Inhibition of Primary Photosynthesis in Desiccating Antarctic Lichens Differing in Their Photobionts, Thallus Morphology, and Spectral Properties
Abstract
1. Introduction
2. Material and Methods
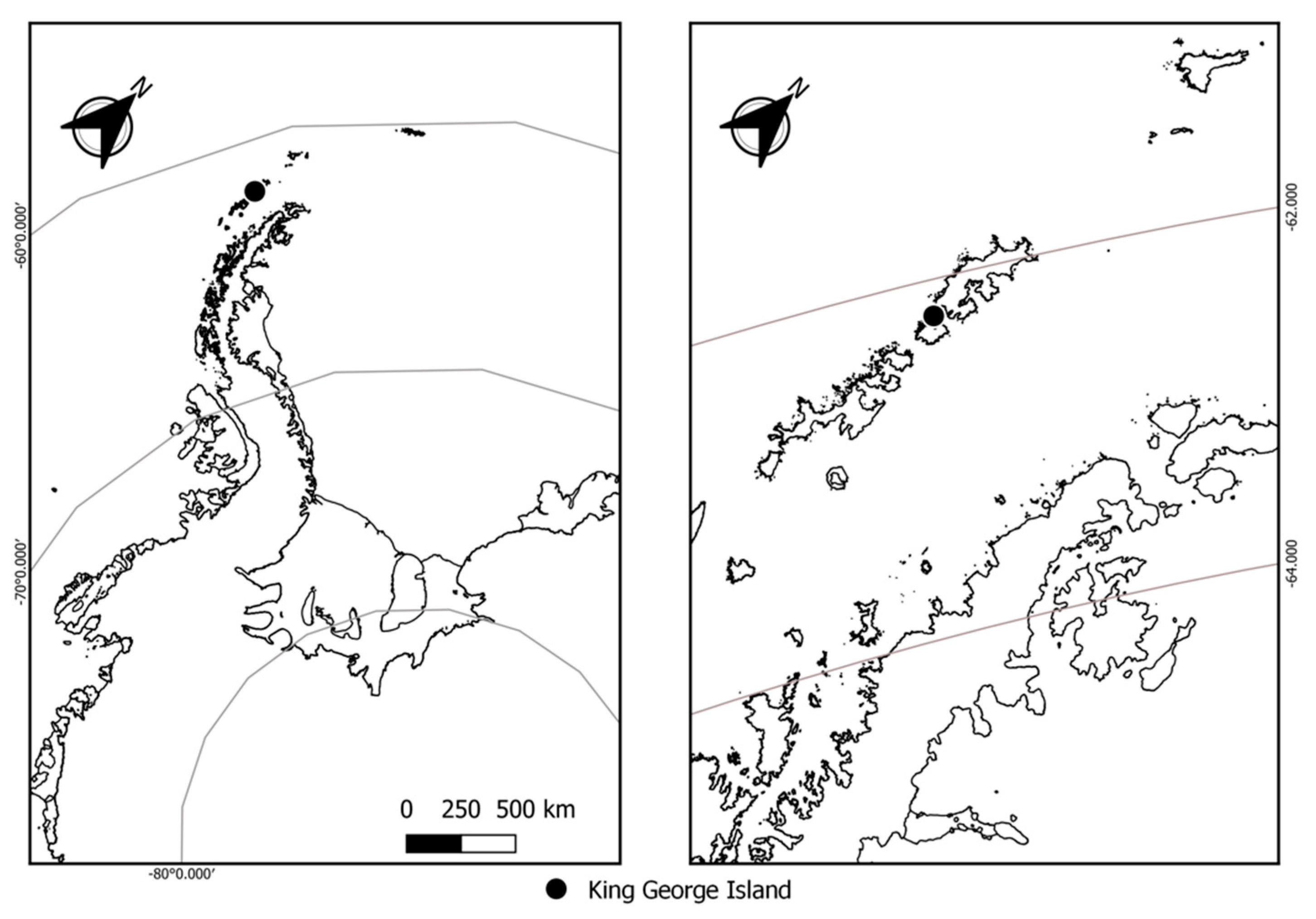
2.1. Site Description, Lichen Species Collection, and Handling
2.2. Species Characteristics
2.3. RWC during Dehydration
2.4. Chlorophyll Fluorescence Measurements
2.5. Spectral Properties in the Wet and Dry States of Thallus
2.6. Statistical Analysis
3. Results
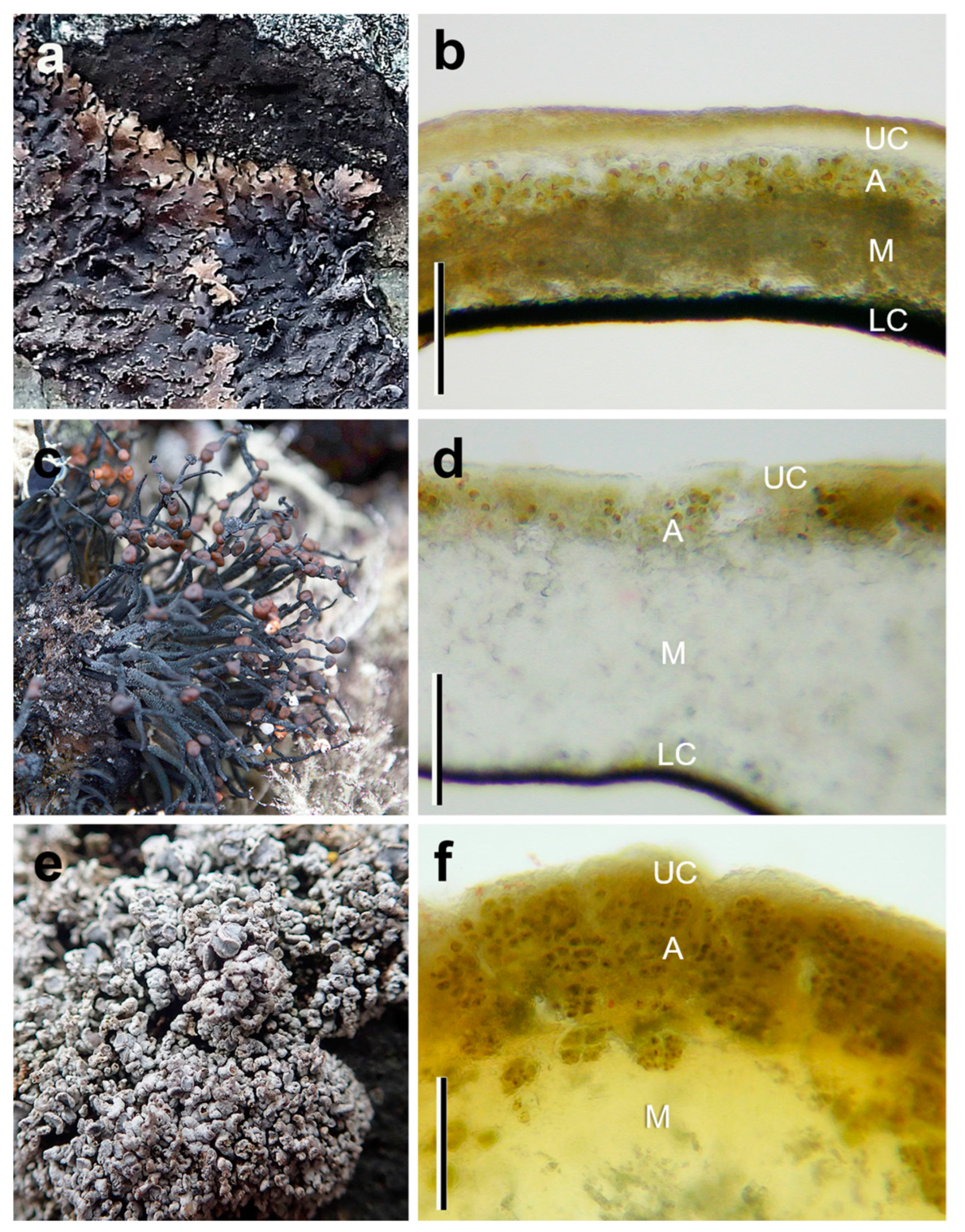
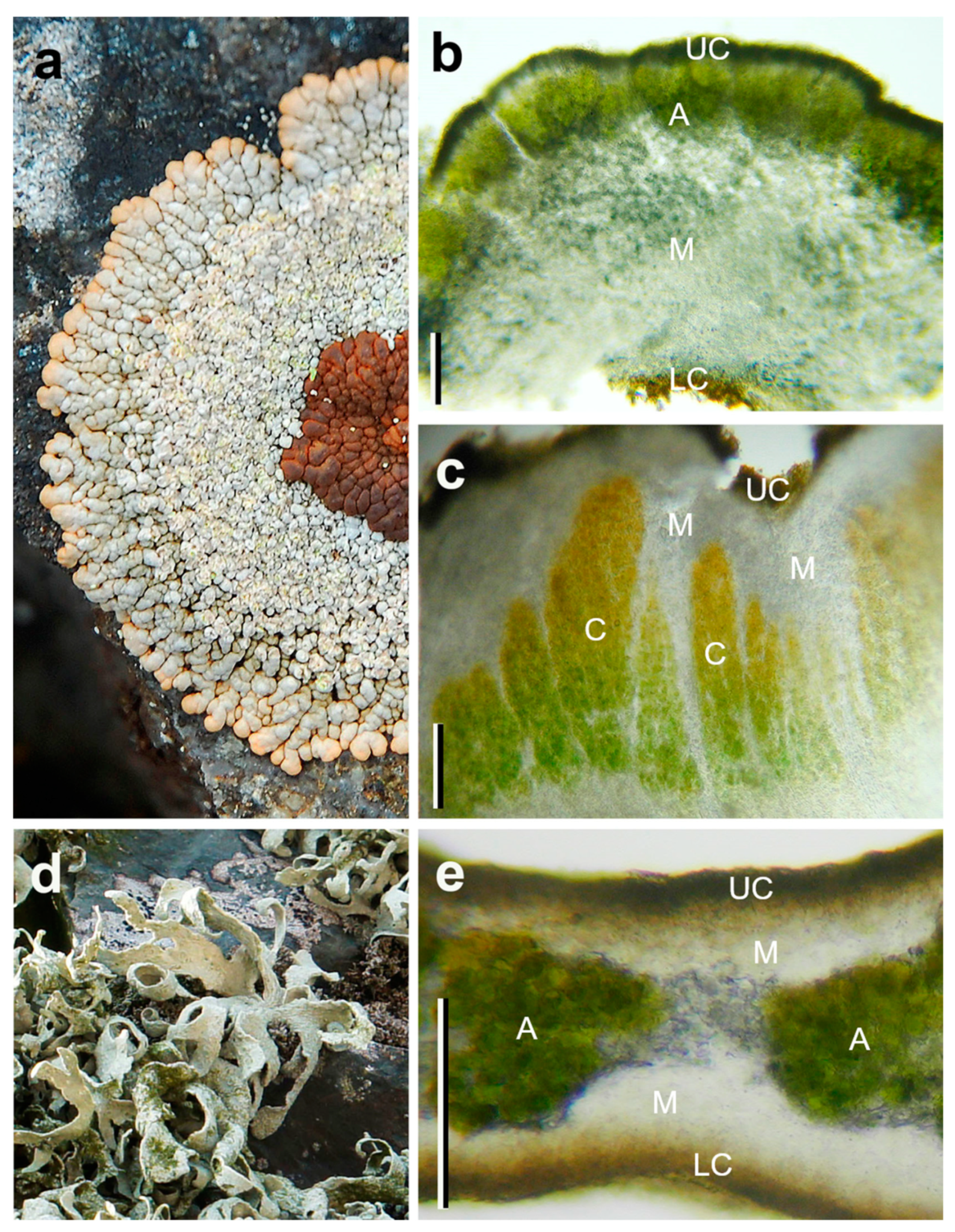
3.1. Anatomy of the Species
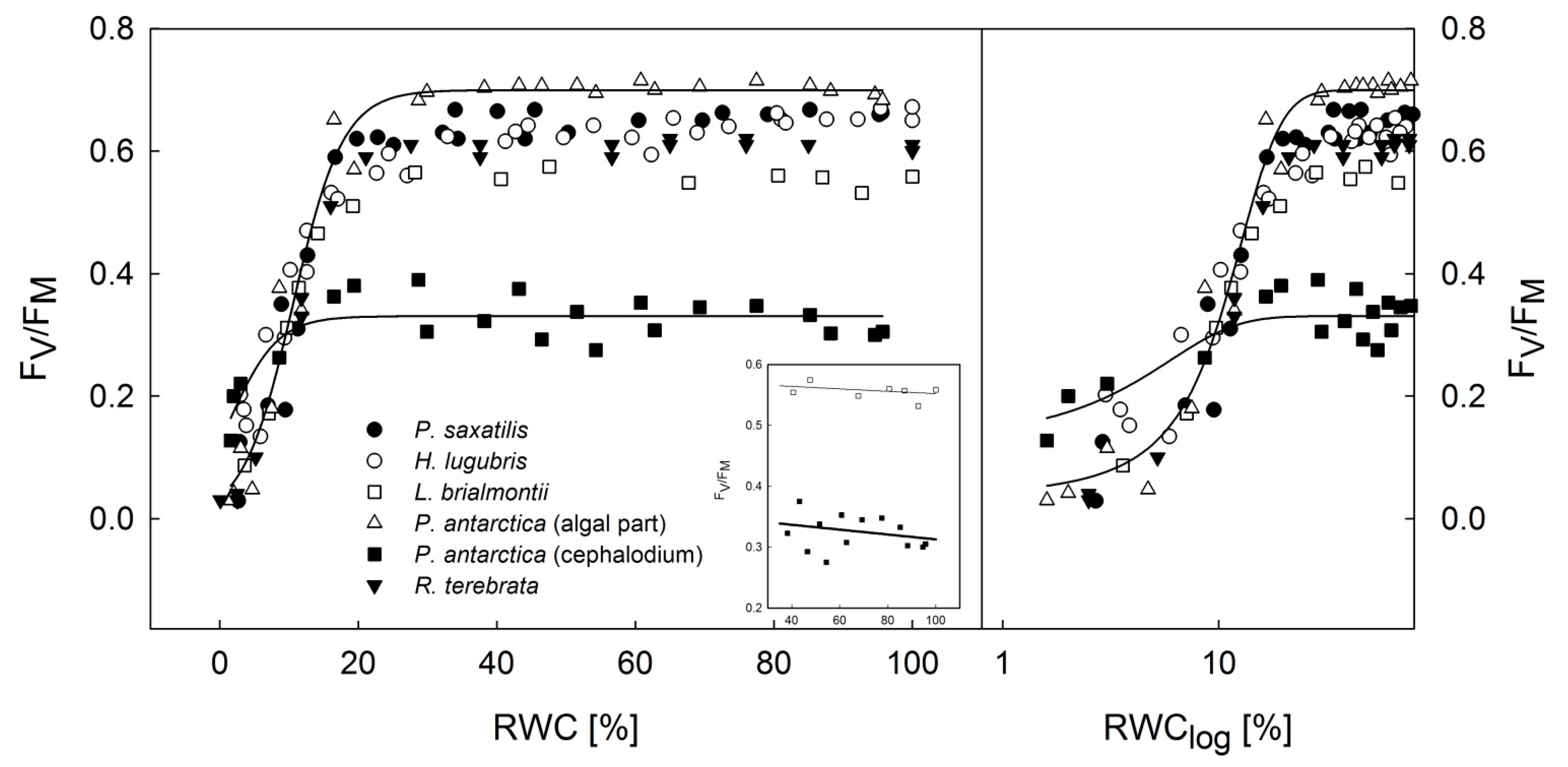
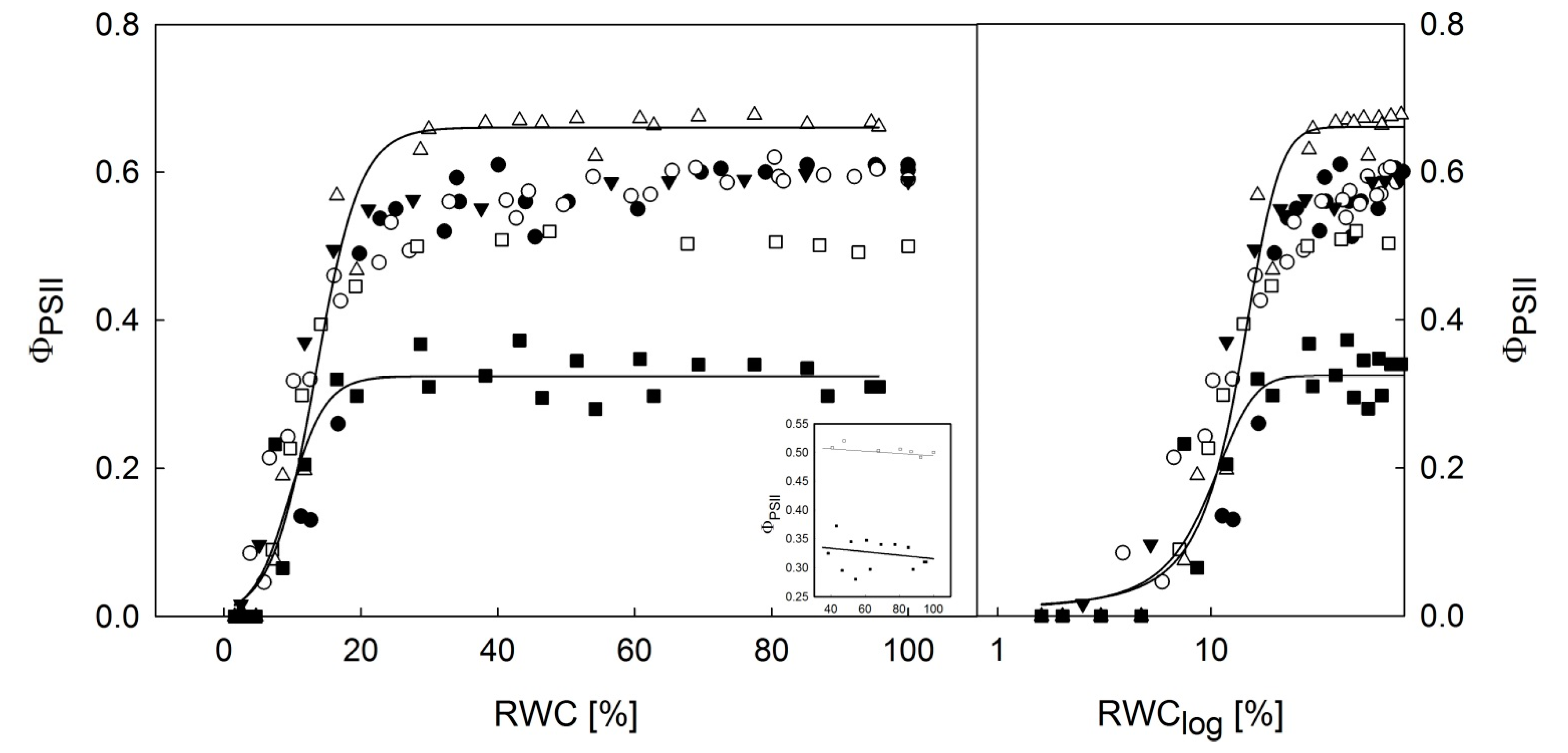
3.2. Dehydration Response Curves of the Potential and Effective Quantum Yield of PSII
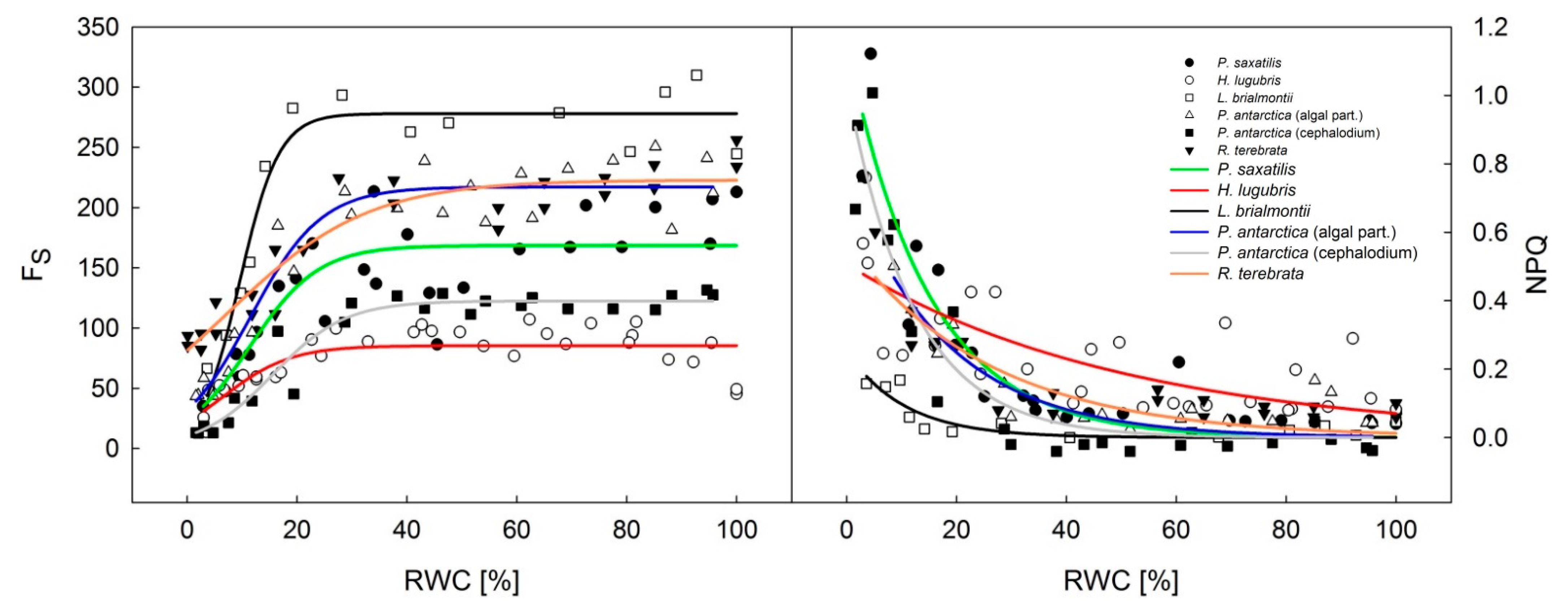
3.3. Slow Kautsky Kinetics Recorded for the Lichens at Different RWCs during Desiccation from Fully a Wet to a Dry State
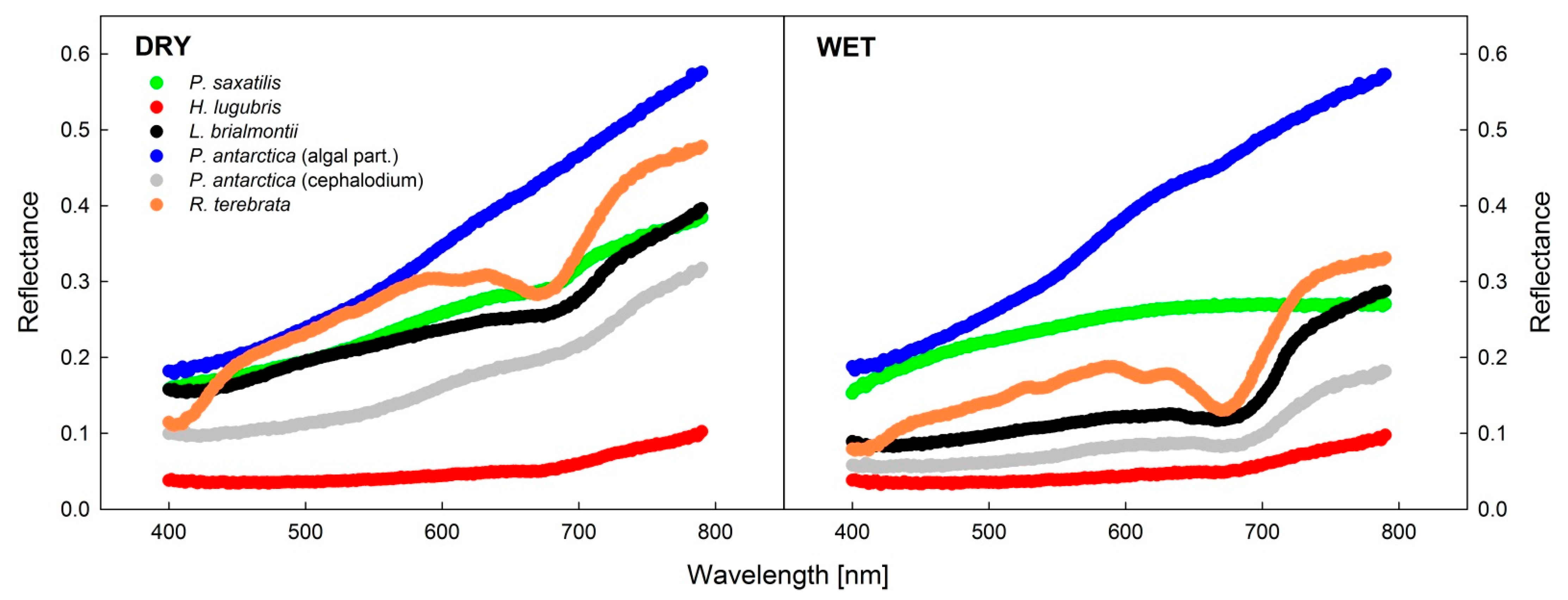
3.4. Analysis of Reflectance Spectra in Dry and Wet States
4. Discussion
4.1. Chlorophyll Fluorescence Parameters during Desiccation
4.2. Kautsky Curve Changes during Desiccation
4.3. Lichen Spectral Properties
5. Conclusions
- Dehydration response curves of potential yield of PSII (FV/FM) revealed that primary photosynthetic processes of PSII remained active in P. antarctica cephalodia, even after severe desiccation (RWC below 10%) compared to the chlorolichens that exhibit a high degree of FV/FM limitation. This might be explained by the effect of the exopolysaccaridic envelope of symbiotic Nostoc commune that helps the cephalodium to maintain higher RWC than average values for the whole thallus including the algal part of the thallus.
- For effective yield of PSII (ΦPSII), the dehydration response curves indicated that H. lugubris showed somewhat higher ΦPSII values at the RWC < 10% than the rest of the investigated species. Together with gradual activation of nonphotochemical quenching (NPQ) during thallus desiccation, this suggests an advantage for the species in terms of efficient primary photosynthetic processes during the final phase of desiccation. This might be a useful mechanism because in the field, the species desiccates rapidly due to the black thallus color. Generally, lichens with dark thalli absorb more light energy and therefore warm up and desiccate more rapidly than species with brighter-colored thalli.
- All species showed a decrease in the ChlF signal with ongoing desiccation and general flattening of the slow Kautsky kinetics curve. As a result of this phenomenon the index parameters derived from the ChlF levels P, S, M, S, such as P/S, P/M S/M generally increased, but the response and indicative value of such parameters for ecophysiological studies must be verified in follow-up studies.
- Spectral reflectance curves recorded in wet and dry states of thalli showed more evident differences in green than black or blackish lichens, with typically lower reflectance in the wet than in the dry state.
- In addition to being used to separate rock-dwelling lichens from those growing on bare soil, species-specific spectral signatures, spectral reflectance methods, and NDVI in particular have the potential to determine lichen physiological activity and water content [78].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Øvstedal, D.; Lewis-Smith, R. Lichens of Antarctica and South Georgia, A Guide to their Identification and Ecology; Cambridge University Press: Cambridge, UK, 2001; p. 411. [Google Scholar]
- Olech, M. Lichens of King George Island, Antarctica; Drukarnia Uniwersytetu Jagiellńskiego: Kraków, Poland, 2004; p. 391. [Google Scholar]
- Kennedy, A.D. Water as a limiting factor in the Antarctic Terrestrial environment: A biogeographical synthesis. Artic Alp. Res. 1993, 25, 308–315. [Google Scholar] [CrossRef]
- Green, T.; Sancho, L.; Pintado, A.; Schroeter, B. Functional and spatial pressures on terrestrial vegetation in Antarctica forced by global warming. Polar Biol. 2011, 34, 1643–1656. [Google Scholar] [CrossRef]
- Green, T.G.A.; Sancho, L.G.; Pintado, A. Ecophysiology of desiccation/rehydration cycles in mosses and lichens. In Plant Desiccation Tolerance; Luttge, U., Beck, E., Bartels, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 215, pp. 89–120. [Google Scholar]
- Schroeter, B.; Scheidegger, C. Water relations in lichens at subzero temperatures: Structural changes and carbon dioxide exchange in the lichen Umbilicaria aprina from continental Antartica. New Phytol. 1995, 131, 275–285. [Google Scholar] [CrossRef]
- Green, T.G.A.; Schroeter, B.; Sancho, L.G. Plant life in Antarctica. In Handbook of Functional Plant Ecology; Pugnaire, F.I., Valladares, F., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2007. [Google Scholar]
- Sancho, L.; Pintado, A.; Green, A. Antarctic studies show lichens to be excellent biomonitors of climate change. Diversity 2019, 11, 42. [Google Scholar] [CrossRef]
- Casanova-Katny, A.; Barták, M.; Gutierrez, C. Open top chamber microclimate may limit photosynthetic processes in Antarctic lichen: Case study from King George Island, Antarctica. Czech. Polar Rep. 2019, 9, 61–77. [Google Scholar] [CrossRef]
- Colesie, C.; Williams, L.; Büdel, B. Water relations in the soil crust lichen Psora decipiens are optimized via anatomical variability. Lichenologist 2017, 49, 483–492. [Google Scholar] [CrossRef]
- Inoue, T.; Kudoh, S.; Uchida, M.; Tanabe, Y.; Inoue, M.; Kanda, H. Factors affecting water availability for high Arctic lichens. Polar Biol. 2017, 40, 853–862. [Google Scholar] [CrossRef]
- Kappen, L. Some aspects of the great success of lichens in Antarctica. Antarct. Sci. 2000, 12, 314–324. [Google Scholar] [CrossRef]
- Colesie, C.; Green, T.G.A.; Raggio, J.; Büdel, B. Summer activity patterns of Antarctic and high alpine lichen- dominated biological soil crusts—Similar but different? Arct. Antarct. Alp. Res. 2016, 48, 449–460. [Google Scholar] [CrossRef]
- Veerman, J.; Vasilev, S.; Paton, G.D.; Ramanauskas, J.; Bruce, D. Photoprotection in the lichen Parmelia sulcata: The origins of desiccation-induced fluorescence quenching. Plant. Physiol. 2007, 145, 997–1005. [Google Scholar] [CrossRef]
- Heber, U.; Bilger, W.; Shuvalov, V.A. Thermal energy dissipation in reaction centers of photosystem II protects desiccated poikilohydric mosses against photooxidation. J. Exp. Bot. 2006, 57, 2993–3006. [Google Scholar] [CrossRef]
- Heber, U.; Azarkovich, M.; Shuvalov, V.A. Activation of mechanisms of photoprotection by desiccation and by light: Poikilohydric photoautotrophs. J. Exp. Bot. 2007, 58, 2745–2759. [Google Scholar] [CrossRef]
- Heber, U. Photoprotection of green plants: A mechanism of ultra-fast thermal energy dissipation in desiccated lichens. Planta 2008, 228, 641–650. [Google Scholar] [CrossRef]
- Heber, U.; Bilger, W.; Turk, R.; Lange, O.L. Photoprotection of reaction centres in photosynthetic organisms: Mechanisms of thermal energy dissipation in desiccated thalli of the lichen Lobaria pulmonaria. New Phytol. 2010, 185, 459–470. [Google Scholar] [CrossRef]
- Riznichenko, G.; Lebedeva, G.; Pogosyan, S.; Sivchenko, M.; Rubin, A. Fluorescence induction curves registered from individual microalgae cenobiums in the process of population growth. Photosynth. Res. 1996, 49, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C.; Govindjee. Photosystem II fluorescence slow changes—Scaling from the past. J. Photochem. Photobiol. B Biol. 2011, 104, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Riznichenko, G.Y.; Rubin, A.B.; Govind, J. Modeling chlorophyll a fluorescence transient: Relation to photosynthesis. Biochemistry 2014, 79, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Seaton, G.G.; Walker, D.D. Chlorophyll fluorescence as a measure of photosynthetic carbon assimilation. Proc. R. Soc. 1990, 242, 29–35. [Google Scholar]
- Noctor, G.; Horton, P. Uncouple titration of energy-dependent chlorophyll fluorescence quenching and photosystem II photochemical yield in intact pea chloroplasts. Biochim. Biophys. Acta 1990, 1016, 228–234. [Google Scholar] [CrossRef]
- Allen, J.F. Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1992, 1098, 275–335. [Google Scholar] [CrossRef]
- Krause, H.G. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant. 1988. [Google Scholar] [CrossRef]
- Kodru, S.; Malavath, T.; Devadasu, E.; Nellaepalli, S.; Stirbet, A.; Subramanyam, R.; Govind, J. The slow S to M rise of chlorophyll a fluorescence reflects transition from state 2 to state 1 in the green alga Chlamydomonas reinhardtii. Photosynth. Res. 2015, 125, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Young, A.J.; Horton, P. Induction of nonphotochemical energy dissipation and absorbance changes in leaves (Evidence for changes in the state of the light-harvesting system of photosystem II in vivo). Plant. Physiol. 1993, 102, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Grieco, M.; Nurmi, P.; Rantala, M.; Suorsa, M.; Aro, E.-M. Regulation of the photosynthetic apparatus under fluctuating growth light. Philos. Trans. R. Soc. Lond. Ser. Biol. Sci. 2012, 367, 3486–3493. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. The slow phase of chlorophyll a fluorescence induction in silico: Origin of S-M fluorescence rise. Photosynth. Res. 2016, 130, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Hazdrová, J.; Hájek, J.; Očenášová, P.; Barták, M.; Skácelová, K.; Adamo, P. Comparative analysis of heterogeneity of primary photosynthetic processes within fruticose lichen thalli: Preliminary study of interspecific differences. Czech. Polar Rep. 2014, 4, 149–157. [Google Scholar] [CrossRef][Green Version]
- Mishra, A.; Hájek, J.; Tuháčková, T.; Barták, M.; Mishra, K.B. Features of chlorophyll fluorescence transients can be used to investigate low temperature induced effects on photosystem II of algal lichens from polar regions. Czech. Polar Rep. 2015, 5, 99–111. [Google Scholar] [CrossRef]
- Nabe, H.; Funabiki, R.; Kashino, Y.; Koike, H.; Satoh, K. Responses to desiccation stress in Bryophytes and an important role of dithiothreitol-insensitive non-photochemical quenching against photoinhibition in dehydrated states. Plant. Cell Physiol. 2007, 48, 1548–1557. [Google Scholar] [CrossRef][Green Version]
- Barták, M.; Hájek, J.; Morkusová, J.; Skácelová, K.; Košuthová, A. Dehydration-induced changes in spectral reflectance indices and chlorophyll fluorescence of Antarctic lichens with different thallus color, and intrathalline photobiont. Acta Physiol. Plant. 2018, 40, 177–187. [Google Scholar]
- Trnková, K.; Barták, M. Desiccation-induced changes in photochemical processes of photosynthesis and spectral reflectance in Nostoc commune (Cyanobacteria, Nostocales) colonies from Antarctica. Phycol. Res. 2017, 65, 44–50. [Google Scholar] [CrossRef]
- Kosugi, M.; Miyake, A.; Kasino, Y.; Shibata, Y.; Satoh, K.; Itoh, S. Lichens assist the drought-induced fluorescence quenching of their photobiont green algae through arabitol. In Photosynthesis Research for Food, Fuel and the Future. Advanced Topics in Science and Technology in China; Springer: Berlin/Heidelberg, Germany, 2013; pp. 514–520. [Google Scholar]
- Rückamp, M.; Braun, M.; Suckro, S.; Blindow, N. Observed glacial changes on the King George Island ice cap, Antarctica, in the last decade. Glob. Planet. Chang. 2011, 79, 99–109. [Google Scholar] [CrossRef]
- Ochyra, R.; Smith, R.I.L.; Bednarek-Ochyra, H. The Illustrated Moss Flora of Antarctica; Cambridge University Press: Cambridge, UK, 2008; p. 704. [Google Scholar]
- Andreyev, M.P. The lichens in the vicinity of Bellingshausen Station, King George Island. Polar Geogr. Geol. 1989, 13, 42–45. [Google Scholar] [CrossRef]
- Beck, A.; Bechteler, J.; Casanova-Katny, A.; Dzhilyanova, I. The pioneer lichen Placopsis in maritime Antarctica: Genetic diversity of their mycobionts and green algal symbionts, and their correlation with deglaciation time. Symbiosis 2019, 79, 1–24. [Google Scholar] [CrossRef]
- Marečková, M.; Barták, M. Effects of short-term low temperature stress on chlorophyll fluorescence transients in Antarctic lichen species. Czech. Polar Rep. 2016, 6, 56–65. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite–1 Syposium. Volume I: Technical Presentations, NASA SP-351, NASA, Washington, DC, USA, 10–14 December 1973; pp. 309–317. [Google Scholar]
- Gamon, J.A.; Peñuelas, J.; Fiels, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Dufková, K.; Barták, M.; Morkusová, J.; Elster, J.; Hájek, J. Screening of growth phases of Antarctic algae and cyanobacteria cultivated on agar plates by chlorophyll fluorescence imaging. Czech. Polar Rep. 2019, 9, 170–181. [Google Scholar] [CrossRef]
- Cho, S.M.; Lee, H.; Hong, S.G.; Lee, J. Study of ecophysiological responses of the antarctic fruticose lichen Cladonia borealis using the PAM fluorescence system under natural and laboratory conditions. Plants 2020, 9, 85. [Google Scholar] [CrossRef]
- Uchida, M.; Nakatsubo, T.; Kanda, H.; Koizumi, H. Estimation of the annual primary production of the lichen Cetrariella delisei in a glacier foreland in the high Arctic, Ny-Ålesund, Svalbard. Polar Res. 2006, 25, 39–49. [Google Scholar]
- Zúñiga-González, P.; Zúñiga-González, G.E.; Pizzaro, M.; Casanova-Katny, A. Soluble carbohydrate content variation in Sanionia uncinata and Polytrichastrum alpinum, two Antarctic mosses with contrasting desiccation capacities. Biol. Res. 2016, 49, 1–9. [Google Scholar] [CrossRef]
- Sancho, L.; De los Ríos, A.; Pintado, A.; Colesie, C.; Raggio, J.; Ascaso, C.; Green, A. Himantormia lugubris, an Antarctic endemic on the edge of the lichen symbiosis. Symbiosis 2020, 82, 49–58. [Google Scholar] [CrossRef]
- Barták, M. Lichen Photosynthesis. Scaling from the cellular to the organism level. In The Structural Basis of Biological Energy Generation. Advances in Photosynthesis and Respiration; Hohmann-Marriott, M.F., Ed.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2014; Volume 39, pp. 379–400. [Google Scholar] [CrossRef]
- Barták, M.; Trnková, K.; Hansen, E.S.; Hazdrová, J.; Skácelová, K.; Hájek, J.; Forbelská, M. Effect of dehydration on spectral reflectance and photosynthetic efficiency in Umbilicaria arctica and U. hyperborea. Biol. Plant. 2015, 59, 357–365. [Google Scholar] [CrossRef]
- Nayaka, S.; Saxena, P. Physiological responses and ecological success of lichen Stereocaulon foliolosum and moss Racomitrium subsecundum growing in same habitat in Himalaya. Indian J. Fundam. Appl. Life Sci. 2014, 4, 167–179. [Google Scholar]
- Hovind, A.B.A.; Phinney, N.H.; Gauslaa, Y. Functional trade-off of hydration strategies in old forest epiphytic cephalolichens. Fungal Biol. 2020, 124, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash, T.H. Desiccation-tolerance in lichens: A review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Heber, U.; Lange, O.L.; Shuvalov, V.A. Conservation and dissipation of light energy as complementary processes: Homoiohydric and poikilohydric autotrophs. J. Exp. Bot. 2006, 57, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.H.; Jahns, P. Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: Characterization and function. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 463–495. [Google Scholar]
- Zorn, M.; Pfeifhofer, H.W.; Grill, D.; Kranner, I. Responses of plastid pigments to desiccation and rehydration in the desert lichen Ramalina maciformis. Symbiosis 2001, 31, 201–212. [Google Scholar]
- MacKenzie, T.D.; MacDonald, T.M.; Dubois, L.A.; Campbell, D.A. Seasonal changes in temperature and light drive acclimation of photosynthetic physiology and macromolecular content in Lobaria pulmonaria. Planta 2001, 214, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Zorn, M.; Turk, B.; Wornik, S.; Beckett, R.P.; Batič, F. Biochemical traits of lichens differing in relative desiccation tolerance. New Phytol. 2003, 160, 167–176. [Google Scholar] [CrossRef]
- Slavnov, C.; Reus, M.; Holzwarth, A.R. Two different mechanisms cooperate in the desiccation-induced excited state quenching in Parmelia lichen. J. Phys. Chem. B 2013, 117, 11326–11336. [Google Scholar] [CrossRef] [PubMed]
- Komura, M.; Yamagishi, A.; Shibata, Y.; Iwasaki, I.; Itoh, S. Mechanism of strong quenching of photosystem II chlorophyll fluorescence under drought stress in a lichen, Physciella melanchla, studied by subpicosecond fluorescence spectroscopy. Biochim. Biophys. Acta 2010, 1797, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Heber, U. Conservation and dissipation of light energy in desiccation-tolerant photoautotrophs, two sides of the same coin. Photosynth. Res. 2012, 113, 5–13. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. Fungal melanins as a sun screen for symbiotic green algae in the lichen Lobaria pulmonaria. Oecologia 2001, 126, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Wieners, P.C.; Mudimu, O.; Bilger, W. Desiccation-induced non-radiative dissipation in isolated green lichen algae. Photosynth. Res. 2012, 113, 239–247. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Tsimilli-Michael, M.; Stamatakis, K. The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: A viewpoint. Photosynth. Res. 2007, 94, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.B.; Vítek, P.; Barták, M. A correlative approach, combining chlorophyll a fluorescence, reflectance, and Raman spectroscopy, for monitoring hydration induced changes in Antarctic lichen Dermatocarpon polyphyllizum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 208, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Tsimilli-Michael, M.; Stamatakis, K.; Papageorgiou, G.C. Dark-to-light transition in Synechococcus sp. PCC 7942 cells studied by fluorescence kinetics assesses plastoquinone redox poise in the dark and photosystem II fluorescence component and dynamics during state 2 to state 1 transition. Photosynth. Res. 2009, 99, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Kaňa, R.; Kotabová, E.; Komárek, O.; Šedivá, B.; Papageorgiou, G.C.; Govindjee; Prášil, O. The slow S to M fluorescence rise in cyanobacteria is due to a state 2 to state 1 transition. Biochim. Biophys. Acta 2012, 1817, 1237–1247. [Google Scholar] [CrossRef]
- Finazzi, G.; Barbagalo, R.P.; Bergo, E.; Barbato, R.; Forti, G. Photoinhibition of Chlamydomonas reinhardtii in State 1 and State 2. Damages to the photosynthetic apparatus under linear and cyclic electron flow. J. Biol. Chem. 2001, 276, 22251–22257. [Google Scholar] [CrossRef]
- Mishra, K.B.; Vítek, P.; Mishra, A.; Hájek, J.; Barták, M. Chlorophyll a fluorescence and Raman spectroscopy can monitor activation/deactivation of photosynthesis and carotenoids in Antarctic lichens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118458. [Google Scholar] [CrossRef]
- Ekman, S. The corticolous and lignicolous species of Bacidia and Bacidina in North America. Opera Bot. 1996, 127, 1–139. [Google Scholar]
- Barták, M.; Hájek, J.; Amarillo, A.M.; Hazdrová, J.; Carreras, H. Changes in spectral reflectance of selected Antarctic and South American lichens caused by dehydration and artificially-induced absence of secondary compounds. Czech. Polar Rep. 2016, 6, 221–230. [Google Scholar] [CrossRef]
- Bechtel, R.; Rivard, B.; Sánchez-Azofeifa, A. Spectral properties of foliose and crustose lichens based on laboratory experiments. Remote Sens. Environ. 2002, 82, 389–396. [Google Scholar] [CrossRef]
- Van Der Veen, C.J.; Csatho, B.M. Spectral characteristics of Greenland Lichens. Géogr. Phys. Quat. 2005, 59, 63–73. [Google Scholar] [CrossRef]
- Kiang, N.Y.; Siefert, J.; Govindjee; Blankenship, R.E. Spectral signatures of photosynthesis. I. Review of earth organisms. Astrobiology 2007, 7, 222–251. [Google Scholar] [CrossRef]
- Kiang, N.Y.; Segura, A.; Tinetti, G.; Govindjee; Blankenship, R.E.; Cohen, M.; Siefert, J.; Crisp, D.; Meadows, V.S. Spectral signatures of photosynthesis. II. Coevolution with other stars and the atmosphere on extrasolar worlds. Astrobiology 2007, 7, 252–274. [Google Scholar] [CrossRef] [PubMed]
- Barták, M.; Mishra, K.B.; Marečková, M. Spectral reflectance indices sense desiccation induced changes in the thalli of Antarctic lichen Dermatocarpon polyphyllizum. Czech. Polar Rep. 2018, 8, 249–259. [Google Scholar] [CrossRef]
- Gloser, J.; Gloser, V. Changes in spectral reflectance of a foliar lichen Umbilicaria hirsuta during desiccation. Biol. Plant. 2007, 51, 395–398. [Google Scholar] [CrossRef]
- Jupa, R.; Hájek, J.; Hazdrová, J.; Barták, M. Interspecific differences in photosynthetic efficiency and spectral reflectance in two Umbilicaria species from Svalbard during controlled desiccation. Czech. Polar Rep. 2012, 2, 31–41. [Google Scholar] [CrossRef]
- Granlund, L.; Keski-Saari, S.; Kumpula, T.; Oksanen, E.; Keinanan, M. Imaging lichen water content with visible to mid-wave infrared (400–5500 nm) spectroscopy. Remote Sens. Environ. 2018, 216, 301–310. [Google Scholar] [CrossRef]

| Lichen Family | Lichen Species | Photobiont | Thallus Morphotype | Ecology and Distribution |
|---|---|---|---|---|
| Parmeliacea | Parmelia saxatilis (L.) Ach | Trebouxioid | Foliose | Cosmopolitan species with southern limit in Antarctica, on rocks, boulders, stones, and mosses, and on dry to moist rock faces. Apothecia not observed in Antarctica. |
| Himantormia lugubris (Hue) I.M. Lamb | Trebouxioid | Fruticose | Endemic in Antarctica. Preferentially saxicolous on acidic rocks and abundant on the southern part of Fildes Peninsula and Ardley Island, where it forms several patches on soil or moss. In nitrophobic communities, growing above 70 m.a.s.l. | |
| Ramalinacea | Lecania brialmontii (Vain.) Zahlbr. | Trebouxioid | Fruticose | Endemic in Antarctica. Preferentially saxicolous, grows on rocks and moist and shaded sites, typical for ornithocoprophilous lichen communities. |
| Ramalina terebrata Hook. F. & Taylor | Trebouxioid | Fruticose | Cosmopolitan species with southern limit in Antarctica. Saxicolous, grows on costal cliffs and large boulders, typical for ornithocoprophilous lichen communities. Apothecia not observed in Antarctica. | |
| Trapeliaceae | Placopsis antarctica, D.J. Galloway, R.I.L. Sm. & Quilhot | Stichococcus antarcticus; or S. allas | Crustose | Endemic in Antarctica. Grows in nitrophobic communities. Cyanobacteria in cephalodia, with species of Nostoc. |
| Spectral Reflectance Indices | Equation | References |
|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | NDVI = (R780 − R670)/(R780 + R670) | [41] Rouse et al. (1974) |
| Photochemical Reflectance Index (PRI) | PRI = (R531 − R570)/(R531 + R570) | [42] Gamon et al. (1992) |
| Lichen Species | R2 for Fs Versus | |
|---|---|---|
| FV/FM | ΦPSII | |
| Parmelia saxatilis | 0.9777 | 0.9804 |
| Himantormia lugubris | 0.5265 | 0.3106 |
| Lecania brialmontii | 0.9768 | 0.9752 |
| Placopsis antarctica—algal part | 0.9966 | 0.9954 |
| Placopsis antarctica—cephalodium | 0.4766 | 0.8825 |
| Ramalina terebrata | 0.9098 | 0.9211 |
| Lichen Species | RWC (%) | Parameters | |||||
|---|---|---|---|---|---|---|---|
| P/S | P/M | S/M | M/T | O/P | O/T | ||
| Lecania brialmontii | 100 | 0.990 | 0.991 | 1.000 | 1.058 | 0.905 | 0.949 |
| 67 | 0.987 | 0.984 | 0.997 | 1.032 | 0.910 | 0.925 | |
| 19 | 0.996 | 1.006 | 1.010 | 1.011 | 0.902 | 0.918 | |
| 9 | 1.000 | 0.998 | 0.998 | 1.037 | 0.907 | 0.939 | |
| 4 | 1.004 | 1.003 | 0.999 | 1.051 | 0.962 | 1.014 | |
| Himantormia lugubris | 100 | 0.973 | 0.911 | 0.936 | 1.081 | 0.942 | 0.927 |
| 65 | 0.984 | 0.930 | 0.945 | 1.071 | 0.937 | 0.933 | |
| 16 | 1.009 | 0.982 | 0.974 | 1.045 | 0.942 | 0.968 | |
| 6 | 1.015 | 1.006 | 0.992 | 1.078 | 0.966 | 1.047 | |
| 3 | 1.004 | 1.007 | 1.003 | 1.002 | 0.995 | 1.004 | |
| P. antarctica algal part | 100 | 1.060 | 1.042 | 0.983 | 1.101 | 0.822 | 0.943 |
| 62 | 1.000 | 0.993 | 0.993 | 1.018 | 0.957 | 0.968 | |
| 19 | 1.015 | 1.022 | 1.007 | 1.105 | 0.931 | 1.051 | |
| 5 | 1.017 | 1.012 | 0.995 | 1.110 | 0.910 | 1.022 | |
| 1 | 1.003 | 0.999 | 0.996 | 1.024 | 0.987 | 1.009 | |
| P. antarctica cephalodium | 100 | 1.008 | 0.991 | 0.983 | 1.005 | 0.950 | 0.947 |
| 62 | 1.001 | 0.995 | 0.995 | 1.017 | 0.958 | 0.969 | |
| 19 | 1.009 | 1.004 | 0.996 | 1.085 | 0.938 | 1.022 | |
| 5 | 1.016 | 1.031 | 1.015 | 1.087 | 0.915 | 1.025 | |
| 1 | 1.027 | 1.010 | 0.984 | 1.001 | 0.932 | 0.943 | |
| Ramalina terebrata | 100 | 0.973 | 0.953 | 0.979 | 1.092 | 0.855 | 0.890 |
| 65 | 0.982 | 0.963 | 0.981 | 1.074 | 0.879 | 0.909 | |
| 16 | 0.970 | 0.956 | 0.986 | 1.097 | 0.899 | 0.944 | |
| 5 | 0.987 | 0.989 | 1.002 | 1.090 | 0.930 | 1.002 | |
| 0.5 | 0.999 | 0.998 | 0.999 | 1.000 | 0.995 | 0.993 | |
| Parmelia saxatilis | 100 | 0.963 | 0.898 | 0.932 | 1.136 | 0.871 | 0.888 |
| 72 | 0.963 | 0.943 | 0.979 | 1.096 | 0.870 | 0.899 | |
| 22 | 0.970 | 0.953 | 0.983 | 1.075 | 0.878 | 0.900 | |
| 9 | 1.007 | 0.981 | 0.974 | 1.058 | 0.941 | 0.976 | |
| 3 | 1.010 | 1.043 | 1.033 | 1.029 | 0.972 | 1.043 | |
| Spectral Index | State of the Thallus | Lichens Species | |||||
|---|---|---|---|---|---|---|---|
| Himantormia lugubris | Lecania brialmontii | Ramalina terebrata | Parmelia saxatilis | Placopsis antarctica (green algae) | Placopsis antarctica cephalodium | ||
| NDVI | Dry | 0.329 a | 0.213 a | 0.212 | 0.152 a | 0.033 a | 0.190 a |
| Wet | 0.23 b | 0.39 b | 0.300 b | 0.060 b | 0.143 b | 0.211 a | |
| PRI | Dry | −0.059 a | −0.038 a | −0.058 a | −0.061 a | 0.003 a | −0.056 a |
| Wet | −0.029 b | −0.039 a | −0.054 a | −0.075 a | −0.082 b | −0.077 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barták, M.; Hájek, J.; Orekhova, A.; Villagra, J.; Marín, C.; Palfner, G.; Casanova-Katny, A. Inhibition of Primary Photosynthesis in Desiccating Antarctic Lichens Differing in Their Photobionts, Thallus Morphology, and Spectral Properties. Microorganisms 2021, 9, 818. https://doi.org/10.3390/microorganisms9040818
Barták M, Hájek J, Orekhova A, Villagra J, Marín C, Palfner G, Casanova-Katny A. Inhibition of Primary Photosynthesis in Desiccating Antarctic Lichens Differing in Their Photobionts, Thallus Morphology, and Spectral Properties. Microorganisms. 2021; 9(4):818. https://doi.org/10.3390/microorganisms9040818
Chicago/Turabian StyleBarták, Miloš, Josef Hájek, Alla Orekhova, Johana Villagra, Catalina Marín, Götz Palfner, and Angélica Casanova-Katny. 2021. "Inhibition of Primary Photosynthesis in Desiccating Antarctic Lichens Differing in Their Photobionts, Thallus Morphology, and Spectral Properties" Microorganisms 9, no. 4: 818. https://doi.org/10.3390/microorganisms9040818
APA StyleBarták, M., Hájek, J., Orekhova, A., Villagra, J., Marín, C., Palfner, G., & Casanova-Katny, A. (2021). Inhibition of Primary Photosynthesis in Desiccating Antarctic Lichens Differing in Their Photobionts, Thallus Morphology, and Spectral Properties. Microorganisms, 9(4), 818. https://doi.org/10.3390/microorganisms9040818






