T-Cell Exhaustion in Mycobacterium tuberculosis and Nontuberculous Mycobacteria Infection: Pathophysiology and Therapeutic Perspectives
Abstract
1. Introduction
2. The Immunologic Background
3. Immune Exhaustion in MTB Infection
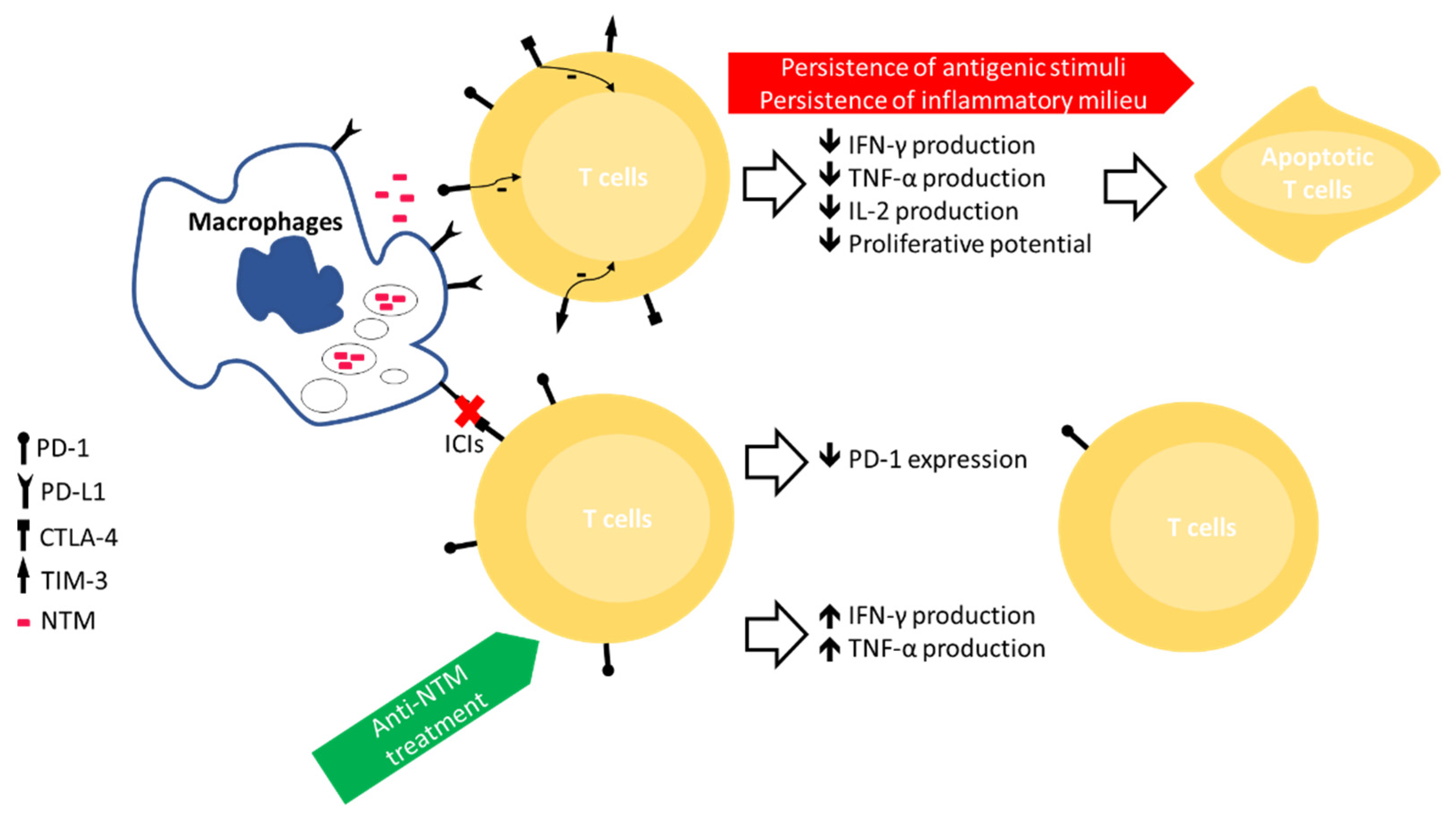
4. Immune Exhaustion in NTM Infection
5. ICIs as Adjuvant in the Treatment of MTB/NTM Infection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef]
- Namkoong, H.; Kurashima, A.; Morimoto, K.; Hoshino, Y.; Hasegawa, N.; Ato, M.; Mitarai, S. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan. Emerg. Infect. Dis. 2016, 22, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; McNelley, E.; Kendall, B.; Marshall-Olson, A.; Morris, C.; Cassidy, M.; Saulson, A.; Hedberg, K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: An emerging public health disease. Am. J. Respir. Crit. Care Med. 2010, 182, 977–982. [Google Scholar] [CrossRef]
- Sexton, P.; Harrison, A.C. Susceptibility to nontuberculous mycobacterial lung disease. Eur. Respir. J. 2008, 31, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Gallimore, A.; Glithero, A.; Godkin, A.; Tissot, A.C.; Plückthun, A.; Elliott, T.; Hengartner, H.; Zinkernagel, R. Induction and Exhaustion of Lymphocytic Choriomeningitis Virus–specific Cytotoxic T Lymphocytes Visualized Using Soluble Tetrameric Major Histocompatibility Complex Class I–Peptide Complexes. J. Exp. Med. 1998, 187, 1383–1393. [Google Scholar] [CrossRef]
- Zajac, A.J.; Blattman, J.N.; Murali-Krishna, K.; Sourdive, D.J.D.; Suresh, M.; Altman, J.D.; Ahmed, R. Viral Immune Evasion Due to Persistence of Activated T Cells Without Effector Function. J. Exp. Med. 1998, 188, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.J.; Zajac, A.J. Ablation of CD8 and CD4 T Cell Responses by High Viral Loads. J. Immunol. 2003, 170, 477–486. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Odorizzi, P.M.; Wherry, E.J. Inhibitory Receptors on Lymphocytes: Insights from Infections. J. Immunol. 2012, 188, 2957–2965. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Boni, C.; Fisicaro, P.; Valdatta, C.; Amadei, B.; Di Vincenzo, P.; Giuberti, T.; Laccabue, D.; Zerbini, A.; Cavalli, A.; Missale, G.; et al. Characterization of Hepatitis B Virus (HBV)-Specific T-Cell Dysfunction in Chronic HBV Infection. J. Virol. 2007, 81, 4215–4225. [Google Scholar] [CrossRef]
- Rehermann, B. Science in medicine Hepatitis C virus versus innate and adaptive immune responses: A tale of coevolution and coexistence. Sci. Med. 2009, 119, 1745–1754. [Google Scholar] [CrossRef]
- Castelli, V.; Lombardi, A.; Palomba, E.; Bozzi, G.; Ungaro, R.; Alagna, L.; Mangioni, D.; Muscatello, A.; Bandera, A.; Gori, A. Immune Checkpoint Inhibitors in People Living with HIV/AIDS: Facts and Controversies. Cells 2021, 10, 2227. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Virgin, H.W.; Wherry, E.J.; Ahmed, R. Redefining Chronic Viral Infection. Cell 2009, 138, 30–50. [Google Scholar] [CrossRef]
- Urdahl, K.B.; Shafiani, S.; Ernst, J.D. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011, 4, 288–293. [Google Scholar] [CrossRef]
- Barry, C.E.; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.A.; Schnappinger, D.; Wilkinson, R.J.; Young, D. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 2009, 7, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Abrahams, D.A.; Lerumo, L.; Janse van Rensburg, E.; Stone, L.; O’rie, T.; Pienaar, B.; de Kock, M.; Kaplan, G.; Mahomed, H.; et al. Functional Capacity of Mycobacterium tuberculosis -Specific T Cell Responses in Humans Is Associated with Mycobacterial Load. J. Immunol. 2011, 187, 2222–2232. [Google Scholar] [CrossRef]
- Shen, L.; Gao, Y.; Liu, Y.; Zhang, B.; Liu, Q.; Wu, J.; Fan, L.; Ou, Q.; Zhang, W.; Shao, L. PD-1/PD-L pathway inhibits M.tb-specific CD4+ T-cell functions and phagocytosis of macrophages in active tuberculosis. Sci. Rep. 2016, 6, 38362. [Google Scholar] [CrossRef]
- Mcnab, F.W.; Berry, M.P.R.; Graham, C.M.; Bloch, S.A.A.; Oni, T.; Wilkinson, K.A.; Wilkinson, R.J.; Kon, O.M.; Banchereau, J.; Chaussabel, D.; et al. Programmed death ligand 1 is over-expressed by neutrophils in the blood of patients with active tuberculosis. Eur. J. Immunol. 2011, 41, 1941–1947. [Google Scholar] [CrossRef]
- Singh, A.; Mohan, A.; Dey, A.B.; Mitra, D.K. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon γ-producing T cells from apoptosis in patients with pulmonary tuberculosis. J. Infect. Dis. 2013, 208, 603–615. [Google Scholar] [CrossRef]
- Jurado, J.O.; Alvarez, I.B.; Pasquinelli, V.; Martínez, G.J.; Quiroga, M.F.; Abbate, E.; Musella, R.M.; Chuluyan, H.E.; García, V.E. Programmed Death (PD)-1:PD-Ligand 1/PD-Ligand 2 Pathway Inhibits T Cell Effector Functions during Human Tuberculosis. J. Immunol. 2008, 181, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.L.; Mayer-Barber, K.D.; Feng, C.G.; Sharpe, A.H.; Sher, A. CD4 T Cells Promote Rather than Control Tuberculosis in the Absence of PD-1–Mediated Inhibition. J. Immunol. 2011, 186, 1598–1607. [Google Scholar] [CrossRef]
- Tousif, S.; Singh, Y.; Prasad, D.V.R.; Sharma, P.; van Kaer, L.; Das, G. T cells from programmed death-1 deficient mice respond poorly to mycobacterium tuberculosis infection. PLoS ONE 2011, 6, e19864. [Google Scholar] [CrossRef]
- Walzl, G.; Ronacher, K.; Hanekom, W.; Scriba, T.J.; Zumla, A. Immunological biomarkers of tuberculosis. Nat. Rev. Immunol. 2011, 11, 343–354. [Google Scholar] [CrossRef]
- Sia, J.K.; Bizzell, E.; Madan-Lala, R.; Rengarajan, J. Engaging the CD40-CD40L pathway augments T-helper cell responses and improves control of Mycobacterium tuberculosis infection. PLoS Pathog. 2017, 13, e1006530. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Valentini, D.; Dodoo, E.; Zumla, A.; Maeurer, M. Anti-PD-1/PD-L1 therapy for infectious diseases: Learning from the cancer paradigm. Int. J. Infect. Dis. 2017, 56, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, P.; Jacques, M.K.; Zhu, C.; Steblenko, K.M.; Stowell, B.L.; Madi, A.; Anderson, A.C.; Kuchroo, V.K.; Behar, S.M. TIM3 Mediates T Cell Exhaustion during Mycobacterium tuberculosis Infection. PLoS Pathog. 2016, 12, e1005490. [Google Scholar] [CrossRef]
- Uplekar, M.; Weil, D.; Lonnroth, K.; Jaramillo, E.; Lienhardt, C.; Dias, H.M.; Falzon, D.; Floyd, K.; Gargioni, G.; Getahun, H.; et al. WHO’s new End TB Strategy. Lancet 2015, 385, 1799–1801. [Google Scholar] [CrossRef]
- Available online: http://www.bacterio.net/mycobacterium.html (accessed on 25 November 2021).
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, e1–e36. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am. J. Respir. Crit. Care Med. 2012, 185, 881–886. [Google Scholar] [CrossRef]
- Adjemian, J.; Frankland, T.B.; Daida, Y.G.; Honda, J.R.; Olivier, K.N.; Zelazny, A.; Honda, S.; Prevots, D.R. Epidemiology of nontuberculous mycobacterial lung disease and Tuberculosis, Hawaii, USA. Emerg. Infect. Dis. 2017, 23, 439–447. [Google Scholar] [CrossRef]
- Kartalija, M.; Ovrutsky, A.R.; Bryan, C.L.; Pott, G.B.; Fantuzzi, G.; Thomas, J.; Strand, M.J.; Bai, X.; Ramamoorthy, P.; Rothman, M.S.; et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am. J. Respir. Crit. Care Med. 2013, 187, 197–205. [Google Scholar] [CrossRef]
- Vankayalapati, R.; Wizel, B.; Samten, B.; Griffith, D.E.; Shams, H.; Galland, M.R.; von Reyn, C.F.; Girard, W.M.; Wallace, R.J.; Barnes, P.F. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J. Infect. Dis. 2001, 183, 478–484. [Google Scholar] [CrossRef]
- Shu, C.-C.; Wang, J.-Y.; Wu, M.-F.; Wu, C.-T.; Lai, H.-C.; Lee, L.-N.; Chiang, B.-L.; Yu, C.-J. Attenuation of lymphocyte immune responses during Mycobacterium avium complex-induced lung disease due to increasing expression of programmed death-1 on lymphocytes. Sci. Rep. 2017, 7, 42004. [Google Scholar] [CrossRef]
- Han, S.A.; Ko, Y.; Shin, S.J.; Jhun, B.W. Characteristics of Circulating CD4+ T Cell Subsets in Patients with Mycobacterium avium Complex Pulmonary Disease. J. Clin. Med. 2020, 9, 1331. [Google Scholar] [CrossRef] [PubMed]
- Wu, U.-I.; Olivier, K.N.; Kuhns, D.B.; Fink, D.L.; Sampaio, E.P.; Zelazny, A.M.; Shallom, S.J.; Marciano, B.E.; Lionakis, M.S.; Holland, S.M. Patients with Idiopathic Pulmonary Nontuberculous Mycobacterial Disease Have Normal Th1/Th2 Cytokine Responses but Diminished Th17 Cytokine and Enhanced Granulocyte-Macrophage Colony-Stimulating Factor Production. Open Forum Infect. Dis. 2019, 6, ofz484. [Google Scholar] [CrossRef]
- Shang, S.; Gibbs, S.; Henao-Tamayo, M.; Shanley, C.A.; McDonnell, G.; Duarte, R.S.; Ordway, D.J.; Jackson, M. Increased virulence of an epidemic strain of mycobacterium massiliense in mice. PLoS ONE 2011, 6, e24726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lutzky, V.P.; Ratnatunga, C.N.; Smith, D.J.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Thomson, R.M.; Bell, S.C.; Miles, J.J. Anomalies in T cell function are associated with individuals at risk of Mycobacterium abscessus complex infection. Front. Immunol. 2018, 9, 1319. [Google Scholar] [CrossRef]
- Wallis, R.S.; Ginindza, S.; Beattie, T.; Arjun, N.; Sebe, M.; Likoti, M.; Edward, V.A.; Rassool, M.; Ahmed, K.; Fielding, K.; et al. Adjunctive host-directed therapies for pulmonary tuberculosis: A prospective, open-label, phase 2, randomised controlled trial. Lancet Respir. Med. 2021, 9, 897–908. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef]
- Elkington, P.T.; Bateman, A.C.; Thomas, G.J.; Ottensmeier, C.H. Implications of tuberculosis reactivation after immune checkpoint inhibition. Am. J. Respir. Crit. Care Med. 2018, 198, 1451–1453. [Google Scholar] [CrossRef]
- Zaemes, J.; Kim, C. Immune checkpoint inhibitor use and tuberculosis: A systematic review of the literature. Eur. J. Cancer 2020, 132, 168–175. [Google Scholar] [CrossRef]
- Sada-Ovalle, I.; Ocaña-Guzman, R.; Pérez-Patrigeón, S.; Chávez-Galán, L.; Sierra-Madero, J.; Torre-Bouscoulet, L.; Addo, M.M. Tim-3 blocking rescue macrophage and T cell function against Mycobacterium tuberculosis infection in HIV+ patients. J. Int. AIDS Soc. 2015, 18, 20078. [Google Scholar] [CrossRef]
- Sada-Ovalle, I.; Chávez-Galán, L.; Torre-Bouscoulet, L.; Nava-Gamiño, L.; Barrera, L.; Jayaraman, P.; Torres-Rojas, M.; Salazar-Lezama, M.A.; Behar, S.M. The Tim3–Galectin 9 Pathway Induces Antibacterial Activity in Human Macrophages Infected with Mycobacterium tuberculosis. J. Immunol. 2012, 189, 5896–5902. [Google Scholar] [CrossRef]
- Phillips, B.L.; Mehra, S.; Ahsan, M.H.; Selman, M.; Khader, S.A.; Kaushal, D. LAG3 expression in active mycobacterium tuberculosis infections. Am. J. Pathol. 2015, 185, 820–833. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Ohashi, P.S. Clinical blockade of PD1 and LAG3-potential mechanisms of action. Nat. Rev. Immunol. 2015, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Rao, M.; Dodoo, E.; Maeurer, M. Potential of immunomodulatory agents as adjunct host-directed therapies for multidrug-resistant tuberculosis. BMC Med. 2016, 14, 89. [Google Scholar] [CrossRef]
- Waterer, G. Beyond antibiotics for pulmonary nontuberculous mycobacterial disease. Curr. Opin. Pulm. Med. 2020, 26, 260–266. [Google Scholar] [CrossRef]
- Fujita, K.; Yamamoto, Y.; Kanai, O.; Okamura, M.; Nakatani, K.; Mio, T. Development of mycobacterium avium complex lung disease in patients with lung cancer on immune checkpoint inhibitors. Open Forum Infect. Dis. 2020, 7, ofaa067. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Yoshida, T.; Shiotsuka, M.; Kobayashi, O.; Iwata, S.; Ohe, Y. Rapid development of pulmonary Mycobacterium avium infection during chemoradiotherapy followed by durvalumab treatment in a locally advanced NSCLC patient. Lung Cancer 2021, 153, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Tamiya, A.; Taniguchi, Y.; Tanaka, T.; Abe, Y.; Isa, S.I.; Tsuyuguchi, K.; Suzuki, K.; Atagi, S. Improvement of mycobacterium abscessus pulmonary disease after nivolumab administration in a patient with advanced non-small cell lung cancer. Intern. Med. 2018, 57, 3625–3629. [Google Scholar] [CrossRef]
- Dumic, I.; Lutwick, L. Successful treatment of rapid growing mycobacterial infections with source control alone: Case series. IDCases 2021, 26, e01332. [Google Scholar] [CrossRef] [PubMed]
- Baseri, B.; Samra, B.; Tam, E.; Chiu, E.; Leaf, A. An Exceptional Responder to Nivolumab in Metastatic Non-Small-Cell Lung Cancer: A Case Report and Literature Review of Long-Term Survivors. Case Rep. Oncol. Med. 2019, 2019, 1816472. [Google Scholar] [CrossRef]
| Age/Gender | Tumour | ICI | Prior Radiotherapy or Chemotherapy | NTM Species Identified | Treatment for NTM Infection | |
|---|---|---|---|---|---|---|
| Fujita et al. 2020 [56] | 78 years, Female | Lung adenocarcinoma | Nivolumab | Standard chemotherapy | M. intracellulare | MAC treatment + nivolumab |
| Fujita et al. 2020 [56] | 80 years, Male | Non-small cell lung cancer | Atezolizumab | Radiotherapy + standard chemotherapy | M. avium + M. intracellulare | MAC treatment + atezolizumab |
| Fujita et al. 2020 [56] | 66 years, Male | Lung squamous cell carcinoma | Nivolumab + Atezolizumab | Standard chemotherapy | M. intracellulare | No medication for severe debilitation |
| Baba et al. 2020 [60] | 80 years, Male | Lung squamous cell carcinoma | Durvalumab | Radiotherapy + standard chemotherapy | M. avium | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, A.; Villa, S.; Castelli, V.; Bandera, A.; Gori, A. T-Cell Exhaustion in Mycobacterium tuberculosis and Nontuberculous Mycobacteria Infection: Pathophysiology and Therapeutic Perspectives. Microorganisms 2021, 9, 2460. https://doi.org/10.3390/microorganisms9122460
Lombardi A, Villa S, Castelli V, Bandera A, Gori A. T-Cell Exhaustion in Mycobacterium tuberculosis and Nontuberculous Mycobacteria Infection: Pathophysiology and Therapeutic Perspectives. Microorganisms. 2021; 9(12):2460. https://doi.org/10.3390/microorganisms9122460
Chicago/Turabian StyleLombardi, Andrea, Simone Villa, Valeria Castelli, Alessandra Bandera, and Andrea Gori. 2021. "T-Cell Exhaustion in Mycobacterium tuberculosis and Nontuberculous Mycobacteria Infection: Pathophysiology and Therapeutic Perspectives" Microorganisms 9, no. 12: 2460. https://doi.org/10.3390/microorganisms9122460
APA StyleLombardi, A., Villa, S., Castelli, V., Bandera, A., & Gori, A. (2021). T-Cell Exhaustion in Mycobacterium tuberculosis and Nontuberculous Mycobacteria Infection: Pathophysiology and Therapeutic Perspectives. Microorganisms, 9(12), 2460. https://doi.org/10.3390/microorganisms9122460







