Combining Visible Light and Non-Focused Ultrasound Significantly Reduces Propionibacterium acnes Biofilm While Having Limited Effect on Host Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bacteria Stocks
2.2. Biofilm Growth Optimization
2.3. Biofilm Preparation for Experiments
2.4. Biofilm Exposure and Evaluation of Viable Bacteria
2.5. Culture of Murine 3T3 and Human Primary Keratinocytes
2.6. Evaluation of Mammalian Cell Viability
2.7. Energy Exposure System
2.8. Statistical Methods
3. Results
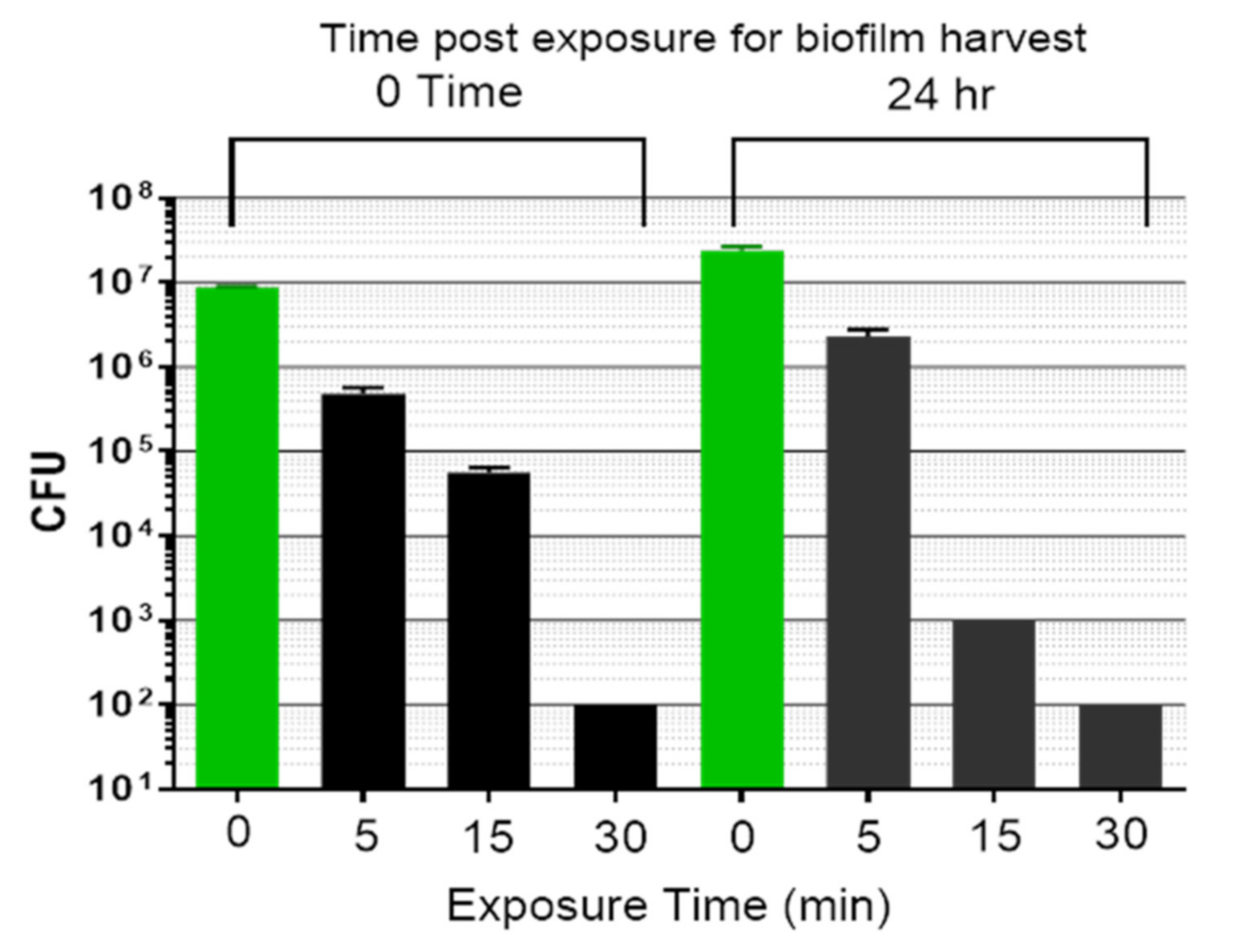
3.1. Synergistic Bactericidal Action Achieved by Combining Light Plus Low Intensity Non-Focused Ultrasound
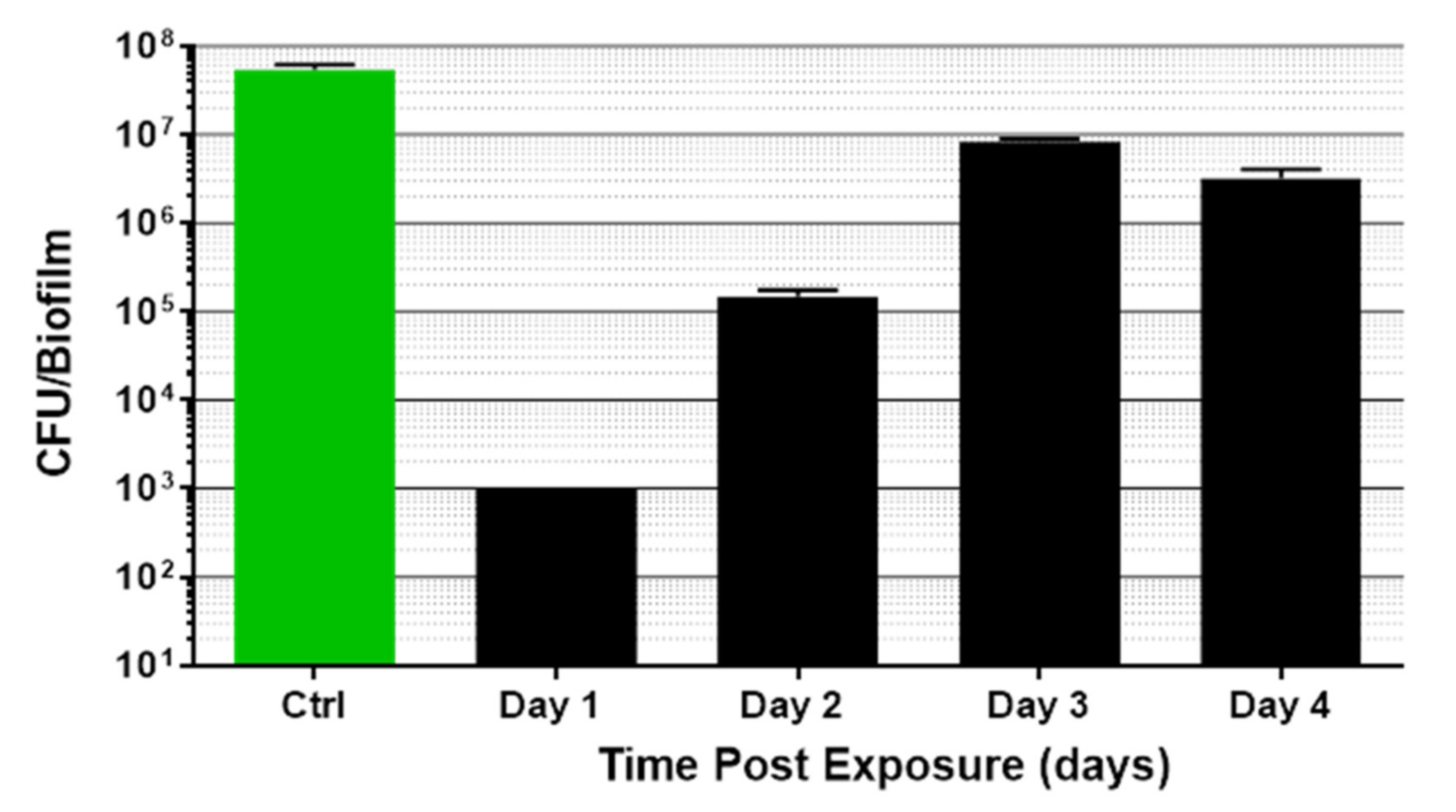
3.2. Kinetics of Biofilm Reduction and Regrowth after Exposure
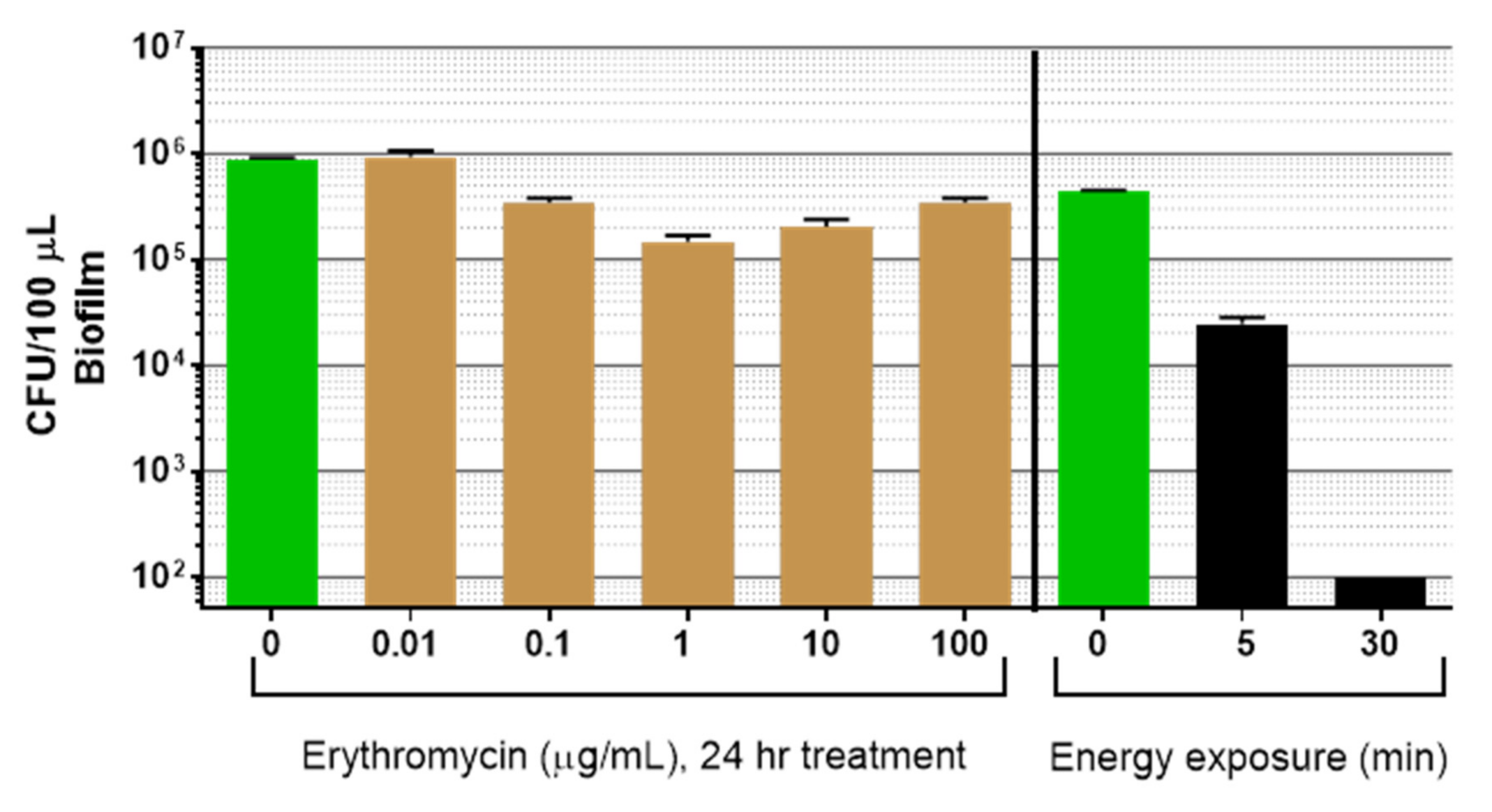
3.3. Comparison of Light Plus Ultrasound to Erythromycin for Biofilm Reduction
3.4. Treatment of Mammalian Cells with Simultaneous Light Plus Low Intensity Ultrasound
4. Discussion
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Musk, D.J.; Hergenrother, P.J. Chemical countermeasures for the control of bacterial biofilms: Effective compounds and promising targets. Curr. Med. Chem. 2006, 13, 2163–2177. [Google Scholar] [CrossRef] [PubMed]
- Kjeldstad, B.; Johnsson, A. An action spectrum for blue and near ultraviolet inactivation of Propionibacterium acnes; with emphasis on a possible porphyrin photosensitization. Photochem. Photobiol. 1986, 43, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori and beyond? Drug Resist Update 2012, 15, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Kjeldstad, B.; Christensen, T. Porphyrin photosensitization of bacteria. Adv. Exp. Med. 1985, 193, 155–159. [Google Scholar]
- Malik, Z.; Hanania, J.; Nitzan, Y. Bactericidal effects of photoactivated porphyrins—An alternative approach to antimicrobial drugs. J. Photochem. Photobiol. 1990, 5, 281–293. [Google Scholar] [CrossRef]
- Lubart, R.; Lipoxski, A.; Nitzan, Y.; Friedmann, H. A possible mechanism for the bactericidal effect of visible light. Laser Ther. 2010, 20, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.N.; Gonzalez, M.L. The practicalities of photodynamic therapy in acne vulgaris. Br. J. Dermatol. 2009, 160, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Kuo, S.; Wang, Y.; Jiang, Y.; Liu, T.Y.; Gallo, R.L.; Huang, C.M. Porphyrin metabolisms in human skin commensal propionibacterium acnes bacteria: Potential application to monitor human radiation risk. Curr. Med. Chem. 2013, 20, 562–568. [Google Scholar] [PubMed]
- Carraro, C.; Pathak, M. Studies on the nature of in vitro and in vivo photosensitisation reactions by psoralens and porphyrins. J. Investig. Dermatol. 1988, 90, 267–275. [Google Scholar] [CrossRef]
- Bartley, J.; Young, D. Ultrasound as a treatment for chronic rhinosinusitis. Med. Hypotheses 2009, 73, 15–17. [Google Scholar] [CrossRef]
- Qian, Z.; Sagers, R.; Pitt, W. Investigation of the mechanism of the bioacoustics effect. J. Biomed. Mater. Res. 1999, 44, 198–205. [Google Scholar] [CrossRef]
- Shi, W.-Y.; Schafer, M.; Dubin, S.; O’Conner, M.; Ozturk, C.; Chou, M.-C. Skin temperature and impedance measurement in ultrasound wound treatment. In Proceedings of the 1996 Fifteenth Southern Biomedical Engineering Conference, Dayton, OH, USA, 29–31 March 1996; pp. 348–350. [Google Scholar]
- Garcia, O.; Schafer, M.E. The effects of nonfocused external ultrasound on tissue temperature and adipocyte morphology. Aesthetic Surg. J. 2013, 33, 117–127. [Google Scholar] [CrossRef]
- Pickering, S.A.; Bayston, R.; Scammell, B.E. Electromagnetic augmentation of antibiotic efficacy in infection of orthopaedic implants. J. Bone Jt. Surg. Br. 2003, 85, 588–593. [Google Scholar] [CrossRef][Green Version]
- Carmen, J.C.; Roeder, B.L.; Nelson, J.L.; Beckstead, B.L.; Runyan, C.M.; Schaalje, G.B.; Robison, R.A.; Pitt, W.G. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo. J. Biomater. Appl. 2004, 18, 237–245. [Google Scholar] [CrossRef]
- Erriu, M.; Blus, C.; Szmukler-Moncler, S.; Buogo, S.; Levi, R.; Barbato, G.; Madonnaripa, D.; Denotti, G.; Piras, V.; Orrù, G. Microbial biofilm modulation by ultrasound. Current concepts and controversies. Ultrason. Sonochem. 2014, 21, 15–22. [Google Scholar] [CrossRef]
- Sugita, Y.; Mizuno, S.; Nakayama, N.; Iwaki, T.; Murakami, E.; Wang, Z.; Endoh, R.; Furuhata, H. Nitric oxide generation directly responds to ultrasound exposure. Ultrasound Med. Biol. 2008, 34, 487–493. [Google Scholar] [CrossRef]
- Suchkova, V.N.; Baggs, R.B.; Sahni, S.K.; Francis, C.W. Ultrasound improves tissue perfusion iischemic tissue through a nitric oxide dependent mechanism. Thromb. Haemost. 2002, 88, 865–870. [Google Scholar] [CrossRef]
- Barraud, N.; Hassett, D.J.; Hwang, S.-H.; Rice, S.A.; Kjelleberg, S.; Webb, J.S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7344–7353. [Google Scholar] [CrossRef]
- Barraud, N.; Storey, M.V.; Moore, Z.P.; Webb, J.S.; Rice, S.A.; Kjelleberg, S. Nitric oxide-mediated dispersal in single- and multi-species biofilms of clinically and industrially relevant microorganisms. Microbial. Biotechnol. 2009, 2, 370–378. [Google Scholar] [CrossRef]
- Schlag, S.; Nerz, C.; Birkenstock, T.A.; Altenberend, F.; Gotz, F. Inhibition of staphylococcal biofilm formation by nitrite. J. Bacteriol. 2007, 189, 7911–7919. [Google Scholar] [CrossRef]
- Jahns, A.C.; Lundskog, B.; Ganceviciene, R.; Palmer, R.H.; Golovleva, I.; Zouboulis, C.C.; McDowell, A.; Patrick, S.; Alexeyev, O.A. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: A case-control study. Br. J. Dermatol. 2012, 167, 50–58. [Google Scholar] [CrossRef]
- Jahns, A.C.; Eilers, H.; Ganceviciene, R.; Alexeyev, O.A. Propionibacterium species and follicular keratinocyte activation in acneic and normal skin. Br. J. Dermatol. 2015, 172, 981–987. [Google Scholar] [CrossRef]
- Sasaki, N.; Takazoe, I. Comparison of the biologic characteristics in two serotypes of Propionibacterium acnes. J. Dent. Res. 1980, 59, 1073. [Google Scholar] [CrossRef]
- Webster, G.F.; Leyden, J.J. Characterization of serum-independent polymorphonuclear leukocyte chemotactic factors produced by Propionibacterium acnes. Inflammation 1980, 4, 261–269. [Google Scholar] [CrossRef]
- Coenye, T.; Peeters, E.; Nelis, H.J. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res. Microbiol. 2007, 158, 386–392. [Google Scholar] [CrossRef]
- Holmberg, A.; Lood, R.; Mörgelin, M.; Söderquist, B.; Holst, E.; Collin, M.; Christensson, B.; Rasmussen, M. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin. Microbiol. Infect. 2009, 15, 787–795. [Google Scholar] [CrossRef]
- Schafer, M.E.; Lewin, P.A. A computerized system for measuring the acoustic output from diagnostic ultrasound equipment. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1988, 35, 102–109. [Google Scholar] [CrossRef]
- Ashkenazi, H.; Malik, Z.; Harth, Y.; Nitzan, Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 2003, 35, 17–24. [Google Scholar] [CrossRef]
- Ross, J.I.; Eady, A.E.; Cove, J.H.; Jones, E.C.; Ratyal, A.H.; Miller, Y.W.; Vyakrnam, S.; Cunliffe, W.J. Clinical resistance to erythromycin and clindamycin in cutaneous propionibacteria isolated from acne patients is associated with mutations in 23S rRNA. Antimicrob. Agents Chemother. 1997, 41, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W. Biofilms and antibiotic therapy: Is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv. Drug Del. Rev. 2005, 57, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.V.; Pitt, W.G. The effect of frequency and power density on the ultrasonically-enhanced killing of biofilm-sequestered Escherichia coli. Colloids Surf. B Biointerfaces 2000, 17, 219–227. [Google Scholar] [CrossRef]
- Bigelow, T.; Northagen, T.; Hill, T.M.; Sailer, F.C. The destruction of Escherichia coli biofilms using high-intensity focused ultrasound. Ultrasound Med. Biol. 2009, 35, 1026–1031. [Google Scholar] [CrossRef]
- Torlak, E.; Sert, D. Combined effect of benzalkonium chloride and ultrasound against Listeria monocytogenes biofilm on plastic surface. Lett. Appl. Microbiol. 2013, 57, 220–226. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Viveiros, J.; Yang, C.; Ahmadi, A.; Ganz, R.A.; Tolkoff, M.J. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob. Agents Chemother. 2005, 49, 2822–2827. [Google Scholar] [CrossRef]
- Maclean, M.; MacGregor, S.; Anderson, J.; Woolsey, G. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J. Photochem. Photobiol. B Biol. 2008, 92, 180–184. [Google Scholar] [CrossRef]
- Guffey, J.S.; Wilborn, J. In vitro bactericidal effects of 405 nm and 470 nm blue light. Photomed. Laser Surg. 2006, 24, 684–688. [Google Scholar] [CrossRef]
- Pitt, W.G.; McBride, M.O.; Roper, R.J.; Sagers, R.D.; Lunceford, J.K. Ultrasonic enhancement of antibiotic action on Gram-negative bacteria. Antimicrob. Agents Chemother. 1994, 38, 2577–2582. [Google Scholar] [CrossRef]
- Rediske, A.M.; Hymas, W.C.; Wilkinson, R.; Pitt, W.G. Ultrasonic enhancement of antibiotic action on several species of bacteria. J. Gen. Appl. Microbiol. 1998, 44, 283–288. [Google Scholar] [CrossRef]
- Runyan, C.; Carmen, J.; Beckstead, B.L.; Nelson, J.L.; Robison, R.A.; Pitt, W.G. Low-frequency ultrasound increases outer membrane permeability of Pseudomonas aeruginosa. J. Gen. Appl. Microbiol. 2006, 52, 295–301. [Google Scholar] [CrossRef]
- Rafferty, S. Nitric oxide synthases of bacteria—And other unicellular organisms. Open Nitric Oxide J. 2011, 3 (Suppl. 1-M4), 25–32. [Google Scholar] [CrossRef]
- Bhambri, S.; Del Rosso, J.Q.; Bhambri, A. Pathogenesis of acne vulgaris: Recent advances. J. Drugs Dermatol. 2009, 8, 615–618. [Google Scholar]
- Berke, R.; Singh, A.; Guralnick, M. Atopic dermatitis: An overview. Am. Fam. Physician 2012, 86, 35–42. [Google Scholar]
- James, W.D. Acne. N. Engl. J. Med. 2005, 352, 1463–1472. [Google Scholar] [CrossRef]
- Thiboutot, D.; Gollnick, H.; Bettoli, V.; Dréno, B.; Kang, S.; Leyden, J.J.; Shalita, A.R.; Lozada, V.T.; Berson, D.; Finlay, A.; et al. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne Group. J. Am. Acad. Dermatol. 2009, 60, S1–S15. [Google Scholar] [CrossRef]
- Leyden, J.J. The evolving role of Propionibacterium acnes in acne. Sem. Cutan. Med. Surg. 2001, 3, 139–143. [Google Scholar] [CrossRef]
- Burkhart, C.G.; Burkhart, C.N. Expanding the microcomedone theory and acne therapeutics: Propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. J. Am. Acad. Dermatol. 2007, 57, 722–724. [Google Scholar] [CrossRef]
- Burkhart, C.N.; Burkhart, C.G. Microbiology’s principle of biofilms as a major factor in the pathogenesis of acne vulgaris. Int. J. Dermatol. 2003, 42, 925–927. [Google Scholar] [CrossRef]
- Allen, H.B.; Vaze, N.D.; Choi, C.; Hailu, T.; Tulbert, B.H.; Cusack, C.A.; Joshi, S.G. The presence and impact of biofilm producing staphylococci in atopic dermatitis. JAMA Dermatol. 2014, 150, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Loike, J.D.; Plitt, A.; Kothari, K.; Zumeris, J.; Budhu, S.; Kavalus, K.; Ray, Y.; Jacob, H. Surface acoustic waves enhance neutrophil killing of bacteria. PLoS ONE 2013, 8, e68334. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Nelmes, R.T.; Gougoulias, N.; Maffulli, N.; Gray, J. The effects of LIPUS on soft-tissue healing: A review of the literature. Br. Med. Bull. 2013, 8, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Bohari, S.P.; Grover, L.M.; Hukins, D.W. Pulsed-low intensity ultrasound enhances extracellular matrix production by fibroblasts encapsulated in alginate. J. Tissue Eng. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T.; Mochida, J.; Miyazaki, T.; Watanabe, T.; Iwabuchi, S.; Ando, K.; Hotta, T.; Sakai, D. Low-intensity pulsed ultrasound stimulates cell proliferation and proteoglycan production in rabbit intervertebral disc cells cultured in alginate. Biomaterials 2006, 27, 354–361. [Google Scholar] [CrossRef]
- Fischer, M.R.; Abel, M.; Lopez Kostka, S.; Rudolph, B.; Becker, D.; von Stebut, E. Blue light irradiation suppresses dendritic cells activation in vitro. Exp. Dermatol. 2013, 22, 558–560. [Google Scholar] [CrossRef]
- Shnitkind, E.; Yaping, E.; Geen, S.; Shalita, A.R.; Lee, W.-L. Anti-inflammatory properties of narrow-band blue light. J. Drugs Dermatol. 2006, 5, 605–610. [Google Scholar]
- Zandi, S.; Kalia, S.; Lui, H. UVA1 Phototherapy: A concise and practical review. Skin Ther. Lett. 2012, 17, 1–8. [Google Scholar]
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.A.; Van Erp, P.E.J.; Van De Kerkhof, P.C.M.; Gerritsen, R.M.J.P. Clinical and histological effects of blue light on normal skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21. [Google Scholar] [CrossRef]
- Becker, D.; Langer, E.; Seemann, M.; Seemann, G.; Fell, I.; Saloga, J.; Grabbe, S.; Von Stebut, E. Clinical efficacy of blue light full body irradiation as treatment option for severe atopic dermatitis. PLoS ONE 2011, 6, e20566. [Google Scholar] [CrossRef]
- Kawada, A.; Aragane, Y.; Kameyama, H.; Sangen, Y.; Tezuka, T. Acne phototherapy with a high-intensity, enhanced, narrow band, blue light source: An open study and in vitro investigation. J. Dermatolog. Sci. 2002, 30, 129–135. [Google Scholar] [CrossRef]
- Monfrecola, G.; Lembo, S.; Cantelli, M.; Ciaglia, E.; Scarpato, L.; Fabbrocini, G.; Balato, A. The effect of visible blue light on the differentiation of dendritic cells in vitro. Biochimie 2014, 101, 252–255. [Google Scholar] [CrossRef]
- Zeina, B.; Greenman, J.; Corry, D.; Purcell, W.M. Antimicrobial photodynamic therapy: Assessment of genotoxic effects on keratinocytes in vitro. Br. J. Dermatol. 2003, 148, 229–232. [Google Scholar] [CrossRef]
- Zeina, B.; Greenman, J.; Corry, D.; Purcell, W.M. Cytotoxic effects of antimicrobial photodynamic therapy on keratinocytes in vitro. Br. J. Dermatol. 2002, 146, 568–573. [Google Scholar] [CrossRef]
- Liebman, J.; Born, M.; Kolb-Bachofen, V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Investig. Dermatol. 2009, 130, 259–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial blue light therapy for multi-drug resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Disease 2014, 209, 1963–1976. [Google Scholar] [CrossRef]
- Patel, A.D.; Rotenberg, S.; Messer, R.L.W.; Wataha, J.C.; Ogbureke, K.U.; McCloud, V.V.; Lockwood, P.; Hsu, S.; Lewis, J.B. Blue light activates phase 2 response proteins and slows growth of A431 epidermoid carcinoma xenografts. Anticancer Res. 2014, 34, 6305–6313. [Google Scholar]
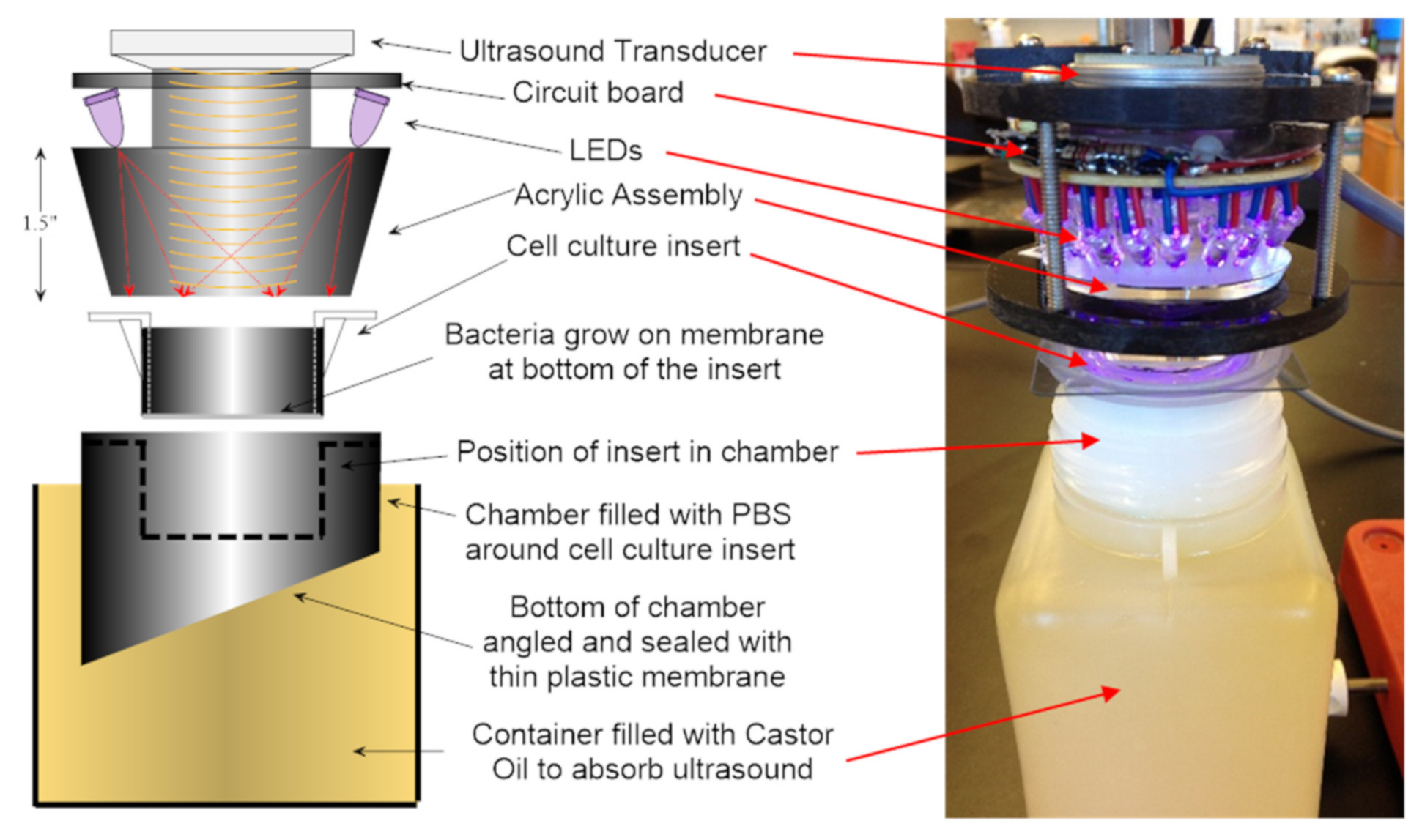
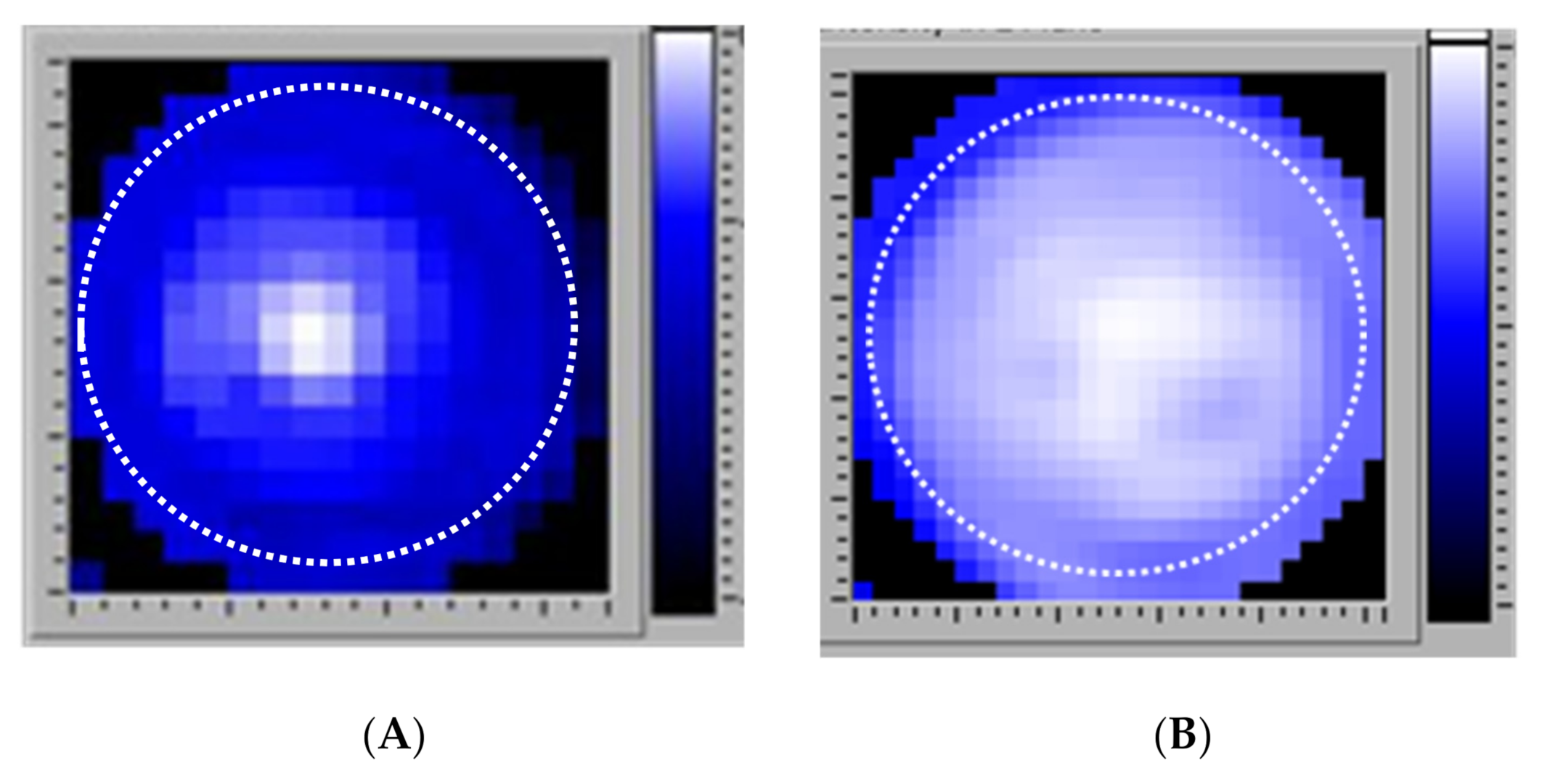
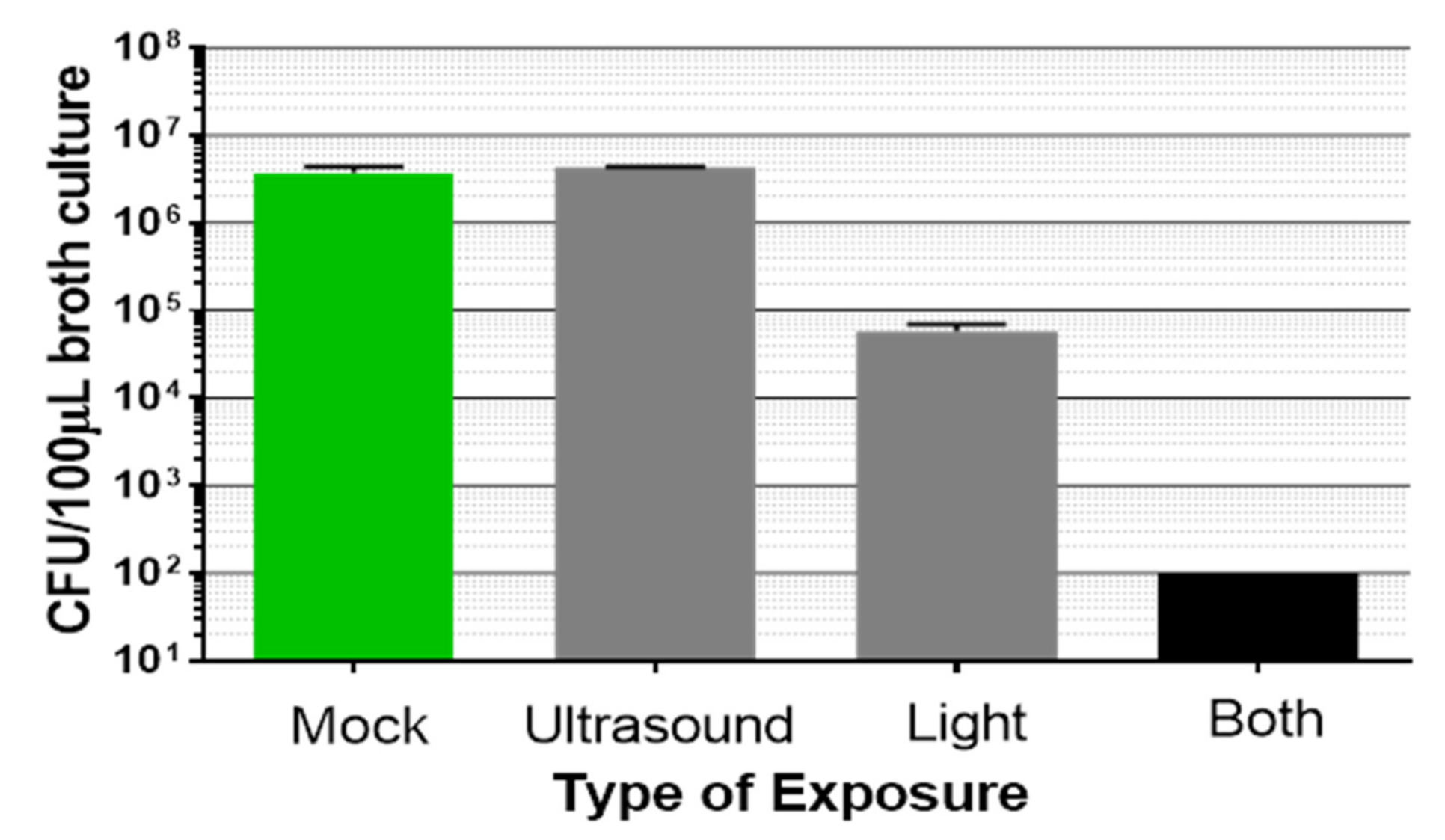
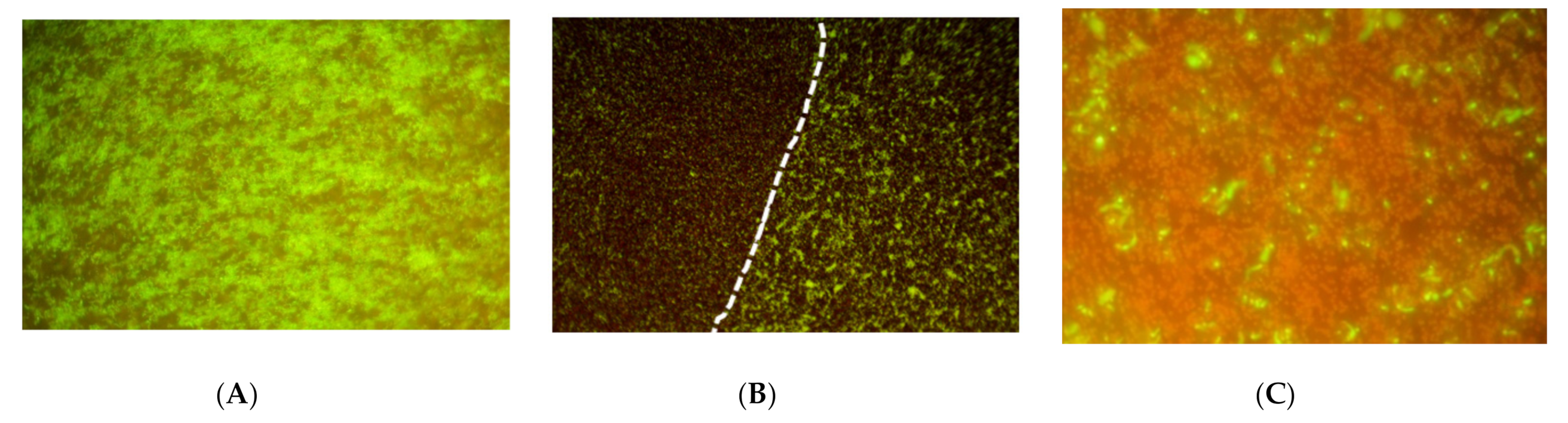


| Bacteria | Inoculum (CFU per Insert) | Adhering Medium | Adhering Time (h) | Growth Medium | Culture Time (Days) | Biofilm (CFU/Insert) |
|---|---|---|---|---|---|---|
| P. acnes, strain 6919 | 1–2 × 108 | DMEM/F10 1:1 (Dulbecco’s mod. Eagle’s med./Ham’s F10), Corning celgro | 18–24 | RCM (reinforced clostridial medium), Oxoid | 4–7 | 107–109 |
| Frequency | Pressure | Energy | Intensity | Wavelength | |
|---|---|---|---|---|---|
| Ultrasound | 456 kHz | 280 kPa | ~80 mW/cm2(Isata.0) | 3.3 mm | |
| Light | 9–144 J/cm2 | 30–100 mW/cm2 | 405 nm |
| Energy Treatment | Time | CFU * |
|---|---|---|
| Mock control | 0 | 2.2 (0.73) × 108 |
| Light and ultrasound simultaneously | 15 min | 2.85 (0.24) × 105 |
| Light and ultrasound simultaneously | 15 min, 15 min | 9.2 (1.7) × 103 |
| Light alone then ultrasound alone | 15 min, 15 min | 2.8 (0.3) × 106 |
| Ultrasound alone then light alone | 15 min, 15 min | 2.6 (0.1) × 106 |
| Light Energy(J/cm2) | Recovered Cell Numbers Day 0 | % Cell Viability Day 0 | Recovered Cell Numbers Day 1 | % Cell Viability Day 1 |
|---|---|---|---|---|
| 12 | 1.8–6.0 × 105 | 95.6–96.6 | 0.7–1.0 × 106 | 96.8–96.8 |
| 23 | 2.2–3.5 × 105 | 90.9–95.0 | 0.7–0.8 × 106 | 96.6–96.8 |
| 46 | 1.4–6.0 × 105 | 89.3–93.3 | 0.5–0.7 × 106 | 96.5–90.3 |
| 58 | 3.0–4.5 × 105 | 88.4–91.1 | 0.4–0.6 × 106 | 93.8–96.5 |
| Mock | 1.8–9.6 × 105 | 95.1–98.0% | 0.3–1.2 × 106 | 94.3–97.7% |
| Light Energy (J/cm2) | Alamar Blue % Reduction Day 0 | Alamar blue % Reduction Day 1 |
|---|---|---|
| 12 | 9–20% | 19–26% |
| 23 | 9–13% | 17–21% |
| 46 | 9–9% | 15–22% |
| 58 | 6–9% | 30–30% |
| Mock | 6–18% | 29–33% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schafer, M.E.; McNeely, T. Combining Visible Light and Non-Focused Ultrasound Significantly Reduces Propionibacterium acnes Biofilm While Having Limited Effect on Host Cells. Microorganisms 2021, 9, 929. https://doi.org/10.3390/microorganisms9050929
Schafer ME, McNeely T. Combining Visible Light and Non-Focused Ultrasound Significantly Reduces Propionibacterium acnes Biofilm While Having Limited Effect on Host Cells. Microorganisms. 2021; 9(5):929. https://doi.org/10.3390/microorganisms9050929
Chicago/Turabian StyleSchafer, Mark E., and Tessie McNeely. 2021. "Combining Visible Light and Non-Focused Ultrasound Significantly Reduces Propionibacterium acnes Biofilm While Having Limited Effect on Host Cells" Microorganisms 9, no. 5: 929. https://doi.org/10.3390/microorganisms9050929
APA StyleSchafer, M. E., & McNeely, T. (2021). Combining Visible Light and Non-Focused Ultrasound Significantly Reduces Propionibacterium acnes Biofilm While Having Limited Effect on Host Cells. Microorganisms, 9(5), 929. https://doi.org/10.3390/microorganisms9050929






