Effects of Cordycepin in Cordyceps militaris during Its Infection to Silkworm Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms, Media, and Silkworm
2.2. Analysis of the Infection
2.3. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
2.4. Phenoloxidase Activity
2.5. RNA-Sequencing (RNA-Seq)
2.6. Statistics Analysis
3. Results and Discussion
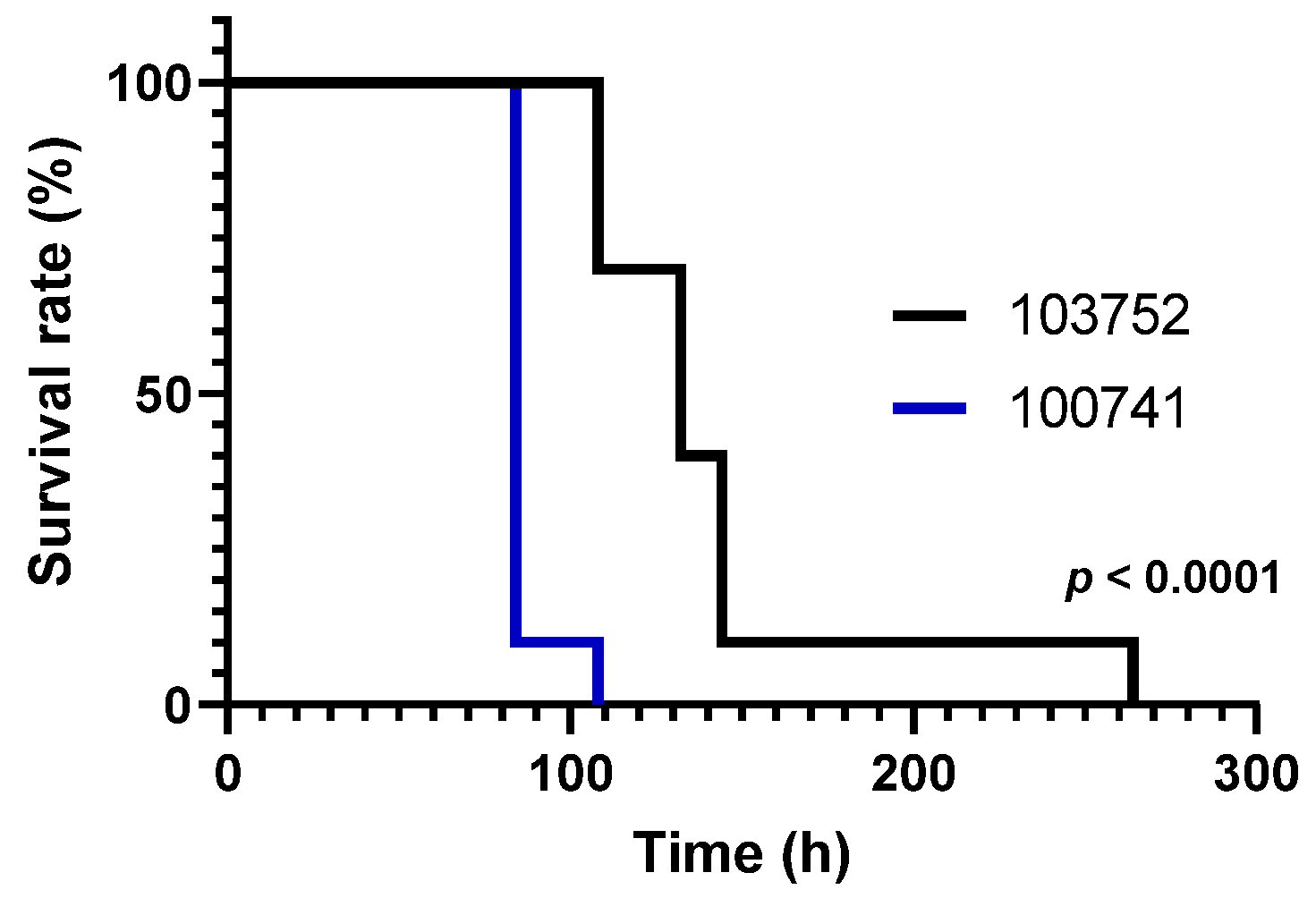
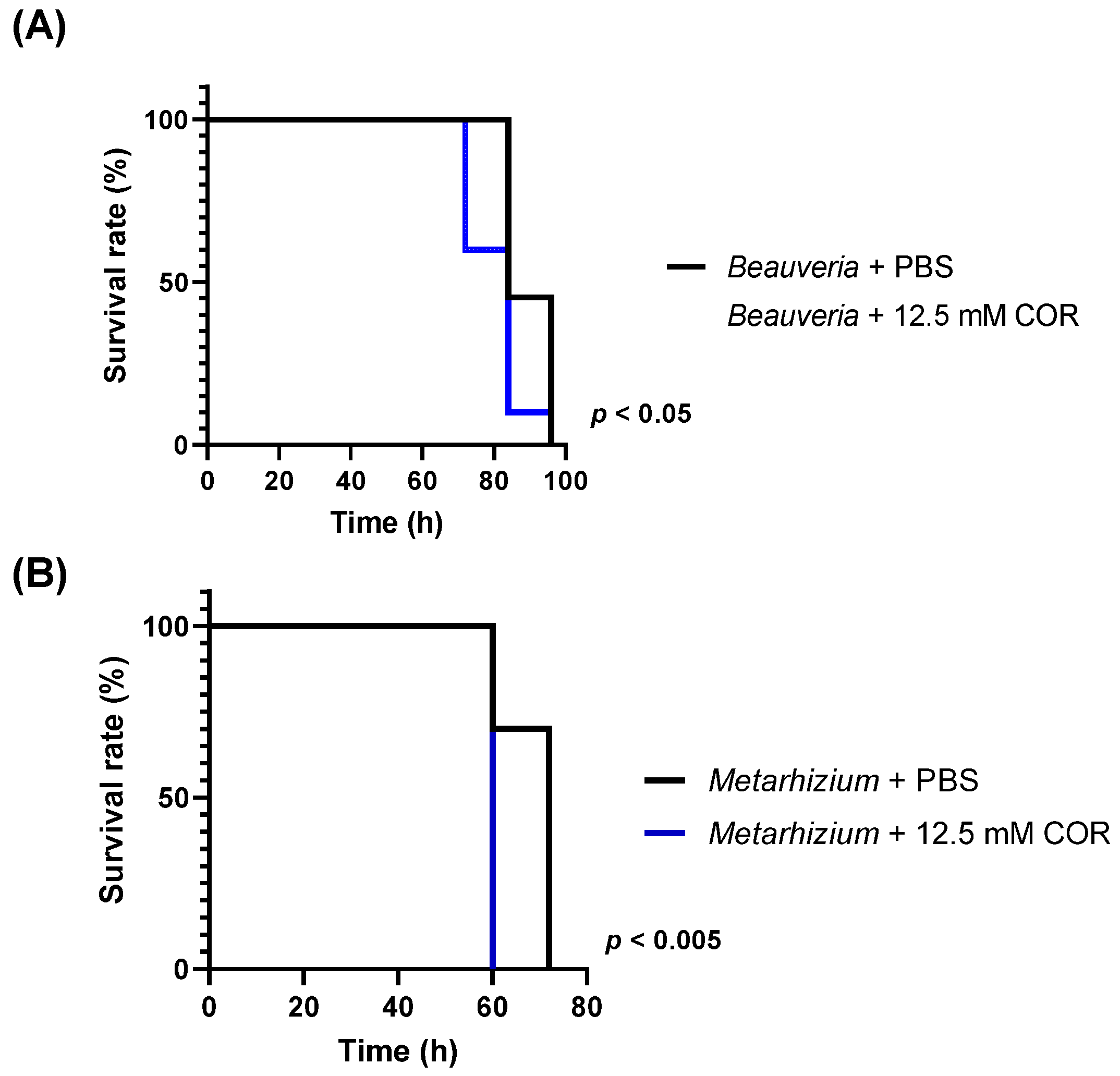
3.1. Viability of Silkworm Larvae after Injection with Conidia
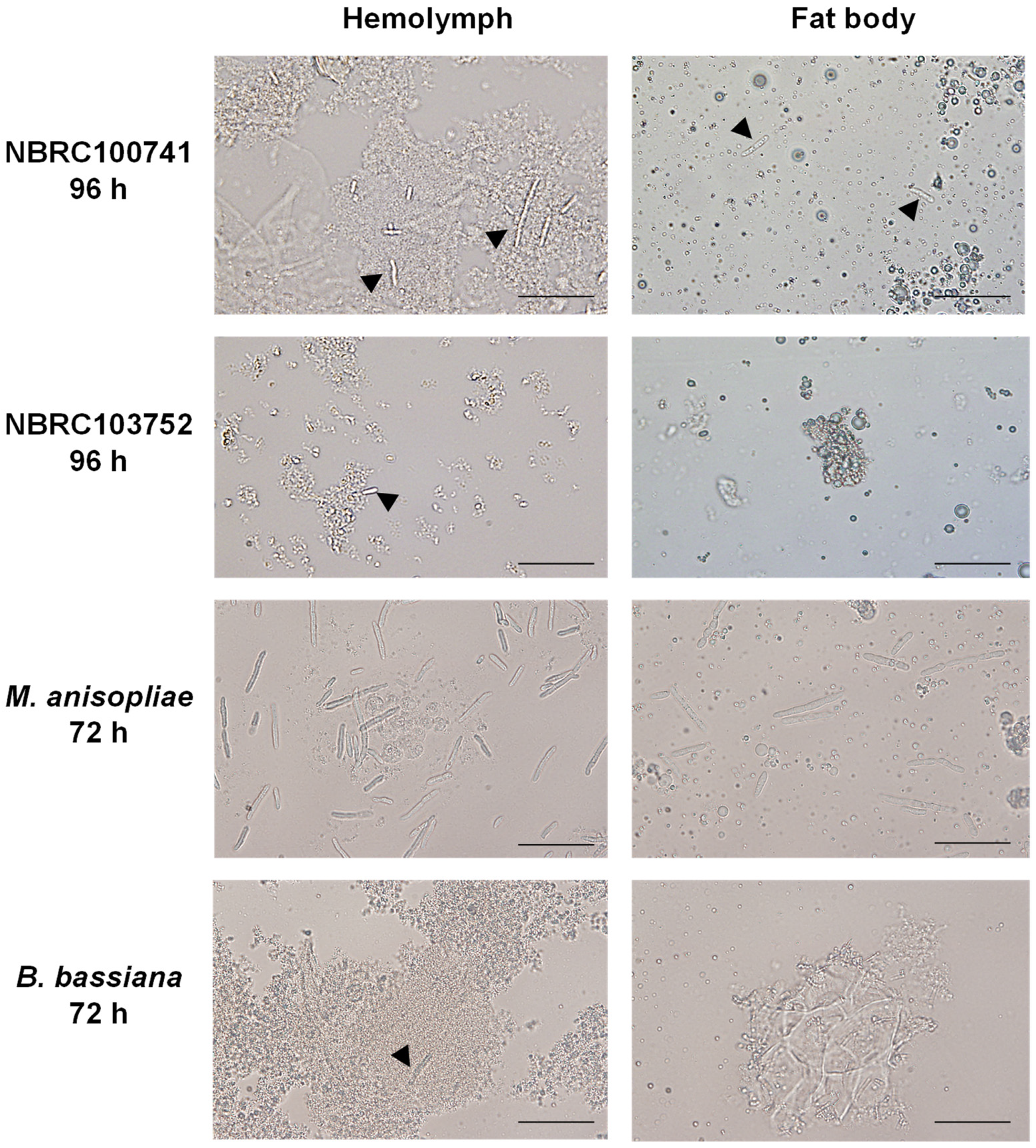
3.2. Morphology of C. militaris in Silkworm Larvae during Its Infection
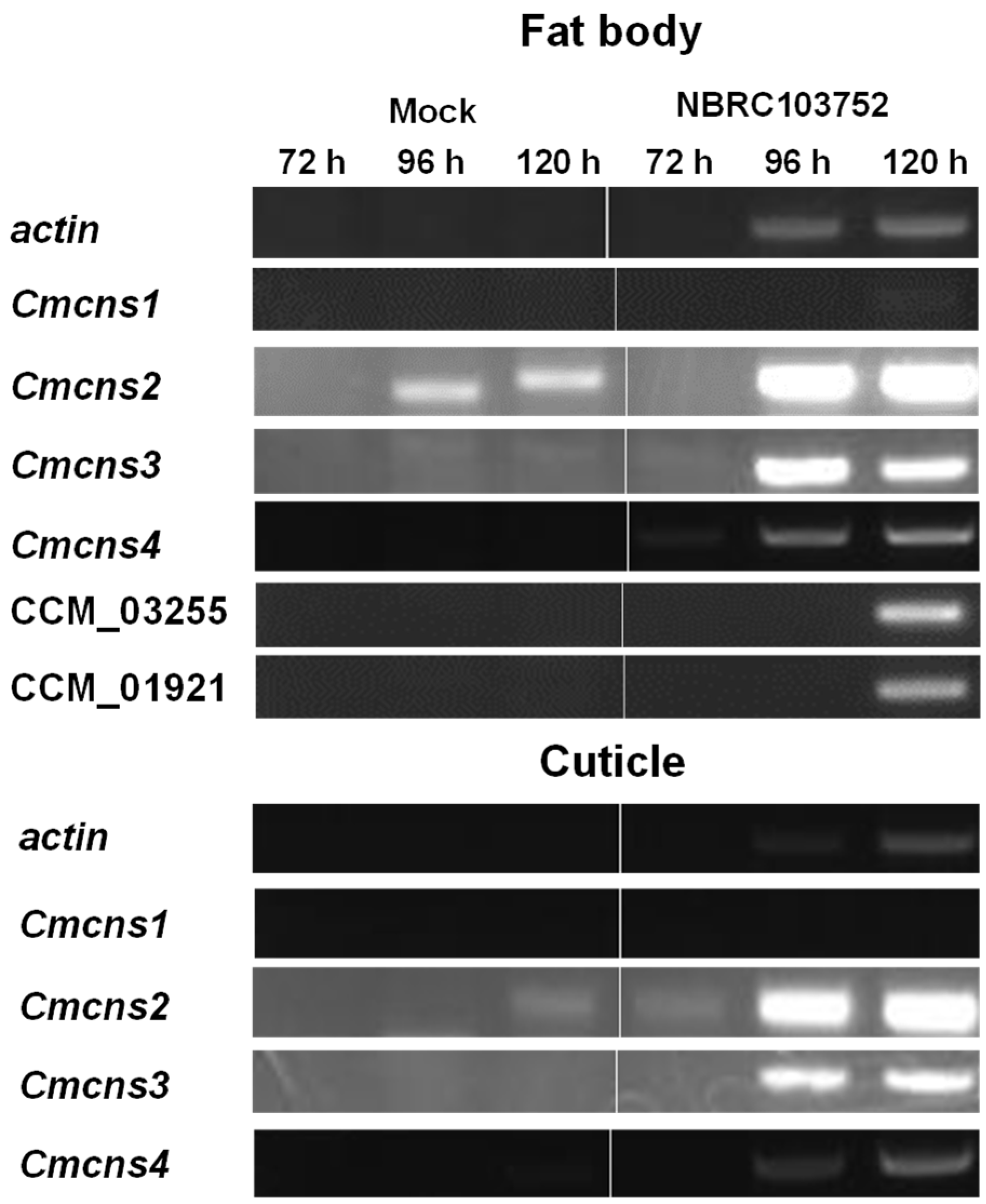
3.3. Cordycepin Biosynthetic Gene Expression of C. militaris in Silkworm Larvae
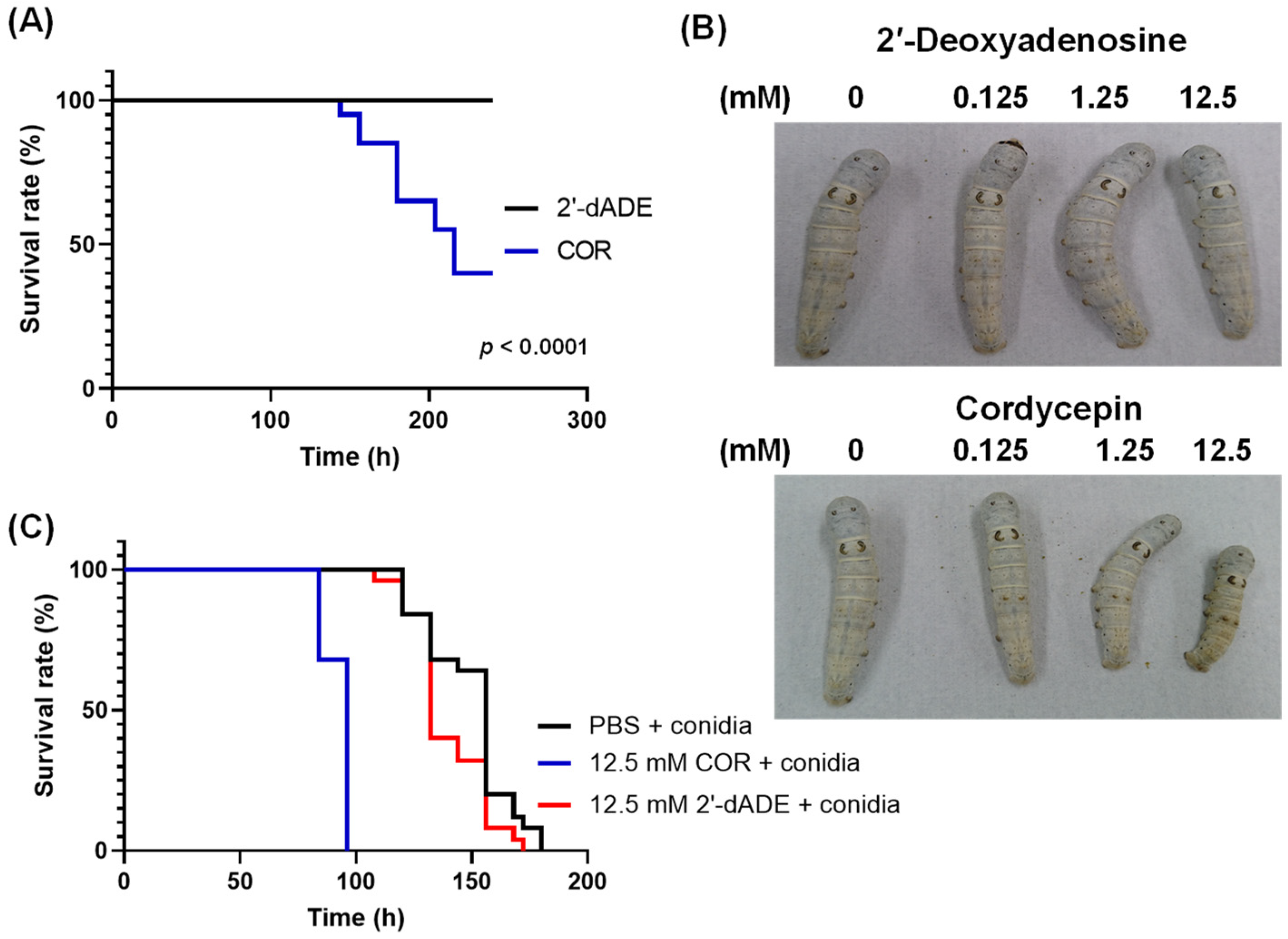
3.4. Influence of Cordycepin on the Growth of Entomopathogenic Fungi
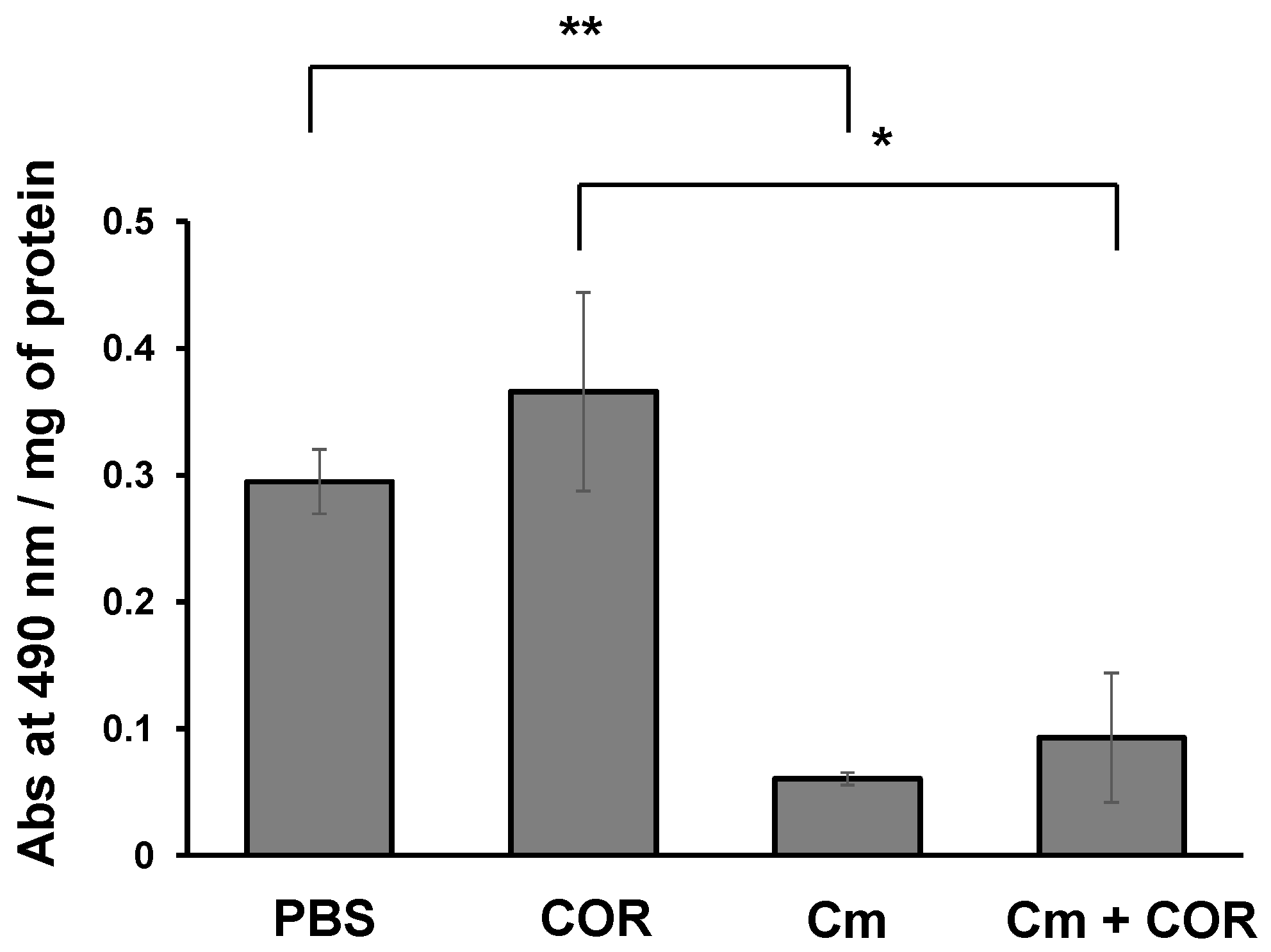
3.5. Phenoloxidase Activity in the Hemolymph of Silkworm Larvae Injected with Cordycepin
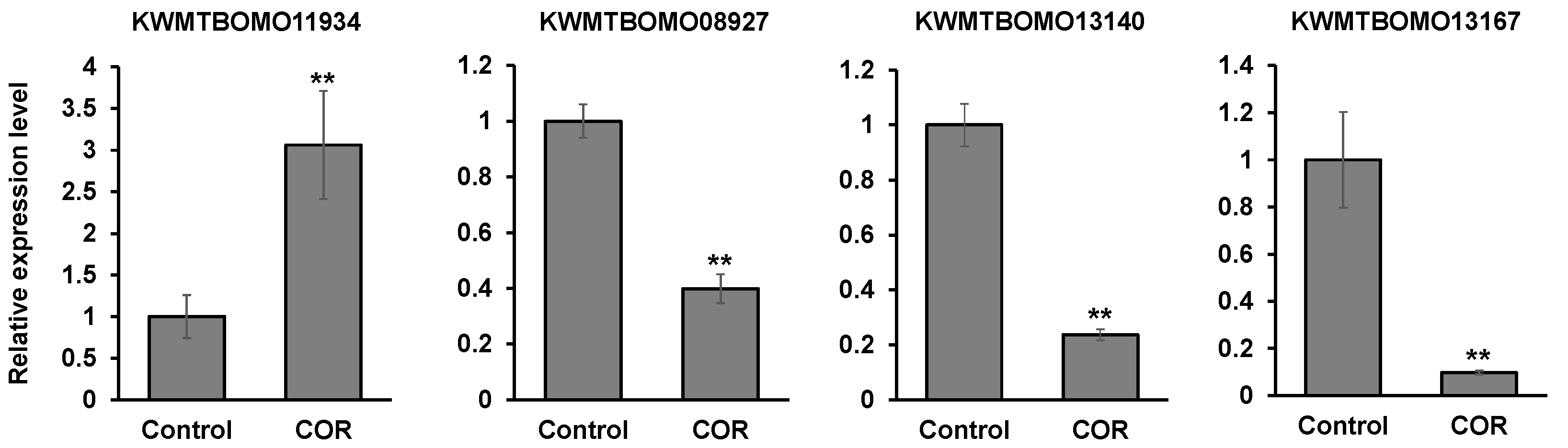
3.6. The Effects of Cordycepin on the Gene Expression in Silkworm Larvae
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Dong, C.; Guo, S.; Wang, W.; Liu, X. Cordyceps industry in China. Mycology 2015, 6, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Lin, J.; Guo, L.; Wang, X.; Tian, S.; Liu, C.; Zhao, Y.; Zhao, R. Advances in research on Cordyceps militaris degeneration. Appl. Microbiol. Biotechnol. 2019, 103, 7835–7841. [Google Scholar] [CrossRef]
- Qin, P.; Li, X.; Yang, H.; Wang, Z.Y.; Lu, D. Therapeutic potential and biological applications of cordycepin and metabolic mechanisms in cordycepin-producing fungi. Molecules 2019, 24, 2231. [Google Scholar] [CrossRef]
- Ko, B.S.; Lu, Y.J.; Yao, W.L.; Liu, T.A.; Tzean, S.S.; Shen, T.L.; Liou, J.Y. Cordycepin regulates GSK-3β/β-catenin signaling in human leukemia cells. PLoS ONE 2013, 8, 76320. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Park, S.J.; Jo, E.; Hwang, I.H.; Lee, K.B.; Kim, S.W.; Kim, D.J.; Joo, J.C.; Hong, S.H.; Lee, M.G.; et al. Cordycepin induces apoptosis of human ovarian cancer cells by inhibiting CCL5-mediated Akt/NF-κB signaling pathway. Cell Death Discov. 2018, 4, 62. [Google Scholar] [CrossRef]
- Liao, Y.; Ling, J.; Zhang, G.; Liu, F.; Tao, S.; Han, Z.; Chen, S.; Chen, Z.; Le, H. Cordycepin induces cell cycle arrest and apoptosis by inducing DNA damage and up-regulation of p53 in Leukemia cells. Cell Cycle 2015, 14, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Jin, C.Y.; Park, C.; Hong, S.H.; Kim, G.Y.; Jeong, Y.K.; Lee, J.D.; Yoo, Y.H.; Choi, Y.H. Induction of apoptosis by cordycepin via reactive oxygen species generation in human leukemia cells. Toxicol. In Vitro 2011, 25, 817–824. [Google Scholar] [CrossRef]
- Kim, J.R.; Yeon, S.H.; Kim, H.S.; Ahn, Y.J. Larvicidal activity against Plutella xylostella of cordycepin from the fruiting body of Cordyceps militaris. Pest Manag. Sci. 2002, 58, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Dalla Rosa, L.; Da Silva, A.S.; Gressler, L.T.; Oliveira, C.B.; Dambrós, M.G.C.; Miletti, L.C.; França, R.T.; Lopes, S.T.A.; Samara, Y.N.; Da Veiga, M.L. Cordycepin (3′-deoxyadenosine) pentostatin (deoxycoformycin) combination treatment of mice experimentally infected with Trypanosoma evansi. Parasitology 2013, 140, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Hatashita, M.; Sakurai, A.; Sakakibara, M. A new approach for improving cordycepin productivity in surface liquid culture of Cordyceps militaris using high-energy ion beam irradiation. Lett. Appl. Microbiol. 2008, 47, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Sari, N.; Suparmin, A.; Kato, T.; Park, E.Y. Improved cordycepin production in a liquid surface culture of Cordyceps militaris isolated from wild strain. Biotechnol. Bioproc. Eng. 2016, 21, 595–600. [Google Scholar] [CrossRef][Green Version]
- Suparmin, A.; Kato, T.; Dohra, H.; Park, E.Y. Insight into cordycepin biosynthesis of Cordyceps militaris: Comparison between a liquid surface culture and a submerged culture through transcriptomic analysis. PLoS ONE 2017, 12, 0187052. [Google Scholar] [CrossRef]
- Suparmin, A.; Kato, T.; Takemoto, H.; Park, E.Y. Metabolic comparison of aerial and submerged mycelia formed in the liquid surface culture of Cordyceps militaris. Microbiologyopen 2019, 8, 00836. [Google Scholar] [CrossRef]
- Woolley, V.C.; Teakle, G.R.; Prince, G.; De Moor, C.H.; Chandler, D. Cordycepin, a metabolite of Cordyceps militaris, reduces immune-related gene expression in insects. J. Invertebr. Pathol. 2020, 177, 107480. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, N. Molecular interactions between entomopathogenic fungi (Hypocreales) and their insect host: Perspectives from stressful cuticle and hemolymph battlefields and the potential of dual RNA sequencing for future studies. Fungal Biol. 2018, 122, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, C.; Cao, L.; Xie, C.; Han, R. Agrobacterium tumefaciens-mediated transformation as a tool for insertional mutagenesis in medicinal fungus Cordyceps militaris. Fungal Biol. 2011, 115, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiravanichpaisal, P.; Cerenius, L.; Lee, B.L.; Söderhäll, I.; Söderhäll, K. Phenoloxidase is an important component of the defense against Aeromonas hydrophila infection in a Crustacean, Pacifastacus leniusculus. J. Biol. Chem. 2007, 282, 33593–33598. [Google Scholar] [CrossRef]
- Kinjo, S.; Monma, N.; Misu, S.; Kitamura, N.; Imoto, J.; Yoshitake, K.; Gojobori, T.; Ikeo, K. Maser: One-stop platform for NGS big data from analysis to visualization. Database 2018, 2018, 27. [Google Scholar] [CrossRef]
- Kershaw, M.J.; Moorhouse, E.R.; Bateman, R.; Reynolds, S.E.; Charnley, A.K. The role of destruxins in the pathogenicity of Metarhizium anisopliae for three species of insect. J. Invertebr. Pathol. 1999, 74, 213–223. [Google Scholar] [CrossRef]
- He, Z.; Luo, L.; Keyhani, N.O.; Yu, X.; Ying, S.; Zhang, Y. The C-terminal MIR-containing region in the Pmt1 O-mannosyltransferase restrains sporulation and is dispensable for virulence in Beauveria bassiana. Appl. Microbiol. 2017, 101, 1143–1161. [Google Scholar] [CrossRef]
- Zhang, A.X.; Mouhoumed, A.Z.; Tong, S.M.; Ying, S.H.; Feng, M.G. BrlA and AbaA govern virulence-required dimorphic switch, conidiation, and pathogenicity in a fungal insect pathogen. mSystems 2019, 4, e00140-19. [Google Scholar] [CrossRef]
- Boucias, D.; Liu, S.; Meagher, R.; Baniszewski, J. Fungal dimorphism in the entomopathogenic fungus Metarhizium rileyi: Detection of an in vivo quorum-sensing system. J. Invertebr. Pathol. 2016, 136, 100–108. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, F.; Shang, Y.; Chen, P.; Lu, Y.; Wang, C. Fungal cordycepin biosynthesis is coupled with the production of the safeguard molecule pentostatin. Cell Chem. Biol. 2017, 24, 1479–1489.e4. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Sieber, C.M.; Von Bargen, K.W.; Studt, L.; Niehaus, E.M.; Espino, J.J.; Huß, K.; Michielse, C.B.; Albermann, S.; Wagner, D.; et al. Deciphering the cryptic genome: Genome-wide analyses of the rice pathogen Fusarium fujikuroi reveal complex regulation of secondary metabolism and novel metabolites. PLoS Pathog. 2013, 9, 1003475. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.; Xie, X.Q.; Shang, Y.; St Leger, R.J.; Zhao, G.P.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xiao, G.; Zheng, P.; Cen, K.; Zhan, S.; Wang, C. Divergent and convergent evolution of fungal pathogenicity. Genome Biol. Evol. 2016, 8, 1374–1387. [Google Scholar] [CrossRef] [PubMed]
- Lobo, L.S.; Luz, W.C.; Fernandes, E.K.K.; Juárez, M.P.; Pedrini, N. Assessing gene expression during pathogenesis: Use of qRT-PCR to follow toxin production from the entomopathogenic fungus Beauveria bassiana during infection and immune response of the insect host Triatoma infestans. J. Invertebr. Pathol. 2015, 128, 14–21. [Google Scholar] [CrossRef]
- Podsiadło, E.; Michalik, K.; Michalik, A.; Szklarzewicz, T. Yeast-like microorganisms in the scale insect Kermes quercus (Insecta, Hemiptera, Coccomorpha: Kermesidae). Newly acquired symbionts? Arthropod. Struct. Dev. 2018, 47, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, V.Y.; Yaroslavtseva, O.N.; Surina, E.V.; Tyurin, M.V.; Dubovskiy, I.M.; Glupov, V.V. Immune reactions of the greater wax moth, Galleria mellonella L. (lepidoptera, pyralidae) larvae under combined treatment of the entomopathogens Cordyceps militaris (L.: Fr.) Link and Beauveria bassiana (Bals.-Criv.) Vuill. (Ascomycota, Hypocreales). Entomol. Rev. 2015, 95, 693–698. [Google Scholar] [CrossRef]
- Zou, Z.; Picheng, Z.; Weng, H.; Mita, K.; Jiang, H. A comparative analysis of serpin genes in the silkworm genome. Genomics 2009, 93, 367–375. [Google Scholar] [CrossRef][Green Version]
- Meekins, D.A.; Kanost, M.R.; Michel, K. Serpins in arthropod biology. Semin. Cell Dev. Biol. 2017, 62, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Xu, X.; De Mandal, S.; Jin, F. Role of serine protease inhibitors in insect-host-pathogen interactions. Arch. Insect Biochem. Physiol. 2019, 102, 21556. [Google Scholar] [CrossRef]
- Lu, A.; Zhang, Q.; Zhang, J.; Yang, B.; Wu, K.; Xie, W.; Luan, Y.X.; Ling, E. Insect prophenoloxidase: The view beyond immunity. Front. Physiol. 2014, 5, 252. [Google Scholar] [CrossRef]
- Kryukov, V.Y.; Kryukova, N.A.; Tomilova, O.G.; Vorontsova, Y.; Chertkova, E.; Pervushin, A.L.; Slepneva, I.; Glupov, V.V.; Yaroslavtseva, O.N. Comparative analysis of the immune response of the wax moth Galleria mellonella after infection with the fungi Cordyceps militaris and Metarhizium robertsii. Microb. Pathog. 2020, 141, 103995. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, L.; Xiang, Z.; He, N. Expression profile of cuticular genes of silkworm, Bombyx mori. BMC Genom. 2010, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.H. Structural cuticular proteins from arthropods: Annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem. Mol. Biol. 2010, 40, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, C.; Shi, Y.; Geng, T.; Lv, D.; Gao, K.; Hou, C.; Guo, X. Silkworm storage protein Bm30K-19G1 has a certain antifungal effects on Beauveria bassiana. J. Invertebr. Pathol. 2019, 163, 34–42. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Y.; Dong, Z.; Guo, P.; Zhao, D.; Li, H.; Hu, H.; Zhou, X.; Chen, H.; Zhao, P. Five silkworm 30K proteins are involved in the cellular immunity against fungi. Insects 2021, 12, 107. [Google Scholar] [CrossRef]
- Shi, X.F.; Li, Y.N.; Yi, Y.Z.; Xiao, X.G.; Zhang, Z.F. Identification and characterization of 30 K protein genes found in Bombyx mori (Lepidoptera: Bombycidae) transcriptome. J. Insect Sci. 2015, 15, 71. [Google Scholar] [CrossRef]
| Name | Sequence (5′ to 3′) |
|---|---|
| Cmcns1-F | CATAGTGGGGACGGGATATG |
| Cmcns1-R | CAAGTGGCTTCTCGCATACA |
| Cmcns2-F | CCCTGCTCCATGACATTTCT |
| Cmcns2-R | CAGCGGAAACAGCTCTTCTT |
| Cmcns3-F | TCCTCAAGCCCACCATCTAC |
| Cmcns3-R | GCTCCTTGTAGACCGTCTCG |
| Cmcns4-F | GTATGACGGCCTTGTTTCGT |
| Cmcns4-R | GCTGAGGACTGCCTCGTAAC |
| CCM_01921-F | GCAAGACCTTTCGCT TCAAC |
| CCM_01921-R | CCTTCTCCAAGTTCG TGCTC |
| CCM_03255-F | ACGGCCACTTGACCTATCAC |
| CCM_03255-R | ACAATCGTAGCCAACCGTTC |
| Cmactin-F | GTCCCCGTCATCATGGTATC |
| Cmactin-R | GGTGTGGTGCCAAATCTTCT |
| BmactA3_F | AAGCCAACGGAATCCACGAA |
| BmactA3_R | CTTCATTGTCGATGGGGCGA |
| KWMTBOMO08927_F | CTCTCCGTCCTGGATTCGTG |
| KWMTBOMO08927_R | TGTGTTGTGTATGGCCCCTC |
| KWMTBOMO11934_F | CGGGGAGGGTAAGGAAATCG |
| KWMTBOMO11934_R | ATCGCCGTATGCGATTCTGT |
| KWMTBOMO13140_F | CTGATGCCGTCATTCTCCGT |
| KWMTBOMO13140_R | CGTCAGGCGCAGAGTATTCA |
| KWMTBOMO13167_F | GGTTGGCGCAGATGGATTTC |
| KWMTBOMO13167_R | CATATCCCGAGTCTGTGGGC |
| Gene | Fold Change * | p-Value | Annotation |
|---|---|---|---|
| KWMTBOMO11934 | 3.87681 | 0.00005 | Low molecular weight lipoprotein 30K-8 |
| KWMTBOMO11934 | 3.90196 | 0.00005 | Low molecular weight lipoprotein 30K-8 isoform |
| KWMTBOMO13140 | −3.25037 | 0.0001 | Cuticular protein RR-1 motif 42 precursor |
| KWMTBOMO13167 | −4.17149 | 0.0001 | Uncharacterized protein LOC101738360 (Insect cuticle protein) |
| KWMTBOMO08927 | −4.07516 | 0.00005 | Serine protease inhibitor 29 isoform X1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, T.; Nishimura, K.; Suparmin, A.; Ikeo, K.; Park, E.Y. Effects of Cordycepin in Cordyceps militaris during Its Infection to Silkworm Larvae. Microorganisms 2021, 9, 681. https://doi.org/10.3390/microorganisms9040681
Kato T, Nishimura K, Suparmin A, Ikeo K, Park EY. Effects of Cordycepin in Cordyceps militaris during Its Infection to Silkworm Larvae. Microorganisms. 2021; 9(4):681. https://doi.org/10.3390/microorganisms9040681
Chicago/Turabian StyleKato, Tatsuya, Konomi Nishimura, Ahmad Suparmin, Kazuho Ikeo, and Enoch Y. Park. 2021. "Effects of Cordycepin in Cordyceps militaris during Its Infection to Silkworm Larvae" Microorganisms 9, no. 4: 681. https://doi.org/10.3390/microorganisms9040681
APA StyleKato, T., Nishimura, K., Suparmin, A., Ikeo, K., & Park, E. Y. (2021). Effects of Cordycepin in Cordyceps militaris during Its Infection to Silkworm Larvae. Microorganisms, 9(4), 681. https://doi.org/10.3390/microorganisms9040681







