Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis cinerea and the Potential of Lactiplantibacillus plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Growth Conditions
2.2. Screening of Antifungal Activity
2.3. Molecular Identification of LAB Species
2.4. Partial Characterization of the Antifungal Activity of LAB Cell-Free Supernatant
2.5. Quantification of Lactic Acid
2.6. Fruit Decay Assay
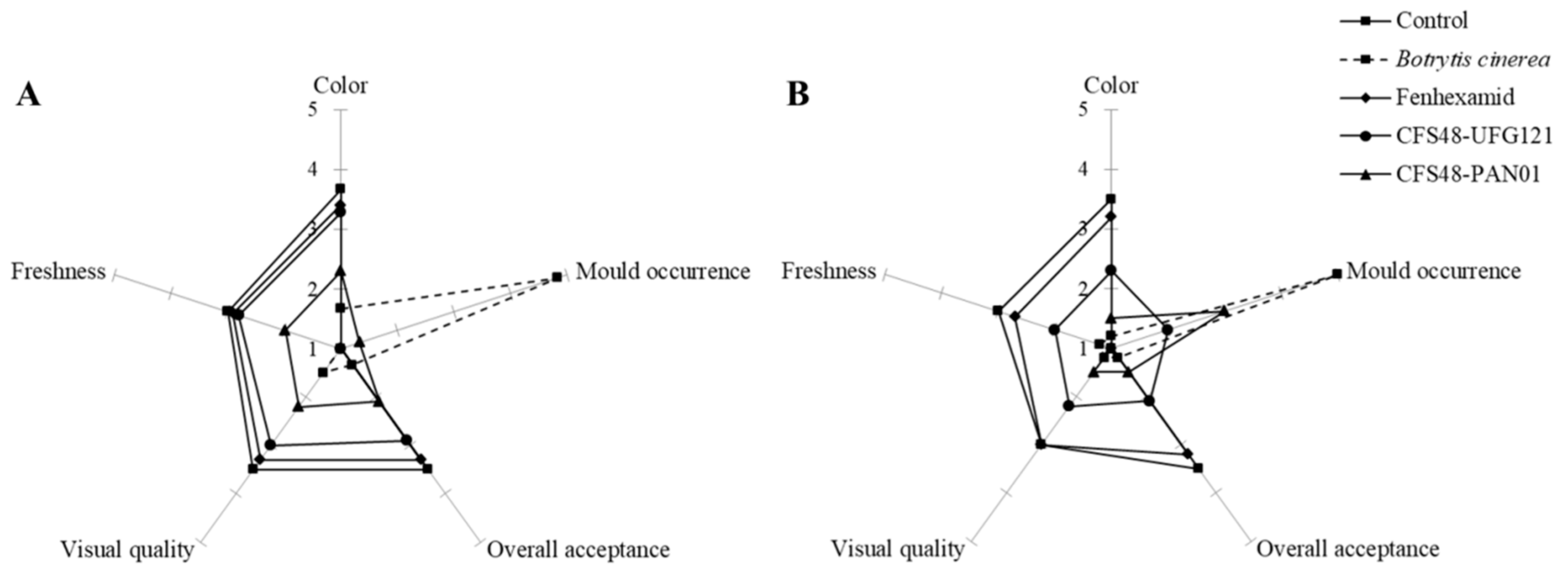
2.7. Sensorial Quality Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Screening of the Antifungal Activity of Lactic Acid Bacteria
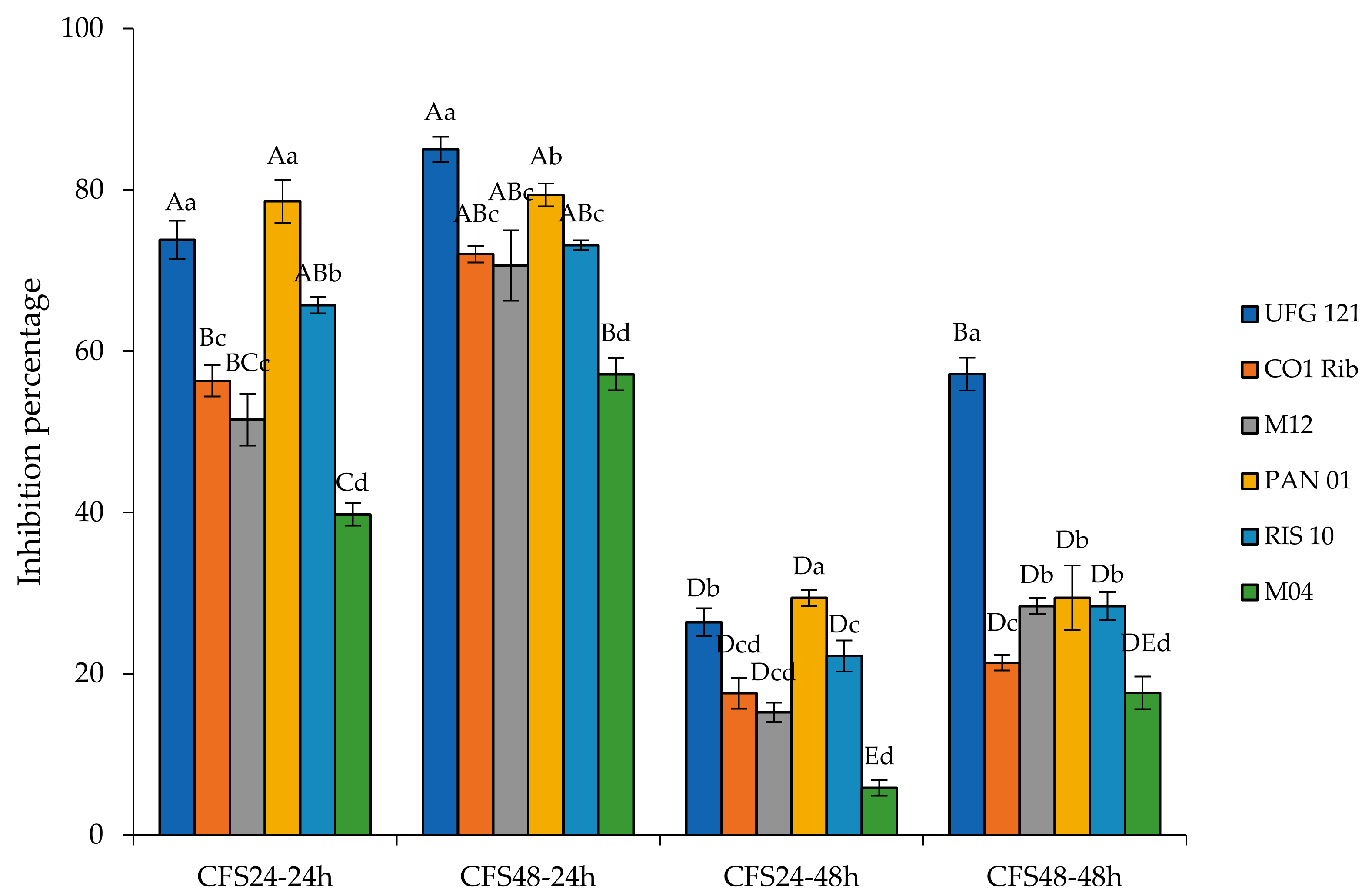
3.2. Anti-Botrytis Activity of Cell-Free Supernatants
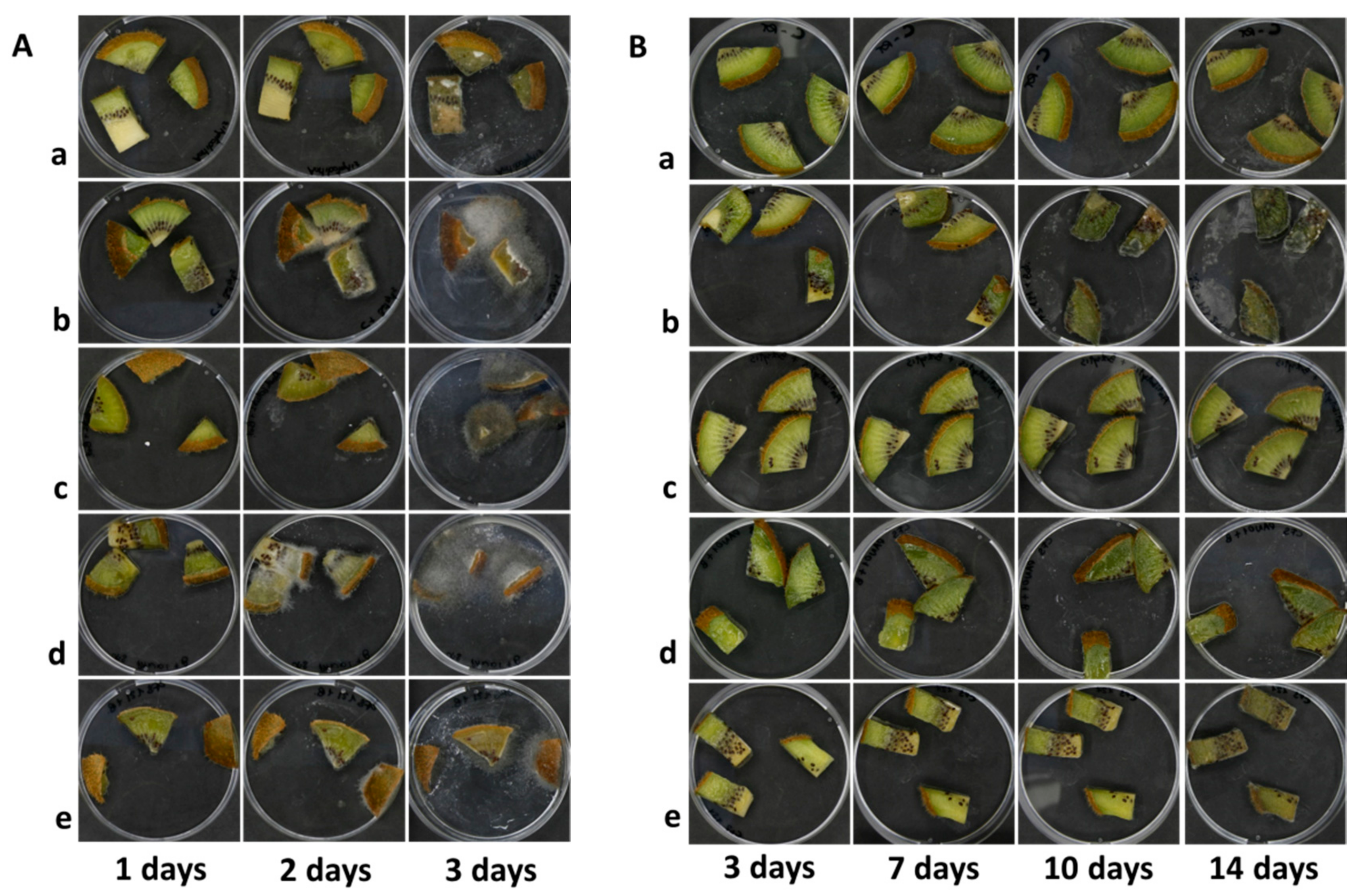
3.3. Anti-Botrytis Activity on Cut Kiwifruits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Zhao, Q.; Lan, T.; Geng, T.; Gao, C.; Yuan, Q.; Zhang, Q.; Xu, P.; Sun, X.; Liu, X.; et al. Comparative Analysis of Physicochemical Characteristics, Nutritional and Functional Components and Antioxidant Capacity of Fifteen Kiwifruit (Actinidia) Cultivars—Comparative Analysis of Fifteen Kiwifruit (Actinidia) Cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef]
- Stonehouse, W.; Gammon, C.S.; Beck, K.L.; Conlon, C.A.; von Hurst, P.R.; Kruger, R. Kiwifruit: Our Daily Prescription for Health. Can. J. Physiol. Pharmacol. 2012, 91, 442–447. [Google Scholar] [CrossRef]
- Hunter, D.C.; Skinner, M.A.; Ferguson, A.R. Chapter 12—Kiwifruit and health. In Fruits, Vegetables, and Herbs; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 239–269. ISBN 978-0-12-802972-5. [Google Scholar]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The Nutritional and Health Attributes of Kiwifruit: A Review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Statistical Database. Available online: http://www.fao.org/faostat/en/#compare (accessed on 3 March 2021).
- Rushing, J.R. Kiwifruit. In The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; USDA Agricultural Research Service: Beltsville, MD, USA, 2016; pp. 371–376. [Google Scholar]
- Cornacchia, R.; Amodio, M.L.; Rinaldi, R.; Colelli, G. Effect of 1-Methylcyclopropene and Controlled Atmosphere on Storage of Kiwifruits. Fresh Prod. 2008, 2, 22–25. [Google Scholar]
- Oliveira, P.M.; Zannini, E.; Arendt, E.K. Cereal Fungal Infection, Mycotoxins, and Lactic Acid Bacteria Mediated Bioprotection: From Crop Farming to Cereal Products. Food Microbiol. 2014, 37, 78–95. [Google Scholar] [CrossRef]
- Dean, R.; Kan, J.a.L.V.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Minas, I.S.; Karaoglanidis, G.S.; Manganaris, G.A.; Vasilakakis, M. Effect of Ozone Application during Cold Storage of Kiwifruit on the Development of Stem-End Rot Caused by Botrytis cinerea. Postharvest Biol. Technol. 2010, 58, 203–210. [Google Scholar] [CrossRef]
- Mari, M.; Spadoni, A.; Ceredi, G. Alternative Technologies to Control Postharvest Diseases of Kiwifruit. Stewart Postharvest Rev. 2015, 11, 1–5. [Google Scholar]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, S.; Leroux, P.; Auclair, C.; Barreau, C.; Hajj, C.A.; Debieu, D. Genetic Analysis of Fenhexamid-Resistant Field Isolates of the Phytopathogenic Fungus Botrytis cinerea. Antimicrob. Agents Chemother. 2008, 52, 3933–3940. [Google Scholar] [CrossRef]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated Management of Postharvest Gray Mold on Fruit Crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; Amodio, M.L.; Colelli, G.; Spano, G.; Russo, P. Microbial-Based Biocontrol Solutions for Fruits and Vegetables: Recent Insight, Patents, and Innovative Trends. Recent Pat. Food Nutr. Agric. 2021, 12, 1. [Google Scholar] [CrossRef]
- Russo, P.; Spano, G.; Capozzi, V. Safety evaluation of starter cultures. In Starter Cultures in Food Production; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 101–128. ISBN 978-1-118-93379-4. [Google Scholar]
- Russo, P.; Fares, C.; Longo, A.; Spano, G.; Capozzi, V. Lactobacillus plantarum with Broad Antifungal Activity as a Protective Starter Culture for Bread Production. Foods 2017, 6, 110. [Google Scholar] [CrossRef]
- Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. Exploration of the Microbial Biodiversity Associated with North Apulian Sourdoughs and the Effect of the Increasing Number of Inoculated Lactic Acid Bacteria Strains on the Biocontrol against Fungal Spoilage. Fermentation 2019, 5, 97. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic Acid Bacteria as Antibacterial Agents to Extend the Shelf Life of Fresh and Minimally Processed Fruits and Vegetables: Quality and Safety Aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Cortés-Zavaleta, O.; López-Malo, A.; Hernández-Mendoza, A.; García, H.S. Antifungal Activity of Lactobacilli and Its Relationship with 3-Phenyllactic Acid Production. Int. J. Food Microbiol. 2014, 173, 30–35. [Google Scholar] [CrossRef]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. Rapid Identification, by Use of the LTQ Orbitrap Hybrid FT Mass Spectrometer, of Antifungal Compounds Produced by Lactic Acid Bacteria. Anal. Bioanal. Chem. 2012, 403, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.B.; Abadias, M.; Anguera, M.; Sabata, J.; Viñas, I. Antagonistic Effect of Probiotic Bacteria against Foodborne Pathogens on Fresh-Cut Pear. LWT Food Sci. Technol. 2017, 81, 243–249. [Google Scholar] [CrossRef]
- Iglesias, M.B.; López, M.L.; Echeverría, G.; Viñas, I.; Zudaire, L.; Abadias, M. Evaluation of Biocontrol Capacity of Pseudomonas graminis CPA-7 against Foodborne Pathogens on Fresh-Cut Pear and Its Effect on Fruit Volatile Compounds. Food Microbiol. 2018, 76, 226–236. [Google Scholar] [CrossRef]
- Leneveu-Jenvrin, C.; Charles, F.; Barba, F.J.; Remize, F. Role of Biological Control Agents and Physical Treatments in Maintaining the Quality of Fresh and Minimally-Processed Fruit and Vegetables. Crit. Rev. Food Sci. Nutr. 2020, 60, 2837–2855. [Google Scholar] [CrossRef] [PubMed]
- Klewicka, E.; Sójka, M.; Klewicki, R.; Kołodziejczyk, K.; Lipińska, L.; Nowak, A. Ellagitannins from Raspberry (Rubus Idaeus L.) Fruit as Natural Inhibitors of Geotrichum Candidum. Molecules 2016, 21, 908. [Google Scholar] [CrossRef] [PubMed]
- Klewicka, E.; Sójka, M.; Ścieszka, S.; Klewicki, R.; Milczarek, A.; Lipińska, L.; Kołodziejczyk, K. The Antimycotic Effect of Ellagitannins from Raspberry (Rubus Idaeus L.) on Alternaria Alternata ŁOCK 0409. Eur. Food Res. Technol. 2020, 246, 1341–1349. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Bioactive Edible Film Based on Konjac Glucomannan and Probiotic Lactobacillus plantarum Strains: Physicochemical Properties and Shelf Life of Fresh-Cut Kiwis. J. Food Sci. 2021, 86, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with Broad Antifungal Activity: A Promising Approach to Increase Safety and Shelf-Life of Cereal-Based Products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, Y.; Wang, J.; Zhang, H.; Qi, W. Production and Characterization of Antifungal Compounds Produced by Lactobacillus plantarum IMAU10014. PLoS ONE 2012, 7, e29452. [Google Scholar] [CrossRef]
- Axel, C.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal Sourdough Lactic Acid Bacteria as Biopreservation Tool in Quinoa and Rice Bread. Int. J. Food Microbiol. 2016, 239, 86–94. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Zhou, T.; Bullerman, L.B. Sourdough Lactic Acid Bacteria as Antifungal and Mycotoxin-Controlling Agents. Food Sci. Technol. Int. 2016, 22, 79–90. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Dal Bello, F.; Clarke, C.I.; Ryan, L.A.M.; Ulmer, H.; Schober, T.J.; Ström, K.; Sjögren, J.; Van Sinderen, D.; Schnürer, J.; Arendt, E.K. Improvement of the Quality and Shelf Life of Wheat Bread by Fermentation with the Antifungal Strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Huh, C.K.; Hwang, T.Y. Identification of Antifungal Substances of Lactobacillus sakei subsp. ALI033 and Antifungal Activity against Penicillium brevicompactum Strain FI02. Prev. Nutr. Food Sci. 2016, 21, 52. [Google Scholar] [CrossRef]
- Axel, C.; Brosnan, B.; Zannini, E.; Peyer, L.C.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal Activities of Three Different Lactobacillus Species and Their Production of Antifungal Carboxylic Acids in Wheat Sourdough. Appl. Microbiol. Biotechnol. 2016, 100, 1701–1711. [Google Scholar] [CrossRef]
- Quattrini, M.; Liang, N.; Fortina, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting Synergies of Sourdough and Antifungal Organic Acids to Delay Fungal Spoilage of Bread. Int. J. Food Microbiol. 2019, 302, 8–14. [Google Scholar] [CrossRef]
- Cheong, E.Y.; Sandhu, A.; Jayabalan, J.; Le, T.T.K.; Nhiep, N.T.; Ho, H.T.M.; Zwielehner, J.; Bansal, N.; Turner, M.S. Isolation of Lactic Acid Bacteria with Antifungal Activity against the Common Cheese Spoilage Mold Penicillium commune and Their Potential as Biopreservatives in Cheese. Food Control 2014, 46, 91–97. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-H.; Ren, L.-Q.; Zhou, Y.; Ye, B.-C. Characterization of Antimicrobial Activity of Three Lactobacillus plantarum Strains Isolated from Chinese Traditional Dairy Food. Food Sci. Nutr. 2019, 7, 1997–2005. [Google Scholar] [CrossRef]
- Kwak, M.-K.; Liu, R.; Kang, S.-O. Antimicrobial Activity of Cyclic Dipeptides Produced by Lactobacillus plantarum LBP-K10 against Multidrug-Resistant Bacteria, Pathogenic Fungi, and Influenza A Virus. Food Control 2018, 85, 223–234. [Google Scholar] [CrossRef]
- Le, N.T.T.; Bach, L.G.; Nguyen, D.C.; Le, T.H.X.; Pham, K.H.; Nguyen, D.H.; Hoang Thi, T.T. Evaluation of Factors Affecting Antimicrobial Activity of Bacteriocin from Lactobacillus plantarum Microencapsulated in Alginate-Gelatin Capsules and Its Application on Pork Meat as a Bio-Preservative. Int. J. Environ. Res. Public. Health 2019, 16, 1017. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Z.; Li, J.; Tao, C.; Feng, Y.; Han, Y. A Novel Endophytic Strain of Lactobacillus plantarum CM-3 with Antagonistic Activity against Botrytis cinerea on Strawberry Fruit. Biol. Control 2020, 148, 104306. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Zheng, Z.; Meng, X.; Cai, Y.; Liu, J.; Hu, Y.; Yan, S.; Wang, X. A Microbial Ecosystem: Agricultural Jiaosu Achieves Effective and Lasting Antifungal Activity against Botrytis cinerea. AMB Express 2020, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, D.; Hamidi-Esfahani, Z. Influence of Bioactive Edible Coatings Loaded with Lactobacillus plantarum on Physicochemical Properties of Fresh Strawberries. Postharvest Biol. Technol. 2019, 156, 110944. [Google Scholar] [CrossRef]
- De Giani, A.; Bovio, F.; Forcella, M.; Fusi, P.; Sello, G.; Di Gennaro, P. Identification of a Bacteriocin-like Compound from Lactobacillus plantarum with Antimicrobial Activity and Effects on Normal and Cancerogenic Human Intestinal Cells. AMB Express 2019, 9, 88. [Google Scholar] [CrossRef]
- Kim, S.W.; Kang, S.I.; Shin, D.H.; Oh, S.Y.; Lee, C.W.; Yang, Y.; Son, Y.K.; Yang, H.-S.; Lee, B.-H.; An, H.-J.; et al. Potential of Cell-Free Supernatant from Lactobacillus plantarum NIBR97, Including Novel Bacteriocins, as a Natural Alternative to Chemical Disinfectants. Pharmaceuticals 2020, 13, 266. [Google Scholar] [CrossRef]
- Ołdak, A.; Zielińska, D.; Łepecka, A.; Długosz, E.; Kołożyn-Krajewska, D. Lactobacillus plantarum Strains Isolated from Polish Regional Cheeses Exhibit Anti-Staphylococcal Activity and Selected Probiotic Properties. Probiotics Antimicrob. Proteins 2020, 12, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Salari, S.; Almani, P.G.N. Antifungal Effects of Lactobacillus acidophilus and Lactobacillus plantarum against Different Oral Candida Species Isolated from HIV/ AIDS Patients: An in Vitro Study. J. Oral Microbiol. 2020, 12, 1769386. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, B.V.; Rao, K.P.; Chennapa, G.; Naik, M.K.; Chandrashekara, K.T.; Sreenivasa, M.Y. Antifungal Attributes of Lactobacillus plantarum MYS6 against Fumonisin Producing Fusarium proliferatum Associated with Poultry Feeds. PLoS ONE 2016, 11, e0155122. [Google Scholar] [CrossRef]
- Xie, C.; Wang, H.; Deng, S.; Xu, X.-L. The Inhibition of Cell-Free Supernatant of Lactobacillus plantarum on Production of Putrescine and Cadaverine by Four Amine-Positive Bacteria in Vitro. LWT Food Sci. Technol. 2016, 67, 106–111. [Google Scholar] [CrossRef]
- Ahmad Rather, I.; Seo, B.J.; Rejish Kumar, V.J.; Choi, U.-H.; Choi, K.-H.; Lim, J.H.; Park, Y.-H. Isolation and Characterization of a Proteinaceous Antifungal Compound from Lactobacillus plantarum YML007 and Its Application as a Food Preservative. Lett. Appl. Microbiol. 2013, 57, 69–76. [Google Scholar] [CrossRef] [PubMed]
- George-Okafor, U.; Ozoani, U.; Tasie, F.; Mba-Omeje, K. The Efficacy of Cell-Free Supernatants from Lactobacillus plantarum Cs and Lactobacillus acidophilus ATCC 314 for the Preservation of Home-Processed Tomato-Paste. Sci. Afr. 2020, 8, e00395. [Google Scholar] [CrossRef]
- Daranas, N.; Roselló, G.; Cabrefiga, J.; Donati, I.; Francés, J.; Badosa, E.; Spinelli, F.; Montesinos, E.; Bonaterra, A. Biological Control of Bacterial Plant Diseases with Lactobacillus plantarum Strains Selected for Their Broad-spectrum Activity. Ann. Appl. Biol. 2019, 174, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, H.; Shin, J.; Huang, L.; Zhang, H.; Qi, W. Activity against Plant Pathogenic Fungi of Lactobacillus plantarum IMAU10014 Isolated from Xinjiang Koumiss in China. Ann. Microbiol. 2011, 61, 879–885. [Google Scholar] [CrossRef]
- Magnusson, J.; Ström, K.; Roos, S.; Sjögren, J.; Schnürer, J. Broad and Complex Antifungal Activity among Environmental Isolates of Lactic Acid Bacteria. FEMS Microbiol. Lett. 2003, 219, 129–135. [Google Scholar] [CrossRef]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic Acid Bacteria—Potential for Control of Mold Growth and Mycotoxins: A Review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Gajbhiye, M.H.; Kapadnis, B.P. Antifungal-Activity-Producing Lactic Acid Bacteria as Biocontrol Agents in Plants. Biocontrol Sci. Technol. 2016, 26, 1451–1470. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicki, R.; Sójka, M.; Bonikowski, R.; Żyżelewicz, D.; Kołodziejczyk, K.; Klewicka, E. Antifungal Activity of Lactobacillus pentosus ŁOCK 0979 in the Presence of Polyols and Galactosyl-Polyols. Probiotics Antimicrob. Proteins 2018, 10, 186–200. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicki, R.; Klewicka, E.; Kołodziejczyk, K.; Sójka, M.; Nowak, A. Antifungal Activity of Lactobacillus Sp. Bacteria in the Presence of Xylitol and Galactosyl-Xylitol. BioMed Res. Int. 2016, 5897486. [Google Scholar] [CrossRef]
- Lipinska-Zubrycka, L.; Klewicki, R.; Sojka, M.; Bonikowski, R.; Milczarek, A.; Klewicka, E. Anticandidal Activity of Lactobacillus Spp. in the Presence of Galactosyl Polyols. Microbiol. Res. 2020, 240, 126540. [Google Scholar] [CrossRef]
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective Mechanisms of Lactic Acid Bacteria against Fungal Spoilage of Food. Curr. Opin. Biotechnol. 2019, 56, 138–146. [Google Scholar] [CrossRef]
- Yoo, J.A.; Lim, Y.M.; Yoon, M.H. Production and Antifungal Effect of 3-Phenyllactic Acid (PLA) by Lactic Acid Bacteria. J. Appl. Biol. Chem. 2016, 59, 173–178. [Google Scholar] [CrossRef]
- Lemos, W.J.F., Jr.; Bovo, B.; Nadai, C.; Crosato, G.; Carlot, M.; Favaron, F.; Giacomini, A.; Corich, V. Biocontrol Ability and Action Mechanism of Starmerella bacillaris (Synonym Candida zemplinina) Isolated from Wine Musts against Gray Mold Disease Agent Botrytis Cinerea on Grape and Their Effects on Alcoholic Fermentation. Front. Microbiol. 2016, 7, 1249. [Google Scholar] [CrossRef]
- Qin, X.; Xiao, H.; Cheng, X.; Zhou, H.; Si, L. Hanseniaspora uvarum Prolongs Shelf Life of Strawberry via Volatile Production. Food Microbiol. 2017, 63, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moyano, S.; Hernández, A.; Galvan, A.I.; Córdoba, M.G.; Casquete, R.; Serradilla, M.J.; Martín, A. Selection and Application of Antifungal VOCs-Producing Yeasts as Biocontrol Agents of Grey Mold in Fruits. Food Microbiol. 2020, 92, 103556. [Google Scholar] [CrossRef]
- Zudaire, L.; Viñas, I.; Plaza, L.; Iglesias, M.B.; Abadias, M.; Aguiló-Aguayo, I. Evaluation of Postharvest Calcium Treatment and Biopreservation with Lactobacillus rhamnosus GG on the Quality of Fresh-Cut ‘Conference’Pears. J. Sci. Food Agric. 2018, 98, 4978–4987. [Google Scholar] [CrossRef]
- Abadias, M.; Altisent, R.; Usall, J.; Torres, R.; Oliveira, M.; Viñas, I. Biopreservation of Fresh-Cut Melon Using the Strain Pseudomonas graminis CPA-7. Postharvest Biol. Technol. 2014, 96, 69–77. [Google Scholar] [CrossRef]
- Russo, P.; Peña, N.; de Chiara, M.L.V.; Amodio, M.L.; Colelli, G.; Spano, G. Probiotic Lactic Acid Bacteria for the Production of Multifunctional Fresh-Cut Cantaloupe. Food Res. Int. 2015, 77, 762–772. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative Strategies Based on the Use of Bio-Control Agents to Improve the Safety, Shelf-Life and Quality of Minimally Processed Fruits and Vegetables. Trends Food Sci. Technol. 2015, 46, 302–310. [Google Scholar] [CrossRef]

| LAB Strains | Source | Species | Reference |
|---|---|---|---|
| M04 | Sourdough-C | L. plantarum | [18] |
| PAN01 | Sourdough-B | L. plantarum | [18] |
| M12 | Sourdough-C | L. plantarum | [18] |
| C01Rib | Sourdough-E | L. plantarum | [18] |
| UFG 121 | Sourdough-A | L. plantarum | [28] |
| RIS10 | Sourdough-D | L. plantarum | [18] |
| LAB Strains | pH | |
|---|---|---|
| CFS24 | CFS48 | |
| M04 | 3.95 | 3.72 |
| PAN01 | 3.57 | 3.54 |
| M12 | 3.76 | 3.63 |
| C01Rib | 3.65 | 3.56 |
| UFG 121 | 3.80 | 3.60 |
| RIS10 | 3.57 | 3.54 |
| UFG 121 | PAN01 | |||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | pH | Lactic Acid (g/L) | L-Lactate (g/L) | D-Lactate (g/L) | pH | Lactic Acid (g/L) | L-Lactate (g/L) | D-Lactate (g/L) |
| 6 | 6.53 | 0.48 ± 0.07 dD | 0.38 ± 0.02 dD | 0.10 ± 0.07 cE | 6.54 | 0.49 ± 0.22 dD | 0.31 ± 0.25 cD | 0.09 ± 0.02 dE |
| 24 | 3.80 | 16.93 ± 0.55 cBC | 14.76 ± 0.50 cC | 2.17 ± 0.65 bBC | 3.57 | 14.70 ± 0.03 cC | 14.14 ± 0.02 bC | 0.56 ±0.07 cD |
| 30 | 3.68 | 20.45 ± 0.74 bB | 17.92 ± 0.74 bB | 2.53 ± 0.76 bB | 3.55 | 16.17 ± 0.19 bcBC | 14.89 ± 0.11 bC | 1.28 ± 0.46 bC |
| 48 | 3.60 | 27.18 ± 0.30 aA | 22.27 ± 0.32 aA | 4.91 ± 0.24 aA | 3.54 | 20.59 ± 0.24 aB | 17.94 ± 0.06 aB | 2.64 ± 0.72 aB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Simone, N.; Capozzi, V.; de Chiara, M.L.V.; Amodio, M.L.; Brahimi, S.; Colelli, G.; Drider, D.; Spano, G.; Russo, P. Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis cinerea and the Potential of Lactiplantibacillus plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit. Microorganisms 2021, 9, 773. https://doi.org/10.3390/microorganisms9040773
De Simone N, Capozzi V, de Chiara MLV, Amodio ML, Brahimi S, Colelli G, Drider D, Spano G, Russo P. Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis cinerea and the Potential of Lactiplantibacillus plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit. Microorganisms. 2021; 9(4):773. https://doi.org/10.3390/microorganisms9040773
Chicago/Turabian StyleDe Simone, Nicola, Vittorio Capozzi, Maria Lucia Valeria de Chiara, Maria Luisa Amodio, Samira Brahimi, Giancarlo Colelli, Djamel Drider, Giuseppe Spano, and Pasquale Russo. 2021. "Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis cinerea and the Potential of Lactiplantibacillus plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit" Microorganisms 9, no. 4: 773. https://doi.org/10.3390/microorganisms9040773
APA StyleDe Simone, N., Capozzi, V., de Chiara, M. L. V., Amodio, M. L., Brahimi, S., Colelli, G., Drider, D., Spano, G., & Russo, P. (2021). Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis cinerea and the Potential of Lactiplantibacillus plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit. Microorganisms, 9(4), 773. https://doi.org/10.3390/microorganisms9040773










