 80% DNA sequence identity to the counterparts from other Chlamydia infecting humans, mismatch in primer sequence. | rpoB |
| 214 | |||||
| Maass et al., 1997 b [34] Yazouli et al., 2018 a [35] | PBMC, AP | HL-1 GTTGTTCATGAAGGCCTACT (45.8 °C) HR-1 TGCATAACCTACGGTGTGTT (42.4 °C) In-1 AGTTGAGCATATTCGTGAGG (46.8 °C) In-2 TTTATTTCCGTGTCGTCCAG (50.2 °C) | 437 | Primers specific to C. pneumoniae, but only 80% DNA sequence identity to the counterparts from other Chlamydia infecting humans, wrong primer sequence insertion of C. | rpoB |
| 128 | |||||
| Messmer et al., 1997 b [36] | B, LT, F, CS, NPS | 1S ACGGAATAATGACTTCGG (44.5 °C) 1A TACCTGGTACGCTCAATT (47.9 °C) 2S ATAATGACTTCGGTTGTTATT (42.9 °C) 2A CGTCATCGCCTTGGTGGGCTT (62.8 °C) | 436 | Primers specific to some Chlamydia; low Tm; extreme Tm difference between the second pair of primers; polymorphism in primer binding site. | 16S rRNA |
| 221 | |||||
| Wilson et al., 1996 b [37] | C | OTF CGATCGCTAATACCGAATGTAGTG
(54.8 °C) OTR TTAGCCAATCTCTCTTATTCCCAG (53.0 °C) INF AAAGCCCACCAAGGCGATG (57.2 °C) INR AAAGTGCTTTACAACCCTAA (45.5 °C) | 317 | Primers specific to C. pneumoniae, but not to other Chlamydia human pathogens; extreme Tm difference between the second pair of primers; polymorphism in primer binding site. | 16S rRNA |
| 178 | |||||
| Black et al., 1994 b [38] Blasi et al., 1996 b [39] | AP, TS | 1 ATAATGACTTCGGTTGTTAT (40.6 °C) 2 TATAAATAGGTTGAGTCAAC (35.9 °C) 3 AGTGTAATTAGGCATCTAATAT (39.9 °C) 4 GCTGTATTTCTACAGTTG (33.6 °C) | 1397 | Primers specific to C. pneumoniae, but not to other Chlamydia human pathogens; low Tm; large PCR product; polymorphism in primer binding site. | 16S rRNA |
| 858 | |||||
| Tong and Sillis, 1992 b [40] Sessa et al., 2001 b [41] Apfalter et al., 2002 b [42] Kumar et al., 2016 b [43] | SP | CP1 TTACAAGCCTTGCCTGTAGG (49.9 °C) CP2 GCGATCCCAAATGTTTAAGGC (55.2 °C) CPC TTATTAATTGATGGTACAATA (37.6 °C) CPD ATCTACGGCAGTAGTATAGTT (40 °C) | 333 | Primers specific to C. pneumoniae, but not to other Chlamydia human pathogens; low Tm; large PCR product; polymorphism in primer binding site. | MOMP ompA |
| NA | 207 | ||||
| Nystrom-Rosander et al., 1997 a [44] | AW | Cpn ATGACAACTGTAGAAATACAGC
(42.0 °C) Cpn B CGCCTCTCTCCTATAAAT (40.7 °C) Cpn 1 CCGCAAGGACATATACACAGG (54.5 °C) Cpn 2 CCAGTTCGGATTGTAGTCTGC (51.4 °C) | 463 | Second pair of primers at the same strand; low Tm; polymorphism in primer binding site. | 16S rRNA |
| 289 | |||||
| Kaltenbock et al., 1997 a [45] Sachse and Hotzel, 2003 b [46] | V | 191 GCIYTITGGGARTGYGGITGYGCIAC (66.5 °C) 371 TTAGAAICKGAATTGIGCRTTIAYGTGIGCIGC (65.2 °C) 201 GGIGCWGMITTCCAATAYGCICARTC (59.0 °C) 336 CCRCAAGMTTTTCTRGAYTTCAWYTTGTTR (59.2 °C) | 582 | Primers specific to Chlamydia human pathogens; despite degeneration, polymorphism in primer binding site. | MOMP ompA |
| 443 | |||||
| Lindholt et al., 1998 b [47] | AW | OutA.TACTGGATCCGCTGCTGCAAACTATACTAC (61.8 °C) OutB CTGTTGCTACGCCAGCGTCTGTTG (62.7 °C) InA GTAGATAGACCTAACCCGGCCTACAATAAG (59.3 °C) InB TAGTACCTTTAACTCCGAATAAACCAACGA (59.0 °C) | 496 | Primers specific to C. pneumoniae, but not to other Chlamydia human pathogens; polymorphism in primer binding site. | MOMP ompa |
| 189 | |||||
| Contini et al., 2018 b [48] Mahony et al., 2000 b [49] | CVT PBMC | CpnA TGACAACTGTAGAAATACAGC (42.0 °C) CpnB CGCCTCTCTCCTATAAAT (40.7 °C) TW50 AGTCCCGCAACGAGCGCA (59.9 °C) TW51 GCTGACACGCCATTACTA (43.7 °C) | 463 | CpnA and CpnB primers unspecific to Chlamydia in silico; specific to C. pneumoniae, but not to other Chlamydia human pathogens; extreme Tm difference. | 16S rRNA |
| 217 | |||||
| Lienard et al., 2011 a [50] | NPS | panCh16F2 CCGCCAACACTGGGACT (51.1 °C) panCh16R2 GGAGTTAGCCGGTGCTTCTTTA (51.9 °C) panCh16S FAMCTACGGGAGGCTCAGTCGAGAATC-BHQ | 208 | Primers designed in conserved regions; proved to be nonspecific by sequencing; 16S RNA gene was amplified from Schaalia odontolytica, Actinomyces sp. | 16S rRNA |
| Method/Source | Dilution of DSM Culture | Number of Cell Equivalents in the Sample/µL | Cell Equivalent in PCR Reaction |
|---|---|---|---|
| LNPCR/cells | 10−4 | 0.5 | 0.25 |
| LNPCR/sputum a | 10−2 * | 100 | 0.034 |
| SNPCR/sputum a | 10−3 | 10 | 0.0034 |
| RTPCR/cells | 10−1 | 0.5 | 2.5 |
| RTPCR/sputum a | 100–1 b | 5 × 103 | 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolejová, M.; Cihová, I.; Sulo, P. Reliable and Sensitive Nested PCR for the Detection of Chlamydia in Sputum. Microorganisms 2021, 9, 935. https://doi.org/10.3390/microorganisms9050935
Smolejová M, Cihová I, Sulo P. Reliable and Sensitive Nested PCR for the Detection of Chlamydia in Sputum. Microorganisms. 2021; 9(5):935. https://doi.org/10.3390/microorganisms9050935
Chicago/Turabian StyleSmolejová, Martina, Iveta Cihová, and Pavol Sulo. 2021. "Reliable and Sensitive Nested PCR for the Detection of Chlamydia in Sputum" Microorganisms 9, no. 5: 935. https://doi.org/10.3390/microorganisms9050935
APA StyleSmolejová, M., Cihová, I., & Sulo, P. (2021). Reliable and Sensitive Nested PCR for the Detection of Chlamydia in Sputum. Microorganisms, 9(5), 935. https://doi.org/10.3390/microorganisms9050935






