Abstract
Oenococcus oeni is the most exploited lactic acid bacterium in the wine industry and drives the malolactic fermentation of wines. Although prophage-like sequences have been identified in the species, many are not characterized, and a global view of their integration and distribution amongst strains is currently lacking. In this work, we analyzed the complete genomes of 231 strains for the occurrence of prophages, and analyzed their size and positions of insertion. Our data show the limited variation in the number of prophages in O. oeni genomes, and that six sites of insertion within the bacterial genome are being used for site-specific recombination. Prophage diversity patterns varied significantly for different host lineages, and environmental niches. Overall, the findings highlight the pervasive presence of prophages in the O. oeni species, their role as a major source of within-species bacterial diversity and drivers of horizontal gene transfer. Our data also have implications for enhanced understanding of the prophage recombination events which occurred during evolution of O. oeni, as well as the potential of prophages in influencing the fitness of these bacteria in their distinct niches.
1. Introduction
The wine-making process starts with the selection of the fruit and the fermentation of sugars into alcohol by yeasts. In most red and dry white wines, malolactic fermentation (MLF), which reduces acidity, increases microbial stability and creates good-quality grape wine, is also a required step. MLF is largely driven by the lactic acid bacterium (LAB) Oenococcus oeni [1]. This bacterium is rarely detected in the natural environment, including the surface of grape berries [2], and its population only increases after crushing. At this step, O. oeni is part of a complex microbiota that still comprises different LAB. Wine conditions become progressively harsher for most bacteria, except for O. oeni, which becomes the sole detectable LAB species isolated from wine when MLF typically occurs, and is indeed the best adapted species to the combined inhibitory effects of low pH, low oxygen, high alcohol, polyphenolic compounds and sulfur dioxide contents [2]. However, intra-species diversity is reported in the O. oeni species and large phenotypic differences among indigenous strains corresponding to variable capacities to withstand stringent wine compositions and impact aromas have been repeatedly observed. This has become the focus of more detailed studies, with the aim to explain microbial phenotypes on a genomic scale. To this aim, collections of strains have been assembled worldwide, from all wine regions, and also from other niches supporting the growth of O. oeni such as apple cider and kombucha, a fermented tea containing very little alcohol [2]. As from 2005 [3], an enormous amount of information has been progressively obtained from whole genome sequencing, showing that O. oeni is a highly diverse species, with genetic adaptation to fermented beverages [4,5,6,7]. Such a specialization to life in wine is visible in the small (1.6 to 2.2 Mbp) genome of O. oeni because the species has lost many metabolic abilities due to its adaptation to an environment rich in amino acids, vitamins and other nutrients [7]. The population structure revealed by MLST and phylogenomic analyses currently recognizes four main phylogenetic groups (referred to as groups A, B, C and D) [7,8,9]. Group A exclusively contains wine strains, and is divided into subgroups which are associated with certain types of wine, such as white wines of Burgundy or Champagne, supporting the idea that group A strains are the most domesticated to wine [10]. Groups B and C contain both cider and wine strains [7]. More recently, strains originating from kombucha tea were shown to form a fourth group named phylogroup D, which may have preceded the advent of other groups [9].
A key factor in the rapid evolution and adaptation of the strains is undoubtedly the hypermutability of the O. oeni species [11]. As reported for a few other species, the absence of the ubiquitous mismatch repair (MMR) genes mutS and mutL results in the accumulation of spontaneous errors in DNA replication, or in reduced stringency in recombination, thus generating high levels of polymorphism, increasing adaptation to environmental fluctuation [12]. The mutator phenotype also promotes horizontal distribution of essential genes for survival, and therefore increases the genetic innovation rate, eliciting environmental adaptation [12]. The species is also devoid of a CRISPR-Cas system, the bacterial immunity defense mechanism against foreign DNA [2,3,4,5,6,7,8,9,10,11]. Given these peculiar characteristics, it is therefore not surprising that mobile genetic elements such as insertion sequences (ISs) [13], plasmids [14] and DNA of (pro)phage origin [15] have been shown to constitute a significant portion of the O. oeni pangenome. The observed prevalence of prophages in bacterial genomes is indeed in agreement with previous cultivation-based assessments of lysogeny reported in the species [16].
Additional insights into the diversity of O. oeni prophages was obtained through the analysis of two major constituents of the site-specific recombination (SSR) unit, corresponding to the phage integrases and attachment sites [4,17]. Alignment of the integrase protein sequences was shown to cluster prophages into four major groups (IntA to IntD), which were related to the integration site in the host chromosome [17]. Thus far, all groups, except IntC, contain mitomycin C-inducible prophages. Accordingly, no IntC phage has been isolated as free phage particles during field campaigns targeting samples from grapes, wines and materials [17,18].
The abundant literature on diverse ecosystems fosters further effort to generate deeper knowledge of lysogeny in the O. oeni species. In particular, there is a convergence of case studies towards a role of prophages in providing their host bacterium with ecologically significant traits [19,20]. The later include immunity against specific phage attack [21], virulence [22] or specific adhesion properties through the modification of the cell surface matrix [20,23], thereby improving bacterial survival under changing conditions. Ecosystem-level consequences of lysogeny have also been documented. For example, prophages modulate the interactions between microbial symbionts and their insect or human hosts [24,25]. The current trend toward understanding phage–host interactions on a global scale also makes a valuable contribution on how phages influence bacterial diversification and ecotype formation [26,27]. The deciphering of the mechanisms of ecological divergence in the Oenococcus genus is highly topical, as three novel species have recently been proposed during exploration of different fermented beverages [2]. This has expanded our knowledge of the horizons of their habitat to other niches such as alcohol production from sugar cane for O. alcoholitolerans [28], shochu distillates for O. kitaharae [29], ciders for O. oeni and O. sicerae [30] and kefirs for O. sicerae [31]. Thanks to the recent increase in the number and diversity of sequenced genomes, we investigated the distribution, organization and insertion of prophages in O. oeni and its related species. Our data will help in understanding the evolutionary trajectories of strains and their phages in fermented beverages, and can be further used to explore whether prophages play a role during the adaptation of their host to wine-making conditions.
2. Material and Methods
2.1. Data Collection and Identification of Candidate Prophage Sequences in Complete Genomes of O. oeni
A total of 231 publicly available genome sequences were obtained from the National Center for Biotechnology Information NCBI, https://www.ncbi.nlm.nih.gov (accessed on 2 January 2021) (Table S1). Corresponding strains have been collected worldwide from different fermented beverages (red, dry and sweet white wines, sparkling wines and more recently cider and kombucha tea). Candidate prophage-like elements were identified using the RAST pipeline which provides an automated approach to phage genome annotation [32]. As suggested by others [33], we performed a manual inspection of the sequences for the presence of signature sequences: attachment sites (att), gene(s) encoding integrase(s), terminases(s), transposases(s), genes coding for structural viral proteins and the sequences of prophage integration sites. In particular, a multi-FASTA file containing the four publicly available integrase protein sequences described among temperate oenophages was used as a reference to blast the draft genomes. In addition, the prophage search tool PHASTER was also used [34].
2.2. Comparisons and Phylogenetic Analyses
For specific ORF phylogenetic analyses, protein sequences were aligned using ClustalOmega at https://toolkit.tuebingen.mpg.de/ (accessed on 2 January 2021).
Phage genome comparisons were conducted using the Genome-BLAST Distance Phylogeny (GBDP) method using VICTOR under settings recommended for prokaryotic viruses [35] at: http://ggdc.dsmz.de/phylogeny-service.php (accessed on 2 January 2021). The resulting intergenomic distances were used to infer a balanced minimum evolution tree with branch support via FASTME including SPR postprocessing for each of the formulas D0, D4 and D6, respectively. Branch support was inferred from 100 pseudo-bootstrap replicates each. Trees were rooted at the midpoint and visualized with FigTree [36] as already described [37].
2.3. PCR
Bacterial strains CRBO11105 and CRBO14210 (accession numbers LKSR01 and LKRV01) were obtained from the Centre de Ressources Biologiques Oenologiques (CRB Oeno; ISVV, Villenave d’Ornon, France). Genomic DNAs from were extracted from cultures by using the Whatman® FTA Clone Saver card technology (Whatman, Sigma-Aldrich, France). PCR amplification reactions were achieved in a 25 µL final volume with 0.2 µM of each primer. The Taq 5X Master Mix kit (New England, Biolabs, Evry, France) was used according to the manufacturer’s recommendations. A BioRad i-Cycler was used for amplification. Bacterial DNA was introduced in the reaction mixture as 1.2 mm FTA discs which were punched out of the center of FTA cards using a Uni-Core Punch (Qiagen Cat. No. WB100028, Whatman®, Fisher Scientific, Schwerte, Germany) and transferred to PCR tubes. The pair of primers targeting the attBB site was described earlier [17].
2.4. Induction with MC
For the induction of phages, mitomycin C was used as the inducing agent (1 μg/mL), with bacterial culture on modified MRS. Overnight cultures were diluted 10-fold in 10 mL of fresh broth, grown to an optical density at 600 nm (OD600) of 0.2 to 0.3 prior to the addition of inducing agent and incubated for 24 h. OD600 was measured periodically.
3. Results and Discussion
3.1. The Genome of O. oeni Is Replete with Putative Prophages
The systematic interrogation of 231 complete genomes assessed from GenBank (NCBI) resulted in the discovery of seemingly intact prophages in 134 strains of O. oeni (58% of the bacterial genomes studied) (Table 1).

Table 1.
Lysogeny in a set of 231 strains representing the four described phylogroups of O. oeni and different combinations of prophage carriage.
Lysogens originated from all types of beverages (wines, ciders, kombucha), and belonged to all four lineages described in O. oeni, including the less numerously represented phylogroups associated with cider (B and C) and kombucha (D). Lysogens mostly harbored a single prophage (64.2%; n = 86). The 48 poly-lysogens contained two (n = 43) or three distinct prophages (n = 5) (Table 1).
This first survey yielded a total of 187 prophages. They showed a genome length ranging from 35 kb to 46.2 kb which, on average, and comprised between 2 and 6.7% of the host chromosome. All prophages encoded identifiable phage-specific functions such as integrases and terminases and tail-associated, portal-associated and lysis-associated proteins. The presence of a large number of predicted proteins characteristic of tailed phages (e.g., terminase, tape measure protein, tail formation and baseplate-related proteins) is consistent with previous observations by transmission electronic microscopy showing that oenophages display the Siphoviridae morphology [16,17,18]. Prophages also demonstrated well-conserved patterns in genome organization. Starting with the gene encoding the integrase, the following order was observed: lysogeny module followed by modules for replication, DNA packaging, head morphogenesis, tail, lysis and finally lysogenic conversion.
During a previous screening and analysis of prophages in a set of 42 publicly available genomes, we reported the existence of four distinct groups of temperate oenophages (IntA-D) [17]. The properties shared by all known members of a group included: (1) high identity of the integrase sequences (>98% at the amino acid level) and (2) tropism for a specific bacterial attachment site corresponding to the 3′ end of a tRNA gene [17]. To get a broader view, we extended our in silico analyses to the 187 newly retrieved prophages. The clustering of the majority of their integrase sequences into the four proposed groups (96%, n = 180) was confirmed. All identified IntA, IntC and IntD integrases were observed to preferentially drive prophage integration into their expected cognate site, encompassing the 3′ end of the tRNAGlu-0506 (attBA), tRNALys-0685 (attBC) and tRNALeu-1359 (attBD) sequences (gene numbers are those present in the PSU1 reference strain), respectively (Figure 1).
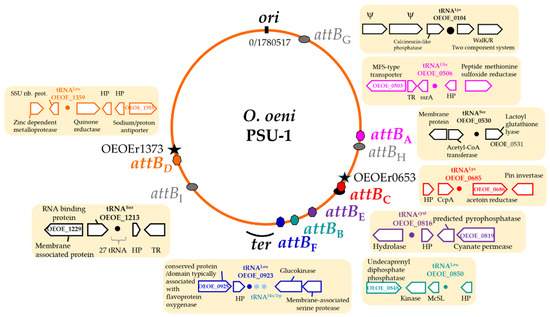
Figure 1.
Site-specific recombination of temperate oenophages in O. oeni. The locations of the attachments sites used by prophages (color) and phage remnants (gray) are shown as a generalized chromosome backbone based upon the PSU-1 genome. Locations of the oriC, Ter site-containing region and rRNA loci (stars) are indicated. Genes flanking all attB sites are indicated.
Intriguing features were observed for some IntB prophages. First, we confirmed that integration at the previously described tRNALeu-0851 (attBB) site is widespread in the group, as this applied to 78% of the IntB prophages [17]. In contrast, 22% showed an unusual localization outside the attBB site. Instead, recombination was shown to occur at the 3′ end of the tRNALeu-0923 gene, located 66 kb away (Figure 1). As this situation occurred in strains CRBO11105 and CRBO14210 from our strain collection, experimental verification of such an unusual integration site was done by PCR. We observed the failure to generate a PCR product using the bacterial chromosomal DNAs as a template and PCR primers flanking the tRNALeu-0923 gene, while an amplicon corresponding to the attL junction between bacterial and phage DNA was amplified in both strains. The IntB phages were therefore integrated at the tRNALeu-0923 site in strains CRBO11105 and CRBO14210. Accordingly, the intact attBB region spanning the tRNALeu-0851 gene was amplified, confirming the absence of prophage at this site in both strains. It is therefore shown that a few IntB prophages become integrated at a 63 bp secondary att site containing the 3′ end of the tRNALeu-0923 gene in O. oeni. This finding is in accordance with previous data suggesting that the IntB phage 10MC could use the tRNALeu-0923 gene as a secondary site during lysogenization assays of the MCW strain [38].
Our genomic survey also indicated that seven of the 187 newly retrieved prophages harbored integrases with lower pairwise sequence identity (60–92%) with the existing IntA-D proteins (Table 2).

Table 2.
Nucleotide and amino acid identities in pairwise comparisons amongst the individual genes of phage integrases in O. oeni.
Three prophages harbored a 100% conserved integrase sequence, which shared 60 to 69% aa identity with the sequences affiliated to the four existing groups. This novel integrase, named IntE, was associated with a 39.5 kb and a 38.9 kb prophage in the cider strains CRBO1384 and CRBO1389, respectively. It was also harbored by a slightly larger 46.3 kb prophage in strain AWRIB663 originating from wine. All three IntE prophages targeted a 11 bp attBE core sequence to lysogenize their host, which consisted of the 3′ end of the unique cysteinyl tRNA0816 gene in O. oeni [3]. The use of a tRNACys as a target for phage integration is rare amongst phages of LAB, but has been reported for a few phages such as TPW22 [39] and PH15 [40].
A second novel integrase, named IntF, was associated with a 41.7 kb resident prophage, found in four out of five strains isolated from kombucha tea. IntF shared a higher protein sequence identity (92%) with IntB and IntC integrases than with the three other groups (60–61%) (Table 2). Surprisingly, the site targeted by IntF prophages (attBF) was the same 63 bp att sequence containing the 3′ end of the tRNALeu-0923 gene, previously described as a putative alternative site for IntB prophages (Figure 1). Hence, prophages with distinct integrases (IntB or IntF) can integrate at the same tRNALeu-0923 site in the chromosome of O. oeni.
Finally, we used mitomycin C (MC) to induce the prophages present in a set of representative strains from the CRB Oeno collection. We first tested 11 mono-lysogens (seven IntA; two IntB; two IntD). We observed a clear lysis for nine strains, and the results obtained for strain IOEB0608 are shown in Figure S1. The obtained lysates formed plaques on strain IOEB277, suggesting that they contain functional phages (results not shown). The strains showing no lysis upon MC addition were IOEB0502 and C23, which harbored an IntA and an IntB phage, respectively. Lastly, we also tested the S28 double lysogen (IntA-IntC). The strain lysed in the presence of MC and plaques were also produced on the sensitive host. Phages were recovered from 20 individual plaques and characterized by PCR with primers targeting the intA and intC genes [17]. Only IntA phages were detected, suggesting that IntC phages do not form active phage particles or that IntA is the faster phage which dictates lysis time.
3.2. Phage Remnants Also Use tRNA Sites in O. oeni
Degeneration of prophages was observed in O. oeni and eight distinct phage-related genomic islands were identified. Their grounding was further supported by the observations of reduced sizes (5.2 kb to 19.5 kb), defective integrase proteins (PRC, PRF1–2, PRI2), the lack of other key phage genes (notably those involved in morphogenesis), the presence of transposase sequences (PRH2, PRI1) and degenerate att sites and/or larger sequence variability reflected by the presence of neighboring pseudogenes (PRG, PRF) (Figure 1 and Figure 2). Such phage-related genomic islands were considered as prophage remnants (PRs).
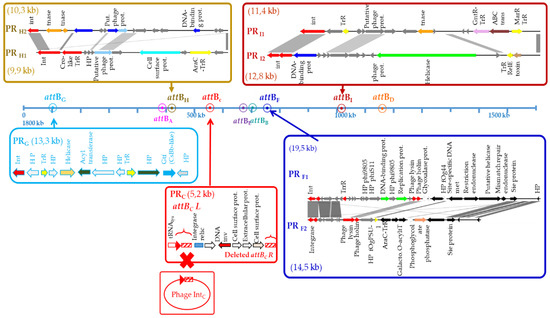
Figure 2.
Localization and genetic organization of prophage remnants in the genome of Oenococcus oeni. Genes encoding hypothetical proteins are represented in gray.
PRG was rare and found in 2.6% of the tested strains. The prevalence of PRH and PRI elements was similar (17.7% and 16.4%, respectively). Only PRC was frequently observed in the species (79.2%) (Table 1).
PRs were consistently integrated in five precise locations within the host genome, which also corresponded to tRNA genes (Figure 1 and Figure 2). Three PRs (PRC and PRF1/F2) were found at the previously characterized host sites used for the SSR of IntC and IntF phages, respectively [17]. However, the resident PRs and prophage sequences shared no homologies. The sequences flanking the five others (PRG, PRH1/2 and PRI1/I2) differed from the sites of the known prophages of O. oeni, and were further named attBG (OEOE_t0104; tRNALys), attBH (OEOE_t0530; tRNASer) and attBI (OEOE_t1213; tRNASer) (Figure 1). The length of the attachment sequences required for SSR were 61, 22 and 19 bp, respectively.
Annotation results indicated that a total of 16% of 122 ORFs found on all eight PRs were assigned to phage protein functions (Figure 2). Most were found in the larger PRH regions (14.5 kb to 19.5 kb). Interestingly, only PRF1 and PRF2 still encode endolysin and holin genes. Several PR-related genes specified surface proteins of unknown function (PRC, PRH1), as well as putative phage resistance mechanisms (Sie, restriction–modification systems) (PRF1, PRF2, PRG, PRI). This suggests that PRs are not inactive DNA remnants and may encode traits useful for the host, as suggested for prophages [17]. In addition, the presence of phage resistance mechanisms in four PRs is an indicator of phage traffic and induced pressure during the making of fermented beverages.
3.3. Chromosomal Location and Genomic Context of Prophages and PR
As observed in many bacterial models, prophage-related regions (complete and PRs) are not randomly distributed in the O. oeni genome. Their presence was rare on the origin-proximal half of the O. oeni chromosome. Only PRG was integrated near the origin of replication, where genes could be, on average, more expressed [41]. There was a significant enrichment in the absolute number of phages and PRs on the right replichore, where phage abundance increased along the ori–ter axis (Figure 1). Interestingly, four attachment sites (attBC, attBE, attBB and attBF sites) were found to lie on a 200 kb segment of the chromosome, near terC. The latter region is of ultimate importance for cellular processes that interact with the chromosome shape and its organization [42,43].
As also previously reported for other bacteria [41], prophage polarization was observed in O. oeni. The genes of oenophages show a preference for co-orientation with the bacterial replication fork. Accordingly, IntD prophages, which are on the left replichore, changed their orientation.
Phages can alter host phenotype by disrupting host genes as a result of integration. Others are known to encode cis-acting element(s) near their attP site that, upon integration, affect the transcription of host neighboring genes [44,45]. We therefore scrutinized the annotated genomes for all nine integration loci in O. oeni and retrieved the genes surrounding the attB sites for functional classification (average distance was 4200 bp) (Figure 1). Integration occurred next to some genes which play important roles in bacterial cellular systems, and specify proteins involved in cell wall metabolism such as the WalK/WalR two-component system [46], response to osmotic stress (mechanosensitive channel of large conductance, MscL), a quality control system monitoring protein synthesis (SsrA) or carbohydrate metabolism (glucokinase, acetoin reductase, catabolite control A (CcpA)). CcpA is involved in the adaptation to changing carbon sources. More recently, its role as a pleiotropic regulator, controlling not only carbohydrate metabolism but also stress response, has been proposed in different LAB under aerobic conditions [47]. The hypothesis that some prophages/PRs may act as switches that regulate the expression of neighboring genes is attractive in O. oeni and deserves further work as this may have practical significance for the production of MLF starter cultures.
Of note, two sites (attBC and attBF) could accommodate a PR, or a PR and a phage in a tandem association. We re-examined the sequences flanking these PRs and/or prophages amongst relevant strains and found that the two expected attL and attR junctions were conserved in tandem associations at the attBD site but not at the attBC locus. The latter consisted of a 78 bp repeated sequence, which may represent the ancestral attBC site. This size is larger than proposed earlier [15,17,38]. In all combinations (PRC alone or tandem association with an IntC prophage), we consistently identified a degenerated attRC sequence in the remnant, which may be indicative of its domestication. PRC had maintained >99% nucleotide similarity across its entire genome, suggesting that it is under strong evolutionary pressure and likely provides an important biological function to the host. Its prevalence in the whole population (75.3%) was higher compared to IntC phages (Table 1). It is likely that the acquisition of PRC preceded that of IntC phages during the evolution of O. oeni. The loss of the attRC site resulting from domestication did not hamper further integration of IntC phages at the intact attLC site (Figure 2). In contrast, the ability of the intact IntC prophages to excise is likely to be impaired as we did not observe any spontaneous or MC-induced excision of the phage in strains containing an IntC phage/PRC tandem association, such as S28.
3.4. Lysogeny Is Widespread among Wine Strains from Phylogroup A
We next explored whether quantitative and qualitative differences in patterns of prophages (as assessed from their integrase and attachment site used for SSR) differ across lineages and niches occupied by strains of O. oeni (Table 1). Most of the currently sequenced O. oeni strains in our set have, to date, been collected from wines and belong to phylogroup A (75%; n = 174). This enables a robust assessment of lysogeny in this particular phylogroup/niche.
Phylogroup A was particularly open to the uptake of foreign DNA of phage origin since 63% of the strains were lysogens (Table 1). Yet, prophages were not distributed uniformly across the different sub-groups described in the lineage. Interestingly, prevalence differed between the AR and AW sub-lineages, corresponding to strains adapted to red and white wines, respectively [10]. Hence, 100% of strains (n = 10) in sub-group AW associated with white wines produced in Burgundy and Champagne were lysogens [9]. In contrast, the proportion was only 40% (n = 10) in the Burgundy red wines subgroup (AR). Lysogeny was less frequent in other sub-groups of strains in phylogroup A. As an example, only one of the seven strains constituting the PSU-1 subgroup was observed to carry a prophage [9], which is lower than the mean value observed in the whole O. oeni species.
Phylogroup A strains appear enriched in some prophages, corresponding to members of the IntA, IntB and IntC groups. Conversely, and with few exceptions, these prophages were less abundant or absent in other lineages (phylogroups B, C and D) (Table 1). Of note, despite different origins of wine and time of collection, all 10 strains from sub-group AW (white wines) exclusively harbored an IntA phage. Eight of ten had the same organization and sequence. This may reflect selection for the acquisition of locally adaptive functions that are transferred by the IntA phage genomes. Alternatively, the IntA phages may have piggybacked on hosts that outcompeted other variants in the process of natural selection in the specific white wines considered [48].
The second pattern seen was the frequent carriage of two distinct prophages in phylogroup A strains, as 45 out of the 47 poly-lysogens detected in the whole O. oeni species belonged to this particular phylogroup. This may suggest that interactions between prophages may be beneficial for the host, by reducing the rate of spontaneous lysis or regulating gene expression under specific wine conditions [27]. This hypothesis is attractive and needs more experimental support. Phylogroup A strains contained the six specific combinations of prophage carriage observed in the whole species: IntA + IntC (n = 17), IntB + IntC (n = 14), IntA + IntD (n = 8), IntA + IntB (n = 3), IntA + IntC + IntD (n = 3) and IntA + IntB + IntC (n = 1) (Table 1). Of note, prophages from the IntD groups were found in 14 lysogens from phylogroup A, of which 11 corresponded to poly-lysogens. Since IntD phages are the only prophages integrated on the left replichore, their presence and possibly low induction rate could balance the prophage integrations on the other replichore and stabilize the genome architecture. Although mono-lysogens for IntD and IntB prophages are found in wine strains, no IntD + IntB double lysogens were detected in phylogroup A, nor in the whole population. Each of these prophages may encode a resistance mechanism to phage superinfection. Alternately, the prophages may be incompatible with each other, or their presence may decrease cell fitness and lead to the extinction of the poly-lysogens in wine.
Further insight into the peculiarity of phylogroup A strains was also provided when we re-examined the distribution of PR elements (Table 1). We found that PRC was present in all strains from phylogroup A, suggesting the domestication of the corresponding ancestral phage in wine strains, possibly leading to niche-specific fitness effects. These genetic signatures might also have applications as they could be used for typing purposes.
Phylogroups B, C and D are less numerously represented in our set of 231 strains (Table 1). Yet, with this caveat in mind, differences in prophage content, with respect to phylogroup A, were found. We detected prophages with lower frequency in phylogroup C (38%) while the value in phylogroup B (55%) was closer to that reported in phylogroup A (60%). Both phylogroups are known to contain strains originating from cider or wine, and the latter have been consistently isolated following completion of AF [9]. Patterns of prophages were also different in phylogroups B and C and IntD phages were the most represented prophages. In particular, they corresponded to 12 out of the 15 prophages identified in mono-lysogens in phylogroup B. Only two poly-lysogens were found. Interestingly, phylogroup C was characterized by the presence of the specific IntE prophages, observed in two cider strains and in strain AWRIB663 from wine. The same characteristic was observed for IntF prophages, as well as PRG, which have, to date, been exclusively found in kombucha strains. Due to the limited number of strains in the B, C and D phylogroups, further investigation and isolation campaigns on ciders, kombucha and possibly other fruits and kefirs are now needed.
3.5. Integrase Phylogeny
We performed a multiple alignment of phage-related integrases from O. oeni. We first included a representative sequence from each of the IntA-F phage groups and all four PR-encoded integrases. The size of the 10 integrases ranged from 340 to 389 aa and we found 25 invariant amino acid residues, of which 10 are located in the C-terminal catalytic domain of the phage recombinases. Amongst them, the catalytic residue tetrad Arg–His–Arg–Tyr (R–H–R–Y), which is needed for DNA cleavage and joining in the integrase family of tyrosine recombinases, was identified (Figure S2).
In order to construct a maximum likelihood tree, we next included integrases from phages infecting the related species O. sicerae from ciders and kefir, O. kitaharae from sake [29,30,31] and other LAB belonging to Pediococcus, Lactiplantibacillus, Fructilactobacillus and Streptococcus genera [49] (Figure 3). The putative O. oeni XerC/XerD recombinases served as an outgroup. Of note, both XerC and XerD from O. oeni resemble the XerS protein which is involved in the cell recombination machinery of Lc. lactis [50].
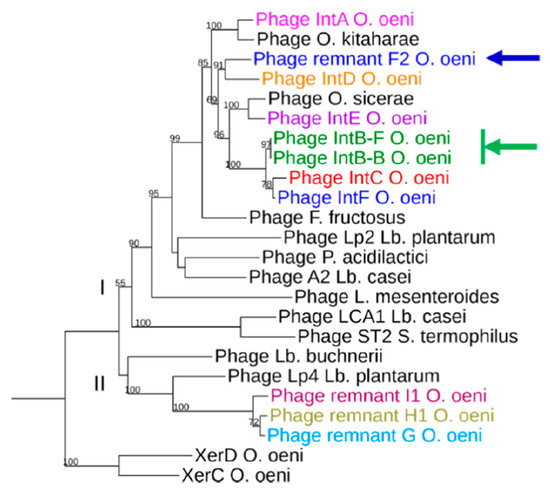
Figure 3.
Phylogenetic tree of integrases found in O. oeni and other LAB. Sequences are distributed in clusters I and II. Their origin is as follows: IntA (IOEB0608); IntB Φ10MC (AAD00268); IntC (IOEBS28); IntD (IOEB9805); IntE CRBO1384; IntF (UBOCC315001); Int PRI1 (IOEBS277); Int PRH1 (AWRIB422); Int PRG (UBOCC315001); Int PRF1 (DIV5-23); Int from phages Lca1 (ABD83428) and A2 (NP_680502); unnamed phage infecting P. acidilactici 7-4 (ZP_06197614); Int from prophages found in Lb. plantarum WCFS1 (NP_785910), STIII (ADN97941) and Lp2 prophage (WP_011101785.1); O. sicerae (WP_128686695.1); O. kitaharae (EHN59022); L. mesenteroides ATCC19254 (ZP_03913849) and Lb. buchneri ATCC 11577 (ZP_03943244). The XerC and XerD proteins were obtained from O. oeni PSU-1 (WP_002817147 and WP_002820463.1) and served as an outgroup. Arrows represent incongruences between integrase phylogeny and attB location of the corresponding prophages in the bacterial genomes. The PRF2-associated integrase-coding gene was split into two ORFs and carried a frameshift mutation due to a missing nucleotide. A single nucleotide was added in the premature stop codon to suppress the nonsense mutation.
As seen in Figure 3, most integrases identified in O. oeni belonged to two major clusters, suggesting distinct evolutionary trajectories for each group. The majority of phage integrases formed a cluster (cluster I) with sequences from phages infecting other LAB (Lb. plantarum, P. acidilactici, Lb. casei and L. mesenteroides). This is consistent with the current literature showing the existence of cross-transmission networks between O. oeni and other LAB, as a variety of species coexist on grapes and must, including, notably, Lactiplantibacillus plantarum [13,49,51]. In addition, recent phylogenomics also resulted in the assignment of Leuconostocaceae and Lactobacillaceae into a single family [49]. A sub-group in cluster I was specific to prophages infecting species of the Oenococcus and Fructilactobacillus genera. In the latter genus, F. fructosus is the only species found in wine [52]. Interestingly, the IntA type integrase was separate, and grouped evolutionarily with a sequence from O. kitaharae. Remarkably, the IntE sequence grouped with the integrase found in a prophage from O. sicereae. The corresponding lysogens (affiliated to the O. oeni and O. sicerae species) were both isolated from cider. It would therefore be interesting to assess whether the oenophages can infect individual hosts from different species in cider and other fermented beverages. We also observed that the three integrases IntB, IntC and IntF, which drive the integration of their cognate phages in the same region in the host chromosome, were more closely related (Figure 1). Lastly, the only integrase from a remnant in cluster I was that from PRF. In contrast, the integrases harbored by PRH1-H2, PRI1-I2 and PRG formed a distinct lineage, and clustered with two integrases associated with other LAB species. The inspection of the alignment showed that these five PR-associated integrases had, notably, an additional domain of 20 amino acids upstream of the tyrosine catalytic residue compared to other oenophage integrases. They may not represent remnants of previous lysogenization by full-length prophages, but rather belong to a unique family of mobile genetic elements.
3.6. Correlation of Phage Phylogeny with attB Location
The correlation of phage integrase phylogeny with attB location on the bacterial chromosome was confirmed with two notable exceptions, which were both related to the occupancy of the attBF site (Figure 1 and Figure 3). This particular locus, close to the replication termini, could indeed accommodate an IntB prophage, or an IntF prophage and/or a PRF remnant in the different strains of our panel. This raised two interesting questions. How did the IntB recombinase display a relaxed specificity, driving the integration of the phage genome (hereby an IntB group member) at distinct locations (attBB or attBF) in the chromosome of distinct strains of O. oeni? Next, we also questioned the reason why PRF integrates at the attBF site, although its cognate integrase was more closely related to members of the IntD than to the IntF group. The rationale behind these observations and questions was that a more detailed analysis of the SSR units of these oenophages and PRs was needed.
3.6.1. SSR of IntF and IntB Prophages
During SSR, cross-activity of a recombinase of one phage with the attachment site of another is proportional to the degree of homology between their integrases and similarity between the core- and arm-type sequences in attachment sites of the respective phages [53]. We therefore clarified the site preference pattern and sequence requirements for the SSR of IntF and IntB phages by analyzing their attP sites. To carry out the analyses, phage attachment core sequences were deduced from the attL and attR junctions within the host chromosome identified in lysogens (accessed from GenBank) and from the attP sequences from the genome of free IntB phages isolated from the enological environment [18,54]. For clarity, the phages belonging to the IntB group were subdivided into the IntB-F and IntB-B sub-groups, depending on the attachment site targeted in the bacterial chromosome (attBB or attBF). Prophages harbored by strain LAB2013 and IOEBB10 were their representatives, respectively. IntF phages were represented by the prophage of strain UBOCC315001.
SSR of IntF phages was found to complement the 13 bp 3′ end of the bacterial tRNALeu 923. This sequence does not encompass the tRNA anticodon loop. The identity block common to the phage and bacterial sequences extended well beyond, and a 63 bp homologous pair was identified (Figure 4A). It is well established that SSR requires a longer sequence at attP than at attB. Tyrosine recombinases usually possess a low binding affinity to the core site of attP and a high binding affinity for the flanking arm regions [53]. We next questioned what sequences within attPF actually support integrase binding in O. oeni. We addressed this question by screening the arm regions for direct repeats (DRs), which could serve as putative arm-binding sites recognized by the N-terminus of the integrase (Figure 4A). A short 10 bp DR was found (5′ATTTGCACAA3′, Figure 4A). It was not evenly distributed on both arms around the core, with two and four repeats on the left and right arms (P1 to P6), respectively (Figure 4A and Table 3). Like other characterized systems amongst LAB phages, the proposed core site exhibited essentially no symmetry and no putative inverted core-binding sites were identified [55]. With the caveat that additional putative binding sites for accessory proteins (excisionase, integration host factor) may be present, we propose that the size of the attP sequence of IntF phages is at least 240 bp. This size is consistent with the well-documented model proposed by Campbell for λ integration in Escherichia coli [56,57].
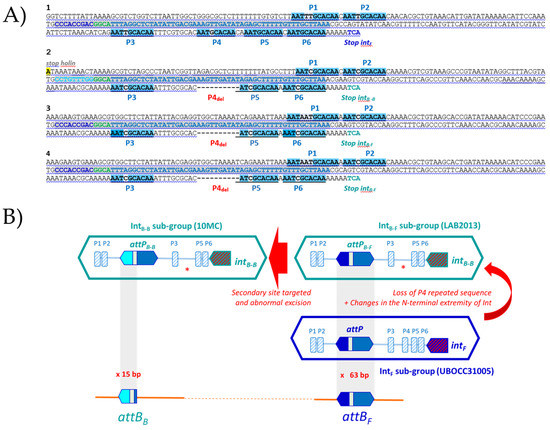
Figure 4.
Interrogation of oenococcal genomes for the SSR units of IntB-B, IntB-F and IntF prophages. (A) Alignments of the attP regions involved in SSR for IntF phages (1 in blue), the proposed sub-types IntB-B and IntB-F phages (3 and 4 in green). 1, IntF prophage from strain UBOCC315005; 2, IntB-B prophage from strain IOEBB10; 3, IntB-F prophage from strain LAB2013; 4, IntB-F phage OE33PA. P1 to P6, Direct repeats. The 63 bp block identity is in gray. (B) Boxed sequences represent the attP sequences of IntB and IntF phages. Arrows indicate the possible complex mechanisms of evolution of SSR units during niche adaptation.

Table 3.
Characteristics of the attB and attP regions from oenophages and phage remnants.
Recombination of IntB-F prophages also required the same 63 bp core sequence, common to attP and attBF. However, the detailed analysis of the whole attPB-F region showed some deletions, resulting in the loss of the P4 repeat on the right arm (Figure 4A, Table 3). As a consequence, there was a reduction in the number of DRs, as well as a modified spacing between the P3 and P5 repeats, which may impact protein–protein and/or protein–DNA interactions during intasome formation. Another striking difference was found when the IntB-F and IntF protein sequences were compared (Figure S2). Despite their high identity (92%), they differed by 46 amino acid (aa) changes, of which 21 (45%) mapped to the 66 aa amino terminus, representing 18% of the integrase. Similar results were observed with the IntB integrases obtained from the free replicating phages OE33PA and B148 previously isolated from wine [17,18]. The size of the IntF N-terminus domain which concentrates the mutations (66 aa) is in agreement with the assignment of the minimal Int fragment of 64 aa that binds to arm-type sites in the lambda model [58].
Altogether, our data are consistent with the existence of different N-termini in IntF and IntB-F integrases, allowing each recombinase to bind the specific sets of DRs on the arm regions in each attP site. The observed mutations may have a broader impact on SSR since the N-terminal domain of Int also plays a role in modulating the activity of core-binding and catalytic domains [59]. In addition, the impact of the mutations associated with the other domains of the integrase protein sequences also needs further work.
Noteworthily, IntF and IntB-F phages were associated with strains from distinct niches, corresponding to kombucha and wine, respectively. It is likely that the presence of slight modifications in their SSR units needs to be examined in light of the distinct niches where their bacterial hosts are evolving. Our observations may reflect the coevolution of the attPF and integrase sequences in response to changing conditions. They may be a signature of distinct evolutionary trajectories across the different niches colonized by O. oeni, and represent signatures of adaptation to wine. We posit that modifications in the attPF site may have progressively arisen during the adaptation of O. oeni to more drastic variations in habitat conditions (wine) and have been coupled to mutations in the IntF sequence. The latter adapted the novel integrase (IntB) to this new and altered site, resulting in the variations observed within the SSR units of IntF and IntB-F phages [60]. Altogether, our observations may reflect the evolutionary processes resulting in niche divergence. If this hypothesis is correct, it questions the existence of selection for the preservation of attBF as an integration site for phages in two distinct niches (kombucha and wine).
Last, it is likely that IntB-F phages went through additional and distinct evolutionary processes in wine, yielding IntB-B phages. Hence, the latter prophages were shown to integrate into a distinct gene, 66 kb away from the attBF site used by IntB-F. Both sites were part of tRNALeu genes. The SSR of IntB-F and IntB-B phages required the same overall region in the phage genomes, but the nature of the sequences involved were different. Striking differences found in IntB-B phages included a reduced number of DRs on arms (5) and the substitution of the 5′ extremity of the 63 bp sequence by a new 9 bp sequence. In addition, the identity block between host and IntB-B phage sequences was reduced to the first 15 bp of the previously proposed 63 bp core (Figure 4B). Since the IntB integrase recombines regardless of the lateral 9 bp sequence in the attP site present in IntB-B and IntB-F phages, the latter nucleotides probably do not represent core-binding sequences for the integrase. The role is probably devoted to the downstream 6 bp sequence that is common to attPB-B and attPB-F sites and where cleavage is likely to occur. The emergence of IntB-B phages is likely to result from mutations leading to a much more substantial increase in the recognition of a secondary site by IntB-F phages followed by an abnormal excision. This hypothesis is consistent with the chromosome jumping model described in lambda [61].
3.6.2. SRR of PRF Elements
The PRF1 and PRF2 remnants are integrated in the attBF site in strains collected from cider and wine and from kombucha (phylogroups B and D, respectively). Yet, their integrase was more related to the IntD than to the IntF type (Figure 3). Both integrase-coding gene were split into two ORFs in PRF1 and PRF2, possibly due to a frameshift mutation (Figure 2). The sequence was manually inspected and a single nucleotide was added in the premature stop codon to suppress the nonsense mutation and the corrected deduced protein sequence was used to build the phylogeny of integrase proteins (Figure 3). We assessed whether an error at this step may have caused an alignment bias, explaining the incongruence of the PRF recombinase in the tree. To verify this, we compared the three int nucleotide sequences found in PRF, IntF and IntD prophages (Figure S3). This confirmed the closer relation of the integrase nucleotide sequences from PRF and IntD prophages. The reason for the discrepancy between the integrase type and integration site of PRF is not known. However, it can be suggested that PRF elements have resulted from modular exchanges in the lysogeny modules between two integrated IntD and IntF oenophages, due to intra-chromosomal homologous recombination. Homologous recombination between two prophages integrated equidistant from the ter region has been reported in S. pyogenes. Such phage-related rearrangements resulted in a large chromosomal inversion of the region between the attachment sites, and the emergence of two novel hybrid prophages with exchanged genes [62]. In O. oeni, intD and intF sequences contain homologous regions. In addition, such homologous recombination events may also be mediated by the bacterial sequences flanking each prophage which both correspond to a tRNALeu gene.
3.7. Most attP Regions in Oenophages Derive from Two Distinct Sequences
Surprisingly, all attP sequences upstream of the integrase gene in IntA, IntB, IntC, IntD and IntF prophages had similar sequences, suggesting a common origin (Figure 5).
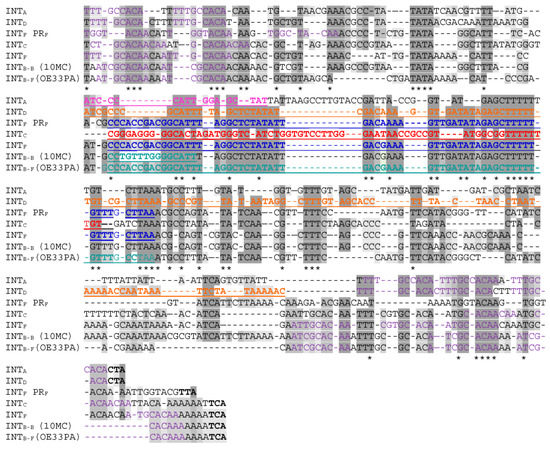
Figure 5.
Alignments of the attP sequences found in the different oenophages. The homologous core sequences involved in recombination with the bacterial chromosome are underlined and in color (the color code is the same as shown in Figure 1). All direct repeats serving as putative binding sites for integrases are in purple. The three last nucleotides correspond to the stop codon of the integrase genes. A clustal alignment was first constructed and gaps were introduced manually.
Despite the presence of several indel events, all core sequences involved in RSS (underlined in Figure 5) had a common feature and were flanked by direct repeat sequences, which are proposed to represent the binding sites for the different recombinases (Table 3). Coevolution of the phage components of SSR units have progressively occurred and involved indels in the core, the slippering of DR sequences and modifications in the N-terminus of the integrase (Figure 5). Through such events, recombinase activity is retained while the core attP sites are progressively adjusted to novel loci in the chromosome of the host. This may indicate long-term coexistence between these phages and the host in fermented beverages.
Of note, the attPs from IntE phages were distinct in their sequence and also lacked DR repeats compared to other oenophages. Future studies should experimentally confirm whether the IntE recombinase can utilize the candidate core sequences for recombination reactions without flanking inverted repeats. The comparative analysis of a set of completely sequenced oenophage genomes has recently demonstrated that prophages are distributed into two clusters of cos (members of IntA and IntB groups) and pac (IntD) phages [63]. Even though the SSR unit of IntE phages has some peculiarities, the latter have not evolved completely independently. As seen in Figure 6, IntE phages are closely related to the cos phages, together with IntC and IntF prophages. Corresponding genomes in this cluster are mosaics, whereby individual phages are constructed as assemblages of modules, many of which are single genes (result not shown). Of note, this cluster harbors prophages which are integrated on the same replichore in the chromosome of O. oeni. In contrast, the cluster corresponding to IntD prophages is less diverse and members share relatively few genes with the other cluster.
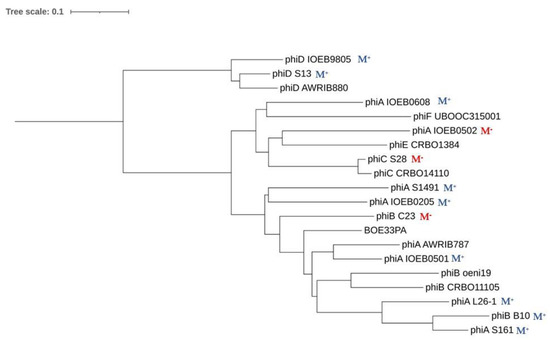
Figure 6.
Phylogenomic Genome BLAST Distance Phylogeny (GBDP) tree of representative oenophages from the CRB Oeno Collection. The tree was generated by VICTOR and visualized with Fig Tree [36]. Int groups are represented by letters (A to F). B10 is the prophage found in strain IOEB B10. M+ and M− indicate the presence or absence of (i) a lysis when the lysogen was grown in the presence of mitomycin C and (ii) plaques when the MC lysate was tested on the sensitive strain IOEB S77.
4. Concluding Remarks
A short minimal doubling time under optimal growth conditions was shown to represent the trait most correlated with lysogeny among bacteria, and the frequency of lysogeny was also increased with bacterial genome size [41]. Clearly, O. oeni stands out from the well-studied model systems used in this study, and probably faces specific ecological conditions which constrain the lytic–lysogeny decision and favor lysogeny and poly-lysogeny. This may generate more benefits than costs for both partners.
Our results also confirmed that tRNA genes are the preferred chromosomal integration sites in O. oeni and that integration tropisms are associated with the phylogeny of the phage integrases. Interestingly, all bacterial attachment sites, except attBD, are located in one replichore (one half of the chromosome) and four lie within a 300 kb region, which is therefore a region of high plasticity in the species. Such lopsided phage integrations into chromosomal DNA may result in an unsymmetrical genome architecture across the replication axis, and induce chromosomal rearrangement for stabilizing the genome architecture. Yet, such rearrangements are not observed in O. oeni [9].
The different niches where the Oenococcus species is found have unique physical, chemical and biological profiles that likely promote speciation of both phages and their bacterial hosts. Accordingly, phages infecting strains associated with kombucha, cider and wines were observed to exhibit differences in their SSR units and more work is now needed to explore their gene repertoire as well, and assess whether they provide the bacterial hosts with additional genes and competitive advantages.
Considering whether prophages (or combinations thereof) have a positive role in the adaptation of O. oeni to wine, it could be expected that strains would domesticate such beneficial prophages, as seen with PR regions, in order to prevent their excision. This is probably the case for IntC prophages, as we could not yet detect excision of phage DNA/particles in past studies. In contrast, many pieces of experimental evidence show that members of the frequently encountered IntA and IntB prophages in wine are still active and can excise. Therefore, it is unclear why IntA and IntB phages are less prone to grounding, compared to members of the IntC group. Reversible lysogeny could be of importance for genome architecture, as the grounding of the various prophages located on the right replichore could be detrimental. In addition, the ability of such prophages to excise could represent a competitive mechanism to eliminate sensitive non lysogenic strains, or to lysogenize them, increasing the tolerance of the population to stressful conditions. Conversely, site-specific integration of IntA and IntB phages may be beneficial under certain circumstances and modulate the expression of essential genes in stressful conditions. These characteristics might reflect the different evolutionary strategies and opposite selection pressures as a consequence of adaptation to diverse niches in which the different phylogroups have evolved.
Supplementary Materials
The following materials are available online at https://www.mdpi.com/article/10.3390/microorganisms9040856/s1. Table S1: Genomes of lysogenic and non-lysogenic strains of O. oeni used in this study; Figure S1: Kinetics of bacteriophage induction from the lysogen O. oeni IOEB0608 with (■) and without (□) mitomycin C treatment (1 µg/mL). Each culture was grown in MRS broth to an OD600 of 0.2–0.3 and then separated into two aliquots, one of which was induced with mitomycin C; Figure S2: Comparisons of the IntF and IntB protein sequences. Mutations are in gray. The four conserved amino acids in the C-terminal region (catalytic domain) of all integrases found in O. oeni are in red; Figure S3: Alignments of the nucleotide sequences of the integrase genes from PRF and IntF and IntD prophages. The yellow color represents deletions in intPRF and intD sequences. The blue color represents deletions only observed in the intF sequence.
Author Contributions
O.C., C.L.M., P.M.L. designed experiments, assembled and analyzed the genomic data and prepared the manuscript. A.C., F.J., C.P., Y.B. performed experimental work. C.L.M. supervised the work progress and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Support for this project was provided by the Conseil Regional Nouvelle Aquitaine and French ANR (grant ANR-2020-CE20-0008-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Grandvalet, C. Oenococcus oeni: Queen of the cellar, nightmare of geneticists. Microbiology 2017, 163, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, M.P.G.; Lucas, P.M. Distribution of Oenococcus oeni populations in natural habitats. Appl. Microbiol. Biotechnol. 2019, 103, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.A.; Rawsthorne, H.; Parker, C.; Tamir, D.; Makarova, K. Genomic analysis of Oenococcus oeni PSU-1 and its relevance to winemaking. FEMS Microbiol. Rev. 2005, 29, 465–475. [Google Scholar] [CrossRef]
- Borneman, A.R.; McCarthy, J.M.; Chambers, P.J.; Bartowsky, E.J. Comparative analysis of the Oenococcus oeni pan genome reveals genetic diversity in industrially-relevant pathways. BMC Genom. 2012, 13, 373. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Vuillemin, M.; Campbell-Sills, H.; Lucas, P.M.; Ballestra, P.; Miot-Sertier, C.; Favier, M.; Coulon, J.; Moine, V.; Doco, T.; et al. Exopolysaccharide (EPS) synthesis by Oenococcus oeni: From genes to phenotypes. PLoS ONE 2014, 9, e98898. [Google Scholar] [CrossRef]
- Sternes, P.R.; Borneman, A.R. Consensus Pan-Genome Assembly of the Specialised Wine Bacterium Oenococcus oeni. BMC Genom. 2016, 17, 813. [Google Scholar]
- Campbell-Sills, H.; Khoury, M.E.; Favier, M.; Romano, A.; Biasioli, F.; Spano, G.; Sherman, D.J.; Bouchez, O.; Coton, E.; Coton, M.; et al. Phylogenomic analysis of Oenococcus oeni reveals specific domestication of strains to cider and wines. Genome Biol. Evol. 2015, 7, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, M.; Campbell-Sills, H.; Salin, F.; Guichoux, E.; Claisse, O.; Lucas, P.M. Biogeography of Oenococcus oeni Reveals Distinctive but Nonspecific Populations in Wine-Producing Regions. Appl. Environ. Microbiol. 2017, 83, e02322-16. [Google Scholar] [CrossRef]
- Lorentzen, M.P.; Campbell-Sills, H.; Jorgensen, T.S.; Nielsen, T.K.; Coton, M.; Coton, E.; Hansen, L.; Lucas, P.M. Expanding the biodiversity of Oenococcus oeni through comparative genomics of apple cider and kombucha strains. BMC Genom. 2019, 20, 330. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Sills, H.; El Khoury, M.; Gammacurta, M.; Miot-Sertier, C.; Dutilh, L.; Vestner, J.; Capozzi, V.; Sherman, D.; Hubert, C.; Claisse, O.; et al. Two different Oenococcus oeni lineages are associated to either red or white wines in Burgundy: Genomics and metabolomics insights. OENO One 2017, 51, 309. [Google Scholar] [CrossRef]
- Marcobal, A.M.; Sela, D.A.; Wolf, Y.I.; Makarova, K.S.; Mills, D.A. Role of hypermutability in the evolution of the genus Oenococcus. J. Bacteriol. 2008, 190, 564–570. [Google Scholar] [CrossRef]
- Meier, P.; Wackernagel, W. Impact of mutS inactivation on foreign DNA acquisition by natural transformation in Pseudomonas stutzeri. J. Bacteriol. 2005, 187, 143–154. [Google Scholar] [CrossRef][Green Version]
- El Gharniti, F.; Dols-Lafargue, M.; Bon, E.; Claisse, O.; Miot-Sertier, C.; Lonvaud, A.; Le Marrec, C. IS30 elements are mediators of genetic diversity in Oenococcus oeni. Int. J. Food Microbiol. 2012, 158, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Favier, M.; Bilhère, E.; Lonvaud-Funel, A.; Moine, V.; Lucas, P.M. Identification of pOENI-1 and related plasmids in Oenococcus oeni strains performing the malolactic fermentation in wine. PLoS ONE 2012, 7, e49082. [Google Scholar] [CrossRef] [PubMed]
- Bon, E.; Delaherche, A.; Bilhère, E.; De Daruvar, A.; Lonvaud-Funel, A.; Le Marrec, C. Oenococcus oeni genome plasticity is associated with fitness. Appl. Environ. Microbiol. 2009, 75, 2079–2090. [Google Scholar] [CrossRef]
- Poblet-Icart, M.; Bordons, A.; Lonvaud-Funel, A. Lysogeny of Oenococcus oeni (syn. Leuconostoc oenos) and study of their induced bacteriophages. Curr. Microbiol. 1998, 36, 365–369. [Google Scholar] [CrossRef]
- Jaomanjaka, F.; Ballestra, P.; Dols-Lafargue, M.; Le Marrec, C. Expanding the diversity of oenococcal bacteriophages: Insights into a novel group based on the integrase sequence. Int. J. Food Microbiol. 2013, 166, 331–340. [Google Scholar] [CrossRef]
- Philippe, C.; Jaomanjaka, F.; Claisse, O.; Laforgue, R.; Maupeu, J.; Petrel, M.; Le Marrec, C. A survey of oenophages during wine making reveals a novel group with unusual genomic characteristics. Int. J. Food Microbiol. 2017, 257, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Howard-Varona, C.; Hargreaves, K.R.; Abedon, S.T.; Sullivan, M.B. Lysogeny in nature: Mechanisms, impact and ecology of temperate phages. ISME J. 2017, 11, 1511–1520. [Google Scholar] [CrossRef]
- Wahl, A.; Battesti, A.; Ansaldi, M. Prophages in Salmonella enterica: A driving force in reshaping the genome and physiology of their bacterial host? Mol. Microbiol. 2019, 111, 303–316. [Google Scholar] [CrossRef]
- Ruiz-Cruz, S.; Parlindungan, E.; Erazo Garzon, A.; Alqarni, M.; Lugli, G.A.; Ventura, M.; van Sinderen, D.; Mahony, J. Lysogenization of a Lactococcal Host with Three Distinct Temperate Phages Provides Homologous and Heterologous Phage Resistance. Microorganisms 2020, 8, 1685. [Google Scholar] [CrossRef]
- Matos, R.C.; Lapaque, N.; Rigottier-Gois, L.; Debarbieux, L.; Meylheuc, T.; Gonzalez-Zorn, B.; Repoila, F.; Lopes, M.d.F.; Serror, P. Enterococcus faecalis prophage dynamics and contributions to pathogenic traits. PLoS Genet. 2013, 9, e1003539. [Google Scholar] [CrossRef]
- Aucouturier, A.; Chain, F.; Langella, P.; Bidnenko, E. Characterization of a Prophage-Free Derivative Strain of Lactococcus lactis ssp. lactis IL1403 Reveals the Importance of Prophages for Phenotypic Plasticity of the Host. Front. Microbiol. 2018, 9, 2032. [Google Scholar] [CrossRef]
- Lynn-Bell, N.L.; Strand, M.R.; Oliver, K.M. Bacteriophage acquisition restores protective mutualism. Microbiology 2019, 165, 985–989. [Google Scholar] [CrossRef] [PubMed]
- De Sordi, L.; Lourenço, M.; Debarbieux, L. “I will survive”: A tale of bacteriophage-bacteria coevolution in the gut. Gut Microbes 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Braga, L.P.P.; Soucy, S.M.; Amgarten, D.E.; da Silva, A.M.; Setubal, J.C. Bacterial Diversification in the light of the interactions with phages: The genetic symbionts and their role in ecological speciation. Front. Ecol. Evol. 2018, 6, 6. [Google Scholar] [CrossRef]
- Szafrański, S.P.; Kilian, M.; Yang, I.; Der Wieden, G.B.; Winkel, A.; Hegermann, J.; Stiesch, M. Diversity patterns of bacteriophages infecting Aggregatibacter and Haemophilus species across clades and niches. ISME J. 2019, 13, 2500–2522. [Google Scholar] [CrossRef] [PubMed]
- Badotti, F.; Moreira, A.P.B.; Tonon, L.A.C.; De Lucena, B.T.L.; Gomes, F.D.C.O.; Krüger, R.; Thompson, C.C.; De Morais, M.A.; Rosa, C.A.; Thompson, F.L. Oenococcus alcoholitolerans sp. nov., a lactic acid bacteria isolated from cachaça and ethanol fermentation processes. Antonie Van Leeuwenhoek 2004, 106, 1259–1267. [Google Scholar] [CrossRef]
- Endo, A.; Okada, S. Oenococcus kitaharae sp. nov., a non-acidophilic and non-malolactic-fermenting Oenococcus isolated from a composting distilled shochu residue. Int. J. Syst. Evol. Microbiol. 2006, 56, 2345–2348. [Google Scholar] [CrossRef]
- Cousin, F.J.; Le Guellec, R.; Chagnot, C.; Goux, D.; Dalmasso, M.; Laplace, J.M.; Cretenet, M. Oenococcus sicerae sp. nov., isolated from French cider. Syst. Appl. Microbiol. 2019, 42, 302–308. [Google Scholar] [CrossRef]
- Verce, M.; De Vuyst, L.; Weckx, S. The metagenome-assembled genome of Candidatus Oenococcus aquikefiri from water kefir represents the species Oenococcus sicerae. Food Microbiol. 2020, 88, 103402. [Google Scholar] [CrossRef]
- McNair, K.; Aziz, R.K.; Pusch, G.D.; Overbeek, R.; Dutilh, B.E.; Edwards, R. Phage Genome Annotation Using the RAST Pipeline. Methods Mol. Biol. 2018, 1681, 231–238. [Google Scholar]
- Czajkowski, R. May the Phage be With You? Prophage-Like Elements in the Genomes of Soft Rot Pectobacteriaceae: Pectobacterium spp. and Dickeya spp. Front. Microbiol. 2019, 10, 138. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Göker, M. VICTOR: Genome-based Phylogeny and Classification of Prokaryotic Viruses. Bioinformatics 2017, 33, 3396–3404. [Google Scholar] [CrossRef]
- Philippe, C.; Krupovic, M.; Jaomanjaka, F.; Claisse, O.; Petrel, M.; Le Marrec, C. Bacteriophage GC1, a novel Tectivirus Infecting Gluconobacter cerinus, an acetic acid bacterium associated with wine-making. Viruses 2018, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- São-José, C.; Santos, S.; Nascimento, J.; Brito-Madurro, A.G.; Parreira, R.; Santos, M.A. Diversity in the lysis-integration region of oenophage genomes and evidence for multiple tRNA loci, as targets for prophage integration in Oenococcus oeni. Virology 2004, 325, 82–95. [Google Scholar] [CrossRef]
- Petersen, A.; Josephsen, J.; Johnsen, M.G. TPW22, a lactococcal temperate phage with a site-specific integrase closely related to Streptococcus thermophilus phage integrases. J. Bacteriol. 1999, 181, 7034–7042. [Google Scholar] [CrossRef]
- Van der Ploeg, J.R. Characterization of Streptococcus gordonii Prophage PH15: Complete Genome Sequence and Functional Analysis of Phage-Encoded Integrase and Endolysin. Microbiology 2008, 154, 2970–2978. [Google Scholar] [CrossRef]
- Bobay, L.M.; Rocha, P.C.; Touchon, M. The Adaptation of Temperate Bacteriophages to Their Host Genomes. Mol. Biol. Evol. 2013, 30, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Touchon, M.; Rocha, E.P. Coevolution of the Organization and Structure of Prokaryotic Genomes. Cold Spring Harb. Perspect. Biol. 2016, 8, a018168. [Google Scholar] [CrossRef]
- Kopejtka, K.; Lin, Y.; Jakubovičová, M.; Koblížek, M.; Tomasch, J. Clustered Core- and Pan-Genome Content on Rhodobacteraceae Chromosomes. Genome Biol. Evol. 2019, 11, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Feiner, R.; Argov, T.; Rabinovich, L.; Sigal, N.; Borovok, I.; Herskovits, A.A. A new perspective on lysogeny: Prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 2015, 13, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.N.; Mettert, E.L.; Fishman-Engel, D.R.; Roggiani, M.; Kiley, P.J.; Goulian, M. Phage integration alters the respiratory strategy of its host. eLife 2019, 8, e49081. [Google Scholar] [CrossRef]
- Takada, H.; Yoshikawa, H. Essentiality and function of WalK/WalR two-component system: The past, present, and future of research. Biosci. Biotechnol. Biochem. 2018, 82, 741–751. [Google Scholar] [CrossRef]
- Li, C.; Sun, J.W.; Zhang, G.F.; Liu, L.B. Effect of the absence of the CcpA gene on growth, metabolic production, and stress tolerance in Lactobacillus delbrueckii ssp. bulgaricus. J. Dairy Sci. 2016, 99, 104–111. [Google Scholar] [CrossRef]
- Knowles, B.; Silveira, C.B.; Bailey, B.A.; Barott, K.; Cantu, V.A.; Cobián-Güemes, A.G.; Coutinho, F.H.; Dinsdale, E.A.; Felts, B.; Furby, K.A.; et al. Lytic to temperate switching of viral communities. Nature 2016, 531, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Le Bourgeois, P.; Bugarel, M.; Campo, N.; Daveran-Mingot, M.L.; Labonté, J.; Lanfranchi, D.; Lautier, T.; Pagès, C.; Ritzenthaler, P. The unconventional Xer recombination machinery of Streptococci/Lactococci. PLoS Genet. 2007, 3, e117. [Google Scholar] [CrossRef]
- Zúñiga, M.; Pardo, I.; Ferrer, S. Transposons Tn916 and Tn925 can transfer from Enterococcus faecalis to Leuconostoc oenos. FEMS Microbiol. Lett. 1996, 135, 179–185. [Google Scholar] [CrossRef]
- Mesas, J.M.; Rodriguez, M.C.; Alegre, M.T. Characterization of lactic acid bacteria from musts and wines of three consecutive vintages of Ribeira Sacra. Lett. Appl. Microbiol. 2011, 52, 258–268. [Google Scholar] [CrossRef]
- Groth, A.C.; Calos, M.P. Phage integrases: Biology and applications. J. Mol. Biol. 2004, 335, 667–678. [Google Scholar] [CrossRef]
- Jaomanjaka, F.; Claisse, O.; Blanche-Barbat, M.; Petrel, M.; Ballestra, P.; Le Marrec, C. Characterization of a new virulent phage infecting the lactic acid bacterium Oenococcus oeni. Food Microbiol. 2016, 54, 167–177. [Google Scholar] [CrossRef]
- Auvray, F.; Coddeville, M.; Ordonez, R.C.; Ritzenthaler, P. Unusual structure of the attB site of the site-specific recombination system of Lactobacillus delbrueckii bacteriophage mv4. J. Bacteriol. 1999, 181, 7385–7389. [Google Scholar] [CrossRef]
- Campbell, A. Phage integration and chromosome structure. A personal history. Annu. Rev. Genet. 2007, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Landy, A.; Ross, W. Viral integration and excision: Structure of the lambda att sites. Science 1977, 197, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Wojciak, J.M.; Sarkar, D.; Landy, A.; Clubb, R.T. Arm-site binding by lambda -integrase: Solution structure and functional characterization of its amino-terminal domain. Proc. Natl. Acad. Sci. USA 2002, 99, 3434–3439. [Google Scholar] [CrossRef]
- Cho, E.H.; Gumport, R.I.; Gardner, J.F. Interactions between integrase and excisionase in the phage lambda excisive nucleoprotein complex. J. Bacteriol. 2002, 184, 5200–5203. [Google Scholar] [CrossRef]
- Suzuki, S.; Yoshikawa, M.; Imamura, D.; Abe, K.; Eichenberger, P.; Sato, T. Compatibility of Site-Specific Recombination Units between Mobile Genetic Elements. iScience 2020, 23, 100805. [Google Scholar] [CrossRef] [PubMed]
- Rutkai, E.; György, A.; Dorgai, L.; Weisberg, R.A. Role of Secondary Attachment Sites in Changing the Specificity of Site-Specific Recombination. J. Bacteriol. 2006, 188, 3409–3411. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Kurokawa, K.; Yamashita, A.; Nakata, M.; Tomiyasu, Y.; Okahashi, N.; Kawabata, S.; Yamazaki, K.; Shiba, T.; Yasunaga, T.; et al. Genome sequence of an M3 strain of Streptococcus pyogenes reveals a large-scale genomic rearrangement in invasive strains and new insights into phage evolution. Genome Res. 2003, 13, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Philippe, C.; Chaïb, A.; Jaomanjaka, F.; Claisse, O.; Lucas, P.M.; Samot, J.; Cambillau, C.; Le Marrec, C. Characterization of the First Virulent Phage Infecting Oenococcus oeni, the Queen of the Cellars. Front. Microbiol. 2021, 11, 596541. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).