Phage Therapy for Mycobacterium Abscessus and Strategies to Improve Outcomes
Abstract
1. Introduction
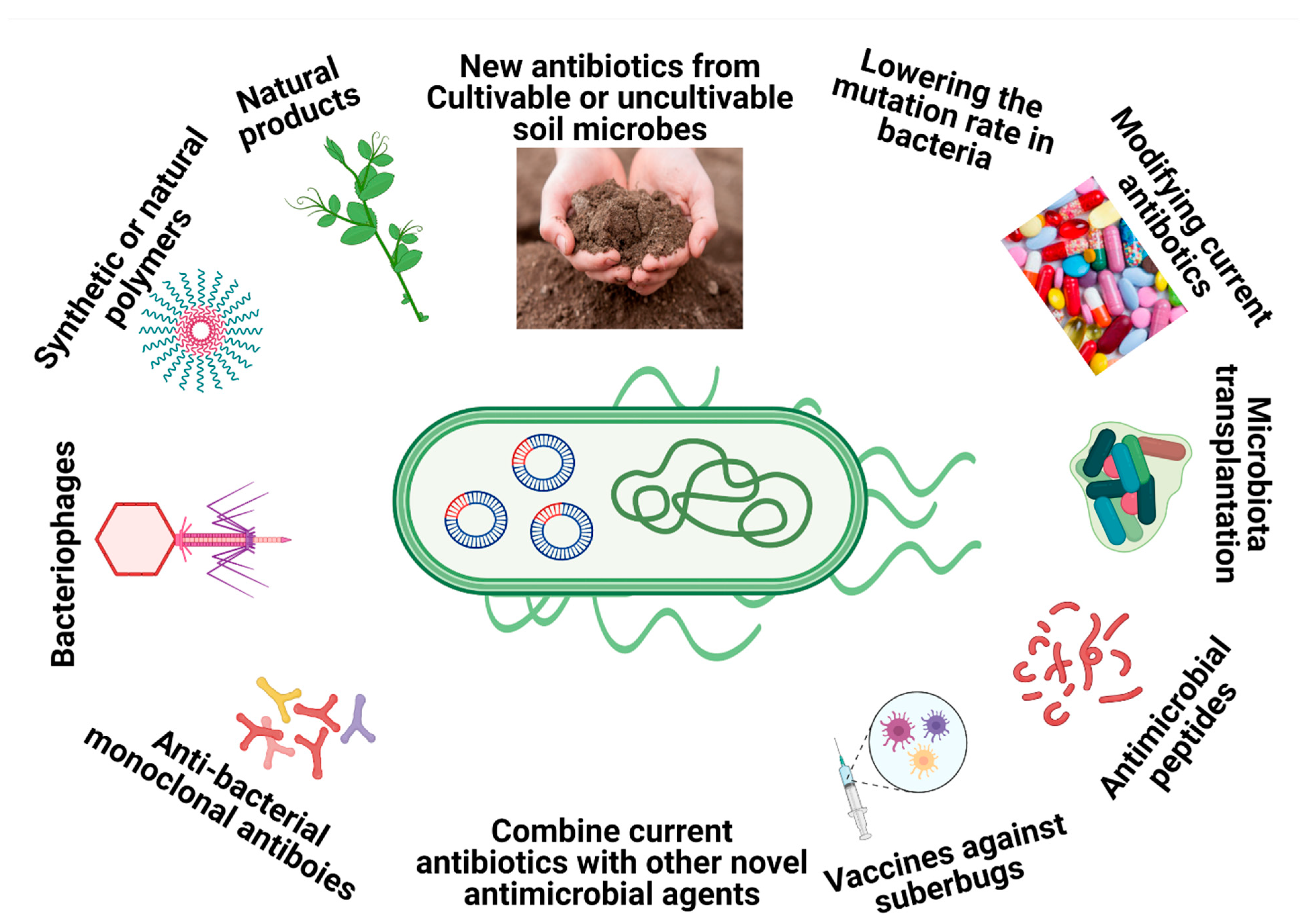
2. New Alternatives for Drug-Resistant M. abscessus
3. The History of Phage Therapy
4. Mycobacteriophages’ Biology and Classification
5. Phage Therapy against Mycobacterial Infections
Studies Related to M. tuberculosis, M. avium, and M. ulcerans
6. M. abscessus-Related Study: A Successful Clinical Model
7. Strategies to Improve Phage Therapy Outcome
7.1. Bacteriophage Cocktails
7.2. Phage Engineering
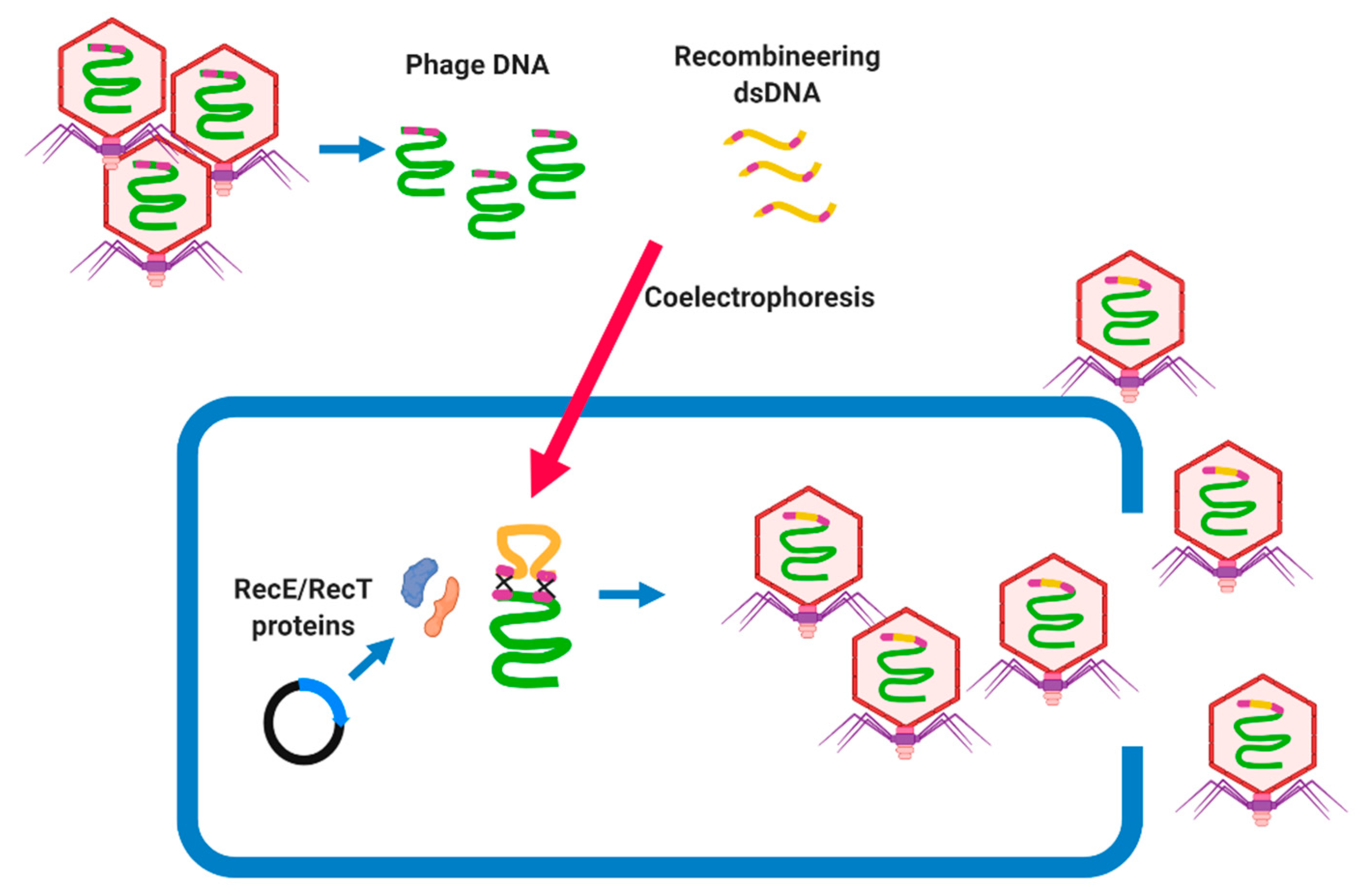
7.2.1. Bacteriophage Recombineering of Electroporated DNA (BRED)
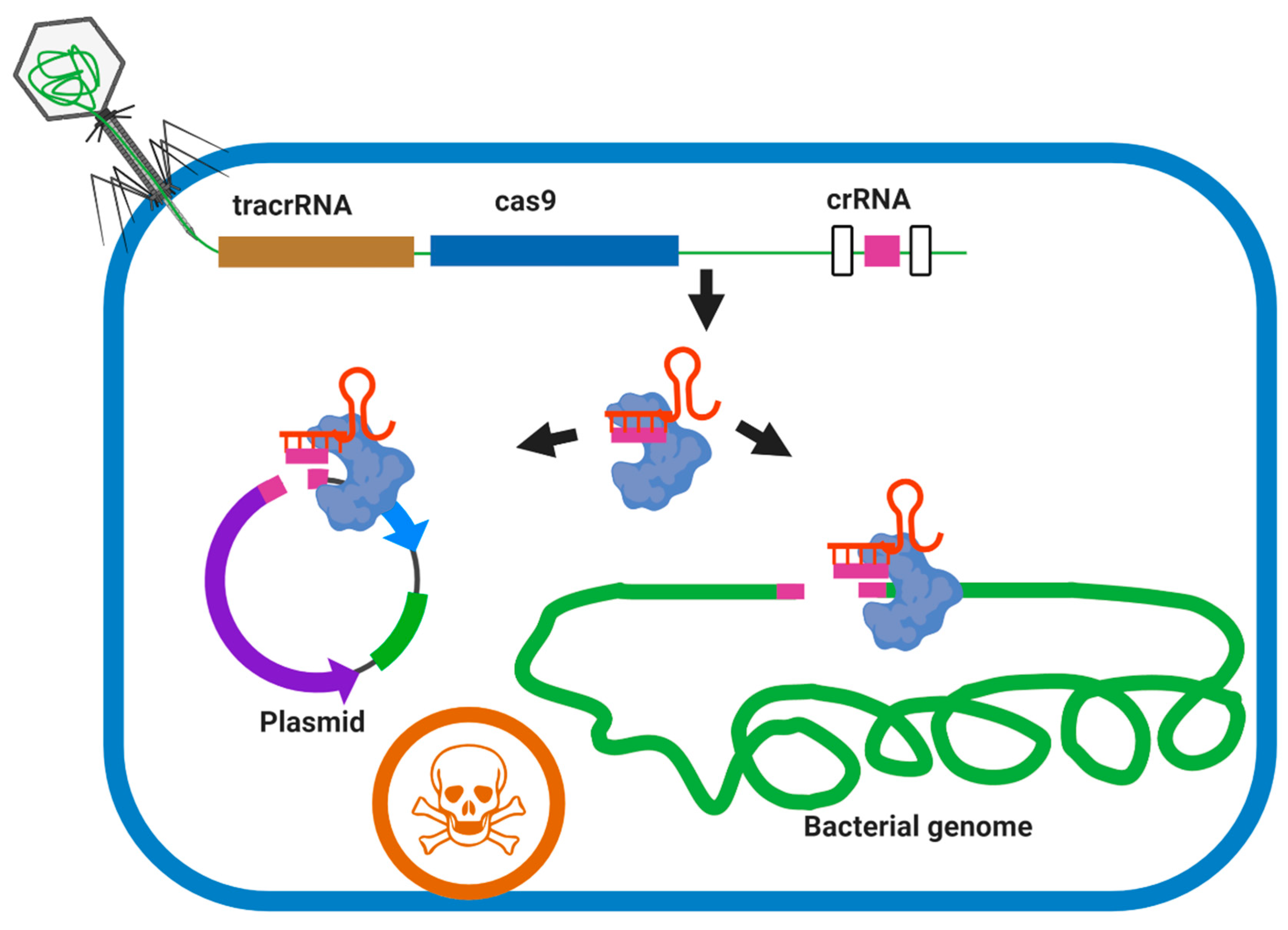
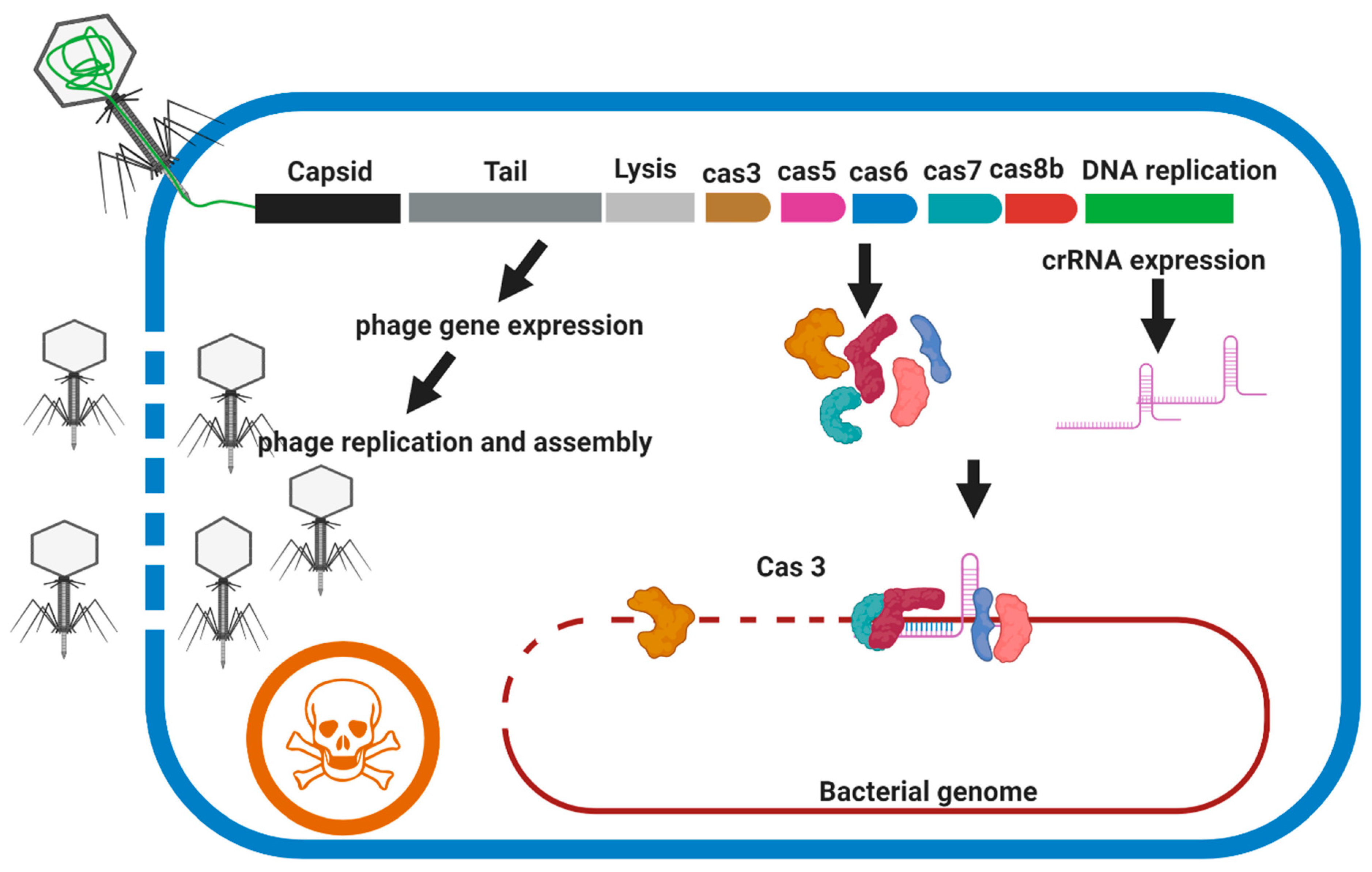
7.2.2. Phage Engineering Using the CRISPR-Cas System
CRISPR–Cas3 (Type I)
CRISPR–Cas9 (Type II)
CRISPR–Cas10 (Type III)
7.2.3. Rebooting Phages Using Assembled Phage Genomic DNA
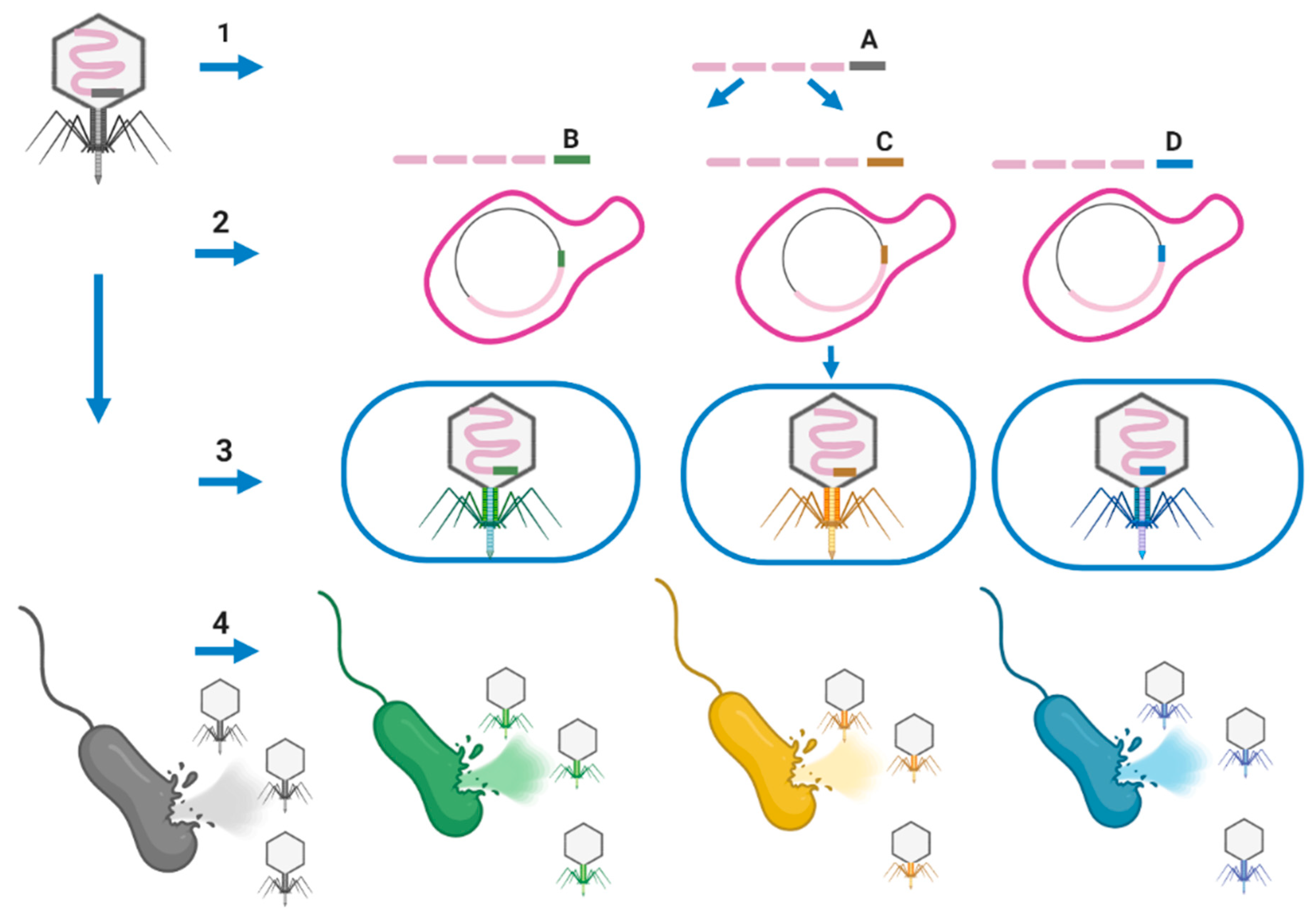
7.3. Arming Mycobacteriophages
8. Phage Resistant Mechanisms in Bacteria
9. Phage Mechanisms to Escape the Bacterial Antiphage System
10. Limitations and Challenges
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, M.-R.; Sheng, W.-H.; Hung, C.-C.; Yu, C.-J.; Lee, L.-N.; Hsueh, P.-R. Mycobacterium abscessus complex infections in humans. Emerg. Infect. Dis. 2015, 21, 1638–1646. [Google Scholar] [CrossRef]
- Tortoli, E.; Kohl, T.A.; Brown-Elliott, B.A.; Trovato, A.; Leão, S.C.; Garcia, M.J.; Vasireddy, S.; Turenne, C.-Y.; Griffith, D.-E.; Philley, J.V. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacteriumabscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4471–4479. [Google Scholar] [CrossRef]
- Bryant, J.M.; Grogono, D.M.; Rodriguez-Rincon, D.; Everall, I.; Brown, K.P.; Moreno, P.; Verma, D.; Hill, E.; Drijkoningen, J.; Gilligan, P. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 2016, 354, 751–757. [Google Scholar] [CrossRef]
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Pasipanodya, J.G.; Ogbonna, D.; Ferro, B.E.; Magombedze, G.; Srivastava, S.; Deshpande, D.; Gumbo, T. Systematic review and meta-analyses of the effect of chemotherapy on pulmonary Mycobacterium abscessus outcomes and disease recurrence. Antimicrob. Agents Chemother. 2017, 66, e01206-17. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.-J.; Jeon, K.; Lee, N.Y.; Kim, B.-J.; Kook, Y.-H.; Lee, S.-H.; Park, Y.K.; Kim, C.K.; Shin, S.J.; Huitt, G.A. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am. J. Respir. Crit. Care Med. 2011, 183, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, B.J.; Kook, Y.; Yun, Y.J.; Shin, J.H.; Kim, B.J.; Kook, Y.H. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol. Immunol. 2010, 54, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.D.; Herrmann, J.-L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 8, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Wee, W.Y.; Dutta, A.; Choo, S.W. Comparative genome analyses of mycobacteria give better insights into their evolution. PLoS ONE 2017, 12, e0172831. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J. Herbal Antibiotics: Moving back into the mainstream as an alternative for Superbugs. Cell. Mol. Biol. 2016, 62, 1–2. [Google Scholar] [PubMed]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Zeiser, E.T.; Becka, S.A.; Park, S.; Wilson, B.M.; Winkler, M.L.; D’Souza, R.; Singh, I.; Sutton, G.; Fouts, E.D.; et al. Ceftazidime-Avibactam in Combination with Fosfomycin: A novel therapeutic strategy against multidrug-resistant Pseudomonas aeruginosa. J. Infect. Dis. 2019, 220, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Domalaon, R.; Idowu, T.; Zhanel, G.G.; Schweizer, F. Antibiotic hybrids: The next generation of agents and adjuvants against gram-negative pathogens? Clin. Microbiol. Rev. 2018, 31, e00077-17. [Google Scholar] [CrossRef]
- Esmatabadi, M.J.D.; Bozorgmehr, A.; Hajjari, S.N.; Sombolestani, A.S.; Malekshahi, Z.V.; Sadeghizadeh, M. Review of new insights into antimicrobial agents. Cell. Mol. Biol. 2017, 63, 40. [Google Scholar] [CrossRef] [PubMed]
- DiGiandomenico, A.; Sellman, B.R. Antibacterial monoclonal antibodies: The next generation? Curr. Opin. Microbiol. 2015, 27, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Morisaki, J.H.; et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; De Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Colameco, S.; Elliot, M.A. Non-coding RNAs as antibiotic targets. Biochem. Pharmacol. 2017, 133, 29–42. [Google Scholar] [CrossRef]
- Ragheb, M.N.; Thomason, M.K.; Hsu, C.; Nugent, P.; Gage, J.; Samadpour, A.N.; Kariisa, A.; Merrikh, C.N.; Miller, S.I.; Sherman, D.R.; et al. Inhibiting the evolution of antibiotic resistance. Mol. Cell 2019, 73, 157–165.e5. [Google Scholar] [CrossRef] [PubMed]
- Ganewatta, M.S.; Rahman, A.; Tang, C. Emerging antimicrobial research against Superbugs: Perspectives from a Polymer Laboratory. J. South Carol. Acad. Sci. 2017, 15, 15. [Google Scholar]
- Rappuoli, R.; Bloom, D.E.; Black, S. Deploy vaccines to fight superbugs. Nat. Cell Biol. 2017, 552, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 2019, 53, 16–21. [Google Scholar] [CrossRef]
- Romero-Calle, D.; Guimarães Benevides, R.; Góes-Neto, A.; Billington, C. Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Comeau, A.M.; Hatfull, G.F.; Krisch, H.M.; Lindell, D.; Mann, N.H.; Prangishvili, D. Exploring the prokaryotic virosphere. Res. Microbiol. 2008, 159, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T. Engineered bacteriophages for treatment of a patient with a dis-seminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Lederberg, J. Smaller fleas. ad infinitum: Therapeutic bacteriophage redux. Proc. Natl. Acad. Sci. USA 1996, 93, 3167–3168. [Google Scholar] [CrossRef]
- Duckworth, D.H. Who discovered bacteriophage? Bacteriol. Rev. 1976, 40, 793. [Google Scholar] [CrossRef]
- D’Herelle, M. Sur un microbe invisible antagoniste des bacilles dysentériques. Acta Kravsi 1917, 165, 373–375. [Google Scholar]
- Myelnikov, D. An Alternative Cure: The Adoption and Survival of Bacteriophage Therapy in the USSR, 1922–1955. J. Hist. Med. Allied Sci. 2018, 73, 385–411. [Google Scholar] [CrossRef] [PubMed]
- Fruciano, D.E.; Bourne, S. Phage as an Antimicrobial Agent: D’herelle’s Heretical Theories and Their Role in the Decline of Phage Prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 19–26. [Google Scholar] [CrossRef]
- Marčuk, L.M.; Nikiforov, V.N.; Ščerbak, J.F.; Levitov, T.A.; Kotljarova, R.I.; Naumšina, M.S.; Davydov, S.U.; Monsur, K.A.; Rahman, M.A.; Latif, M.A.; et al. Clinical studies of the use of bacteriophage in the treatment of cholera. Bull. World Health Organ. 1971, 45, 77–83. [Google Scholar] [PubMed]
- Monsur, K.A.; Rahman, M.A.; Huq, F.; Islam, M.N.; Northrup, R.S.; Hirschhorn, N. Effect of massive doses of bacteriophage on excretion of vibrios, duration of diarrhoea and output of stools in acute cases of cholera. Bull. World Health. Organ. 1970, 42, 723–732. [Google Scholar] [PubMed]
- Tsulukidze, A. Experience of Use of Bacteriophages in the Conditions of War Traumatism; Gruzmedgiz: Tbilisi, Georgia, 1941. [Google Scholar]
- Summers, W.C. Bacteriophage Therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef]
- Luria, S.E.; Delbrück, M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 1943, 28, 491–511. [Google Scholar] [PubMed]
- D’Herelle, F. Bacteriophage as a Treatment in Acute Medical and Surgical Infections*. Bull. NY Acad. Med. 1931, 7, 329–348. [Google Scholar]
- Hatfull, G.F. Mycobacteriophages. Microbiol. Spectr. 2018, 6, 1029–1055. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F. Actinobacteriophages: Genomics, Dynamics, and Applications. Annu. Rev. Virol. 2020, 7, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Jacobs-Sera, D.; Marinelli, L.J.; Bowman, C.; Broussard, G.W.; Bustamante, C.G.; Boyle, M.M.; Petrova, Z.O.; Dedrick, R.M.; Pope, W.H.; Advancing, S.E.A.P.H. On the nature of mycobacteriophage diversity and host preference. Virology 2012, 434, 187–201. [Google Scholar] [CrossRef]
- Hatfull, G.F. Mycobacteriophages: Genes and Genomes. Annu. Rev. Microbiol. 2010, 64, 331–356. [Google Scholar] [CrossRef]
- Hatfull, G.F. Complete genome sequences of 138 mycobacteriophages. Am. Soc. Microbiol. 2012, 86, 2382–3284. [Google Scholar] [CrossRef]
- Rybniker, J.; Kramme, S.; Small, P.L. Host range of 14 mycobacteriophages in Mycobacterium ulcerans and seven other mycobacteria including Mycobacterium tuberculosis–application for identification and susceptibility testing. J. Med. Microbiol. 2006, 55, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, F.; Galí, N.; Domínguez, J.; Berlanga, P.; Blanco, S.; Orús, P.; Martín, R. Usefulness of a new mycobac-teriophage-based technique for rapid diagnosis of pulmonary tuberculosis. J. Clin. Microbiol. 2003, 41, 2867–2871. [Google Scholar] [CrossRef][Green Version]
- Albert, H.; Heydenrych, A.; Brookes, R.; Mole, R.J.; Harley, B.; Subotsky, E.; Henry, R.; Azevedo, V. Performance of a rapid phage-based test, FASTPlaqueTB™, to diagnose pulmonary tuberculosis from sputum specimens in South Africa. Int. J. Tuberc. Lung Dis. 2002, 6, 529–537. [Google Scholar] [CrossRef]
- Zhu, C.; Cui, Z.; Zheng, R.; Yang, H.; Jin, R.; Qin, L.; Liu, Z.; Wang, J.; Hu, Z. A Multi-Center Study to Evaluate the Performance of Phage Amplified Biologically Assay for Detecting TB in Sputum in the Pulmonary TB Patients. PLoS ONE 2011, 6, e24435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galí, N.; Domínguez, J.; Blanco, S.; Prat, C.; Alcaide, F.; Coll, P.; Ausina, V. The Mycobacteria Research Group of Barcelona Use of a Mycobacteriophage-Based Assay for Rapid Assessment of Susceptibilities of Mycobacterium tuberculosis Isolates to Isoniazid and Influence of Resistance Level on Assay Performance. J. Clin. Microbiol. 2006, 44, 201–205. [Google Scholar] [CrossRef]
- Hemvani, N.; Patidar, V.; Chitnis, D. A simple and economical in-house phage technique for the rapid detection of rifampin, isoniazid, ethambutol, streptomycin, and ciprofloxacin drug resistance in Mycobacterium tuberculosis, directly on decontaminated sputum samples. Int. J. Infect. Dis. 2012, 16, e332–e336. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.E.; Jurgensen, S.; Sarkis, G.J.; Hatfull, G.F.; Jacobs, W.R., Jr. Construction of D29 shuttle phasmids and luciferase reporter phages for detection of mycobacteria. Gene 1996, 183, 129–136. [Google Scholar] [CrossRef]
- Van Kessel, J.C.; Hatfull, G.F. Recombineering in Mycobacterium tuberculosis. Nat. Methods 2006, 4, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Schürch, A.C.; van Soolingen, D. DNA fingerprinting of Mycobacterium tuberculosis: From phage typing to whole-genome sequencing. Infect. Genet. Evol. 2012, 12, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.D., Jr.; Woodley, C.L. Phage-type patterns of Mycobacterium tuberculosis from Southeast Asian immigrants. Am. Rev. Respir. Dis. 1983, 127, 348–349. [Google Scholar]
- Bates, J.H.; Fitzhugh, J.K. Subdivision of the species Mycobacterium tuberculosis by mycobacteriophage typing. Am. Rev. Respir. Dis. 1967, 96, 7–10. [Google Scholar]
- Engel, H.; Berwald, L.; Grange, J.; Kubin, M. Phage typing of Mycobacterium kansasii. Tubercle 1980, 61, 11–19. [Google Scholar] [CrossRef]
- Crawford, J.T.; Fitzhugh, J.K.; Bates, J.H. Phage typing of the Mycobacterium avium-intracellulare-scrofulaceum complex. Am. Rev. Respir. Dis. 1981, 124, 559–562. [Google Scholar] [PubMed]
- Sula, L.; Sulová, J.; Stolcpartová, M. Therapy of experimental tuberculosis in guinea pigs with mycobacterial phages DS-6A, GR-21 T, My-327. Czechoslov. Med. 1981, 4, 209–214. [Google Scholar]
- Broxmeyer, L.; Sosnowska, D.; Miltner, E.; Chacón, O.; Wagner, D.; McGarvey, J.; Barletta, R.G.; Bermudez, L.E. Killing of Mycobacterium avium and Mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent mycobacterium: A model for phage therapy of intracellular bacterial pathogens. J. Infect. Dis. 2002, 186, 1155–1160. [Google Scholar] [CrossRef]
- Danelishvili, L.; Young, L.S.; Bermudez, L.E. In vivo efficacy of phage therapy for Mycobacterium avium infec-tion as delivered by a nonvirulent mycobacterium. Microb. Drug Resist. 2006, 12, 1–6. [Google Scholar] [CrossRef]
- Walsh, D.S.; Portaels, F.; Meyers, W.M. Buruli ulcer: Advances in understanding Mycobacterium ulcerans infection. Dermatol Clin. 2011, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Trigo, G.; Martins, T.G.; Fraga, A.G.; Longatto-Filho, A.; Castro, A.G.; Azeredo, J.; Pedrosa, J. Phage Therapy Is Effective against Infection by Mycobacterium ulcerans in a Murine Footpad Model. PLOS Neglected Trop. Dis. 2013, 7, e2183. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F. Mycobacteriophages: Windows into Tuberculosis. PLOS Pathog. 2014, 10, e1003953. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Bustamante, C.A.G.; Garlena, R.A.; Pinches, R.S.; Cornely, K.; Hatfull, G.F. Mycobacteriophage ZoeJ: A broad host-range close relative of mycobacteriophage TM4. Tuberculosis 2019, 115, 14–23. [Google Scholar] [CrossRef]
- Pope, W.H.; Bowman, C.A.; Russell, D.A.; Jacobs-Sera, D.; Asai, D.J.; Cresawn, S.G.; Jacobs, W.R., Jr.; Hendrix, R.W.; Lawrence, J.G.; Hatfull, G.F. Whole genome comparison of a large collection of mycobacteriophages re-veals a continuum of phage genetic diversity. eLife 2015, 4, e06416. [Google Scholar] [CrossRef] [PubMed]
- Jancevski, A.; Dubois, H.; Scola, S.; Lebel, E.; Pinches, S.; Brown, M.; Cavedon, W.; Fernando, M.; Perri, C.; Rogers, S. Isolation and Characterization of Mycobacteriophage ZoeJ, a K2 Cluster Phage. FASEB J. 2015, 29, 575-14. [Google Scholar]
- Sampson, T.; Broussard, G.W.; Marinelli, L.J.; Jacobs-Sera, D.; Ray, M.; Ko, C.-C.; Russell, D.; Hendrix, R.W.; Hatfull, G.F. Mycobacteriophages BPs, Angel and Halo: Comparative genomics reveals a novel class of ultra-small mobile genetic elements. Microbiology 2009, 155, 2962–2977. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Futur. Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Abedon, S.T. Phage therapy pharmacology: Phage cocktails. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 78, pp. 1–23. [Google Scholar]
- Hall, A.R.; De Vos, D.; Friman, V.-P.; Pirnay, J.-P.; Buckling, A. Effects of sequential and simultaneous applications of bacteriophages on populations of Pseudomonas aeruginosa in vitro and in wax moth larvae. Appl. Environ. Microbiol. 2012, 78, 5646–5652. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, D.E., Jr.; Singh, P.; Strable, E.; Brown, S.; Manchester, M.; Finn, M. Plasma clearance of bacteriophage Qβ particles as a function of surface charge. J. Am. Chem. Soc. 2008, 130, 1328–1334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown-Jaque, M.; Calero-Cáceres, W.; Espinal, P.; Rodríguez-Navarro, J.; Miró, E.; González-López, J.J.; Corne-jo, T.; Hurtado, J.C.; Navarro, F.; Muniesa, M. Antibiotic resistance genes in phage particles isolated from human faeces and induced from clinical bacterial isolates. Int. J. Antimicrob. Agents 2018, 51, 434–442. [Google Scholar] [CrossRef]
- Schroven, K.; Aertsen, A.; Lavigne, R. Bacteriophages as drivers of bacterial virulence and their potential for biotechnological exploitation. FEMS Microbiol. Rev. 2021, 45, 041. [Google Scholar] [CrossRef] [PubMed]
- Holmfeldt, K.; Middelboe, M.; Nybroe, O.; Riemann, L. Large variabilities in host strain susceptibility and phage host range govern interactions between lytic marine phages and their Flavobacterium hosts. Appl. Environ. Microbiol. 2007, 73, 6730–6739. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Cleto, S.; Sillankorva, S.; Azeredo, J.; Lu, T.K. Genetically engineered phages: A review of advances over the last decade. Microbiol. Mol. Biol. Rev. 2016, 80, 523–543. [Google Scholar] [CrossRef]
- Marinelli, L.J.; Piuri, M.; Swigoňová, Z.; Balachandran, A.; Oldfield, L.M.; Van Kessel, J.C.; Hatfull, G.F. BRED: A simple and powerful tool for constructing mutant and recombinant bacteriophage genomes. PLoS ONE 2008, 3, e3957. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Marinelli, L.J.; Newton, G.L.; Pogliano, K.; Pogliano, J.; Hatfull, G.F. Functional requirements for bacteriophage growth: Gene essentiality and expression in mycobacteriophage Giles. Mol. Microbiol. 2013, 88, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Lee, J.-H.; Yoon, H.; Kang, D.-H.; Ryu, S. Genomic investigation of lysogen formation and host lysis systems of the Salmonella temperate bacteriophage SPN9CC. Appl. Environ. Microbiol. 2014, 80, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Deveau, H.; Garneau, J.E.; Moineau, S. CRISPR/Cas System and Its Role in Phage-Bacteria Interactions. Annu. Rev. Microbiol. 2010, 64, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Fan, X.; Xie, J. Comparative genomic structures of Mycobacterium CRISPR-Cas. J. Cell. Biochem. 2012, 113, 2464–2473. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.-Y.; Yan, H.-Q.; Ren, G.-X.; Zhao, J.-P.; Guo, X.-P.; Sun, Y.-C. CRISPR-Cas12a-Assisted recombineering in bacteria. Appl. Environ. Microbiol. 2017, 83, 83. [Google Scholar] [CrossRef]
- Choudhary, E.; Thakur, P.; Pareek, M.; Agarwal, N. Gene silencing by CRISPR interference in mycobacteria. Nat. Commun. 2015, 6, 6267. [Google Scholar] [CrossRef]
- Pope, W.H.; Jacobs-Sera, D.; Best, A.A.; Broussard, G.W.; Connerly, P.L.; Dedrick, R.M.; Kremer, T.A.; Offner, S.; Ogiefo, A.H.; Pizzorno, M.C.; et al. Cluster J mycobacteriophages: Intron splicing in capsid and tail genes. PLoS ONE 2013, 8, e69273. [Google Scholar] [CrossRef][Green Version]
- Kiro, R.; Shitrit, D.; Qimron, U. Efficient engineering of a bacteriophage genome using the type I-E CRISPR-Cas system. RNA Biol. 2014, 11, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Box, A.M.; McGuffie, M.J.; O’Hara, B.J.; Seed, K.D. Functional analysis of bacteriophage immunity through a type I-E CRISPR-Cas system in Vibrio cholerae and its application in bacteriophage genome engineering. J. Bacteriol. 2015, 198, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Martel, B.; Moineau, S. CRISPR-Cas: An efficient tool for genome engineering of virulent bacteriophages. Nucleic Acids Res. 2014, 42, 9504–9513. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Batra, H.; Dong, J.; Chen, C.; Rao, V.B.; Tao, P. Genetic engineering of bacteriophages against infectious diseases. Front. Microbiol. 2019, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Dietrich, S.; Hoppert, M.; Hertel, R. A CRISPR-Cas9-based toolkit for fast and precise in vivo genetic engineering of Bacillus subtilis phages. Viruses 2018, 10, 241. [Google Scholar] [CrossRef]
- Bari, S.M.N.; Walker, F.C.; Cater, K.; Aslan, B.; Hatoum-Aslan, A. Strategies for editing virulent Staphylococcal phages using CRISPR-Cas10. ACS Synth. Biol. 2017, 6, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Jamal, M.; Chen, X.; Zhou, W.; Yang, B.; Zou, Y.; Xu, W.; Lei, Y.; Wu, C.; Cao, X. Reprogramming the endogenous type III-A CRISPR-Cas system for genome editing, RNA interference and CRISPRi screening in Mycobacterium tuberculosis. BioRxiv 2020. [Google Scholar] [CrossRef]
- Ando, H.; Lemire, S.; Pires, D.P.; Lu, T.K. Engineering Modular Viral Scaffolds for Targeted Bacterial Population Editing. Cell Syst. 2015, 1, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Kilcher, S.; Studer, P.; Muessner, C.; Klumpp, J.; Loessner, M.J. Cross-genus rebooting of custom-made, syn-thetic bacteriophage genomes in L-form bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.; Rupf, B.; Tala, M.; Qabrati, X.; Ernst, P.; Shen, Y.; Sumrall, E.; Heeb, L.; Plückthun, A.; Loessner, M.J. Reprogramming bacteriophage host range through structure-guided design of chimeric receptor binding proteins. Cell Reports 2019, 29, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Naser, S.A.; McCarthy, C.M.; Smith, G.B.; Tupponce, A.K. Low temperature protocol for efficient transformation of Mycobacterium smegmatis spheroplasts. Curr. Microbiol. 1993, 27, 153–156. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Klumpe, H.E.; Luo, M.L.; Selle, K.; Barrangou, R.; Beisel, C.L. Programmable removal of bacterial strains by use of genome-targeting CRISPR-Cas systems. mBio 2014, 5, e00928-13. [Google Scholar] [CrossRef]
- Bikard, D.; Euler, C.W.; Jiang, W.; Nussenzweig, P.M.; Goldberg, G.W.; Duportet, X.; Fischetti, V.A.; Marraffini, L.A. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 2014, 32, 1146–1150. [Google Scholar] [CrossRef]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Selle, K.; Fletcher, J.R.; Tuson, H.; Schmitt, D.S.; McMillan, L.; Vridhambal, G.S.; Rivera, A.J.; Montgomery, S.A.; Fortier, L.-C.; Barrangou, R. In vivo targeting of Clostridioides difficile using phage-delivered CRISPR-Cas3 antimicrobials. mBio 2020, 11, e00019-20. [Google Scholar] [CrossRef] [PubMed]
- Hampton, H.G.; Watson, B.N.J.; Fineran, P.C. The arms race between bacteria and their phage foes. Nat. Cell Biol. 2020, 577, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Genet. 2010, 8, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Gentile, G.M.; Wetzel, K.S.; Dedrick, R.M.; Montgomery, M.T.; Garlena, R.A.; Jacobs-Sera, D.; Hatfull, G.F. More Evidence of Collusion: A new prophage-mediated viral defense system encoded by mycobacteriophage Sbash. mBio 2019, 10, e00196-19. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.; Gouret, P.; Chabrol, O.; Pontarotti, P.; Drancourt, M. Mycobacteriophage-drived diversification of Mycobacterium abscessus. Biol. Direct 2014, 9, 19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ram, G.; Chen, J.; Ross, H.F.; Novick, R.P. Precisely modulated pathogenicity island interference with late phage gene transcription. Proc. Natl. Acad. Sci. USA 2014, 7, 14536–14541. [Google Scholar] [CrossRef] [PubMed]
- Barsom, E.K.; Hatfull, G.F. Characterization of a Mycobacterium smegmatis gene that confers resistance to phages L5 and D29 when overexpressed. Mol. Microbiol. 1996, 21, 159–170. [Google Scholar] [CrossRef]
- Meyer, J.R.; Dobias, D.T.; Medina, S.J.; Servilio, L.; Gupta, A.; Lenski, R.E. Ecological speciation of bacterio-phage lambda in allopatry and sympatry. Science 2016, 354, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B.J.C.M.C. Bacteriophages and phage-derived proteins–application approaches. Curr. Med. Chem. 2015, 22, 1757–1773. [Google Scholar] [CrossRef] [PubMed]
- Pleška, M.; Guet, C.C. Effects of mutations in phage restriction sites during escape from restriction–modification. Biol. Lett. 2017, 13, 20170646. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Mahony, J.; Ainsworth, S.; Nauta, A.; van Sinderen, D. Bacteriophage orphan DNA methyltransferases: Insights from their bacterial origin, function, and occurrence. Appl. Environ. Microbiol. 2013, 79, 7547–7555. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Huang, X. Anti-CRISPR proteins targeting the CRISPR-Cas system enrich the toolkit for genetic engineering. FEBS J. 2020, 287, 626–644. [Google Scholar] [CrossRef]
- Rauch, B.J.; Silvis, M.R.; Hultquist, J.F.; Waters, C.S.; McGregor, M.J.; Krogan, N.J.; Bondy-Denomy, J. Inhibition of CRISPR-Cas9 with Bacteriophage Proteins. Cell 2017, 168, 150–158.e10. [Google Scholar] [CrossRef]
- Seed, K.D.; Lazinski, D.W.; Calderwood, S.B.; Camilli, A. A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nat. Cell Biol. 2013, 494, 489–491. [Google Scholar] [CrossRef]
- Tao, P.; Wu, X.; Rao, V. Unexpected evolutionary benefit to phages imparted by bacterial CRISPR-Cas9. Sci. Adv. 2018, 4, eaar4134. [Google Scholar] [CrossRef]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef]
- Hagens, S.; Habel, A.; Von Ahsen, U.; Von Gabain, A.; Bläsi, U. Therapy of experimental Pseudomonas infections with a nonreplicating genetically modified phage. Antimicrob. Agents Chemother. 2004, 48, 3817–3822. [Google Scholar] [CrossRef] [PubMed]


| Application | Purpose/Reported Phages |
|---|---|
| Diagnostic markers | Diagnosis of pulmonary tuberculosis (PhageTek MB kit) [43] |
| Diagnosis of pulmonary tuberculosis (FASTPlaqueTB™) [44] | |
| Diagnosis of pulmonary tuberculosis (phage amplified assay: PhaB) [45] | |
| Drug-resistant | Detection of isoniazid resistance (D29) [46] and Rifampin, isoniazid, ethambutol, streptomycin, and ciprofloxacin (D29) [47] |
| Genetic manipulation | Shuttle plasmids (L5, D29) [48], luciferase reporter phages (D29) [48], Recombineering (Che9c) [49] |
| Molecular typing | M. tuberculosis complex (GS4E) [50,51,52], M. kansasii (AX1, C3, KA3,6 and 8, D34A, D303-304, D345C) [53], M. avium (JF1-4, D302, and AN1-9) [54] |
| Therapeutic application | M. tuberculosis [55,56,57], M. avium [56,57], M. ulcerans [58], M. abscessus [25] |
| Host | Phage Cluster | Subcluster | Phage Name |
|---|---|---|---|
| M. tuberculosis | A | A1 | Bxb1 and U2 |
| A2 | L5 a, D29 a, Turbido | ||
| A3 | Bxz2 a, Microwolf, Rockstar, Vix | ||
| B b | B1 | Scoot17c | |
| B2 | Qyrzyla | ||
| G | - | Angel, Avrafan, BPs, Halo, Liefie, Bo4 | |
| K | K1 | Adephagia, CrimD, Jaws | |
| K2 | TM4 c | ||
| K3 | Pixie | ||
| K5 | Fionnbharth | ||
| Singleton | - | Dori | |
| F | F1 | Ms6 | |
| - | - | DS-6A, GR-21/T, My-327, BTCU-1, SWU1 d | |
| M. scrofulaceum | D | D1 | PBI1 |
| B | B1 | PG2 e | |
| V | - | Wildcat e | |
| M. fortuitum, M. chelonae | B | B4 | Cooper |
| M. avium | K | K2 | ZoeJ |
| M. abscessus subsp. massiliense | Singleton | - | Muddy (strain GD01) |
| Phages | Source | Cluster/Subcluster | Length (kb)/Number of Coding Genes | Used in PT | Effectiveness Against GD01 Strain (PFU) a | GD01 Survivors | Effectiveness on Other Strains b |
|---|---|---|---|---|---|---|---|
| Muddy | Decomposed aubergine [62] | Singleton | 48/72 | Wild | Effective (<101–1010) | No | Ineffective |
| ZoeJ | Soil [63] | K/K2 | 57/92 | Engineered (ZoeJΔ45) | Ineffective at low concentration (102–1010) | Yes | Ineffective |
| PBs | Soil [64] | G/G1 | 42/63 | Engineered, mutant (BPsΔ33HTH-HRM10) | Ineffective at low concentration (108–1010) | Yes | Ineffective |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemi Shahraki, A.; Mirsaeidi, M. Phage Therapy for Mycobacterium Abscessus and Strategies to Improve Outcomes. Microorganisms 2021, 9, 596. https://doi.org/10.3390/microorganisms9030596
Hashemi Shahraki A, Mirsaeidi M. Phage Therapy for Mycobacterium Abscessus and Strategies to Improve Outcomes. Microorganisms. 2021; 9(3):596. https://doi.org/10.3390/microorganisms9030596
Chicago/Turabian StyleHashemi Shahraki, Abdolrazagh, and Mehdi Mirsaeidi. 2021. "Phage Therapy for Mycobacterium Abscessus and Strategies to Improve Outcomes" Microorganisms 9, no. 3: 596. https://doi.org/10.3390/microorganisms9030596
APA StyleHashemi Shahraki, A., & Mirsaeidi, M. (2021). Phage Therapy for Mycobacterium Abscessus and Strategies to Improve Outcomes. Microorganisms, 9(3), 596. https://doi.org/10.3390/microorganisms9030596







