The Effect of Visible Light on Cell Envelope Subproteome during Vibrio harveyi Survival at 20 °C in Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Vibrio harveyi Strain and Inocula Preparation
2.2. Survival Experiments
2.3. Cell Counting and Estimation of Bacterial Size
2.4. Isolation of Membrane Proteins by Using Sodium Carbonate Extraction
2.5. Protein Identification and Quantification
3. Results
3.1. Analysis of V. harveyi Persistence at 20 °C
3.2. Changes of Membrane Subproteome during Permanence at 20 °C
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker-Austin, C.; Trinanes, J.; Gonzalez-Escalona, N.; Martinez-Urtaza, J. Non-Cholera Vibrios: The Microbial Barometer of Climate Change. Trends Microbiol. 2017, 25, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071. [Google Scholar] [CrossRef]
- Du, M.; Chen, J.; Zhang, X.; Li, A.; Li, Y. Characterization and resuscitation of viable but nonculturable Vibrio alginolyticus VIB283. Arch. Microbiol. 2007, 188, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Ishiwa, A.; Arakawa, E.; Makino, S.-I.; Okada, Y.; Yamamoto, S.; Igimi, S. Gene expression profile of Vibrio cholerae in the cold stress-induced viable but non-culturable state. Environ. Microbiol. 2006, 9, 869–879. [Google Scholar] [CrossRef]
- Parada, C.; Orruño, M.; Kaberdin, V.; Bravo, Z.; Barcina, I.; Arana, I. Changes in the Vibrio harveyi Cell Envelope Subproteome during Permanence in Cold Seawater. Microb. Ecol. 2016, 72, 549–558. [Google Scholar] [CrossRef]
- Sun, F.; Chen, J.; Zhong, L.; Zhang, X.-H.; Wang, R.; Guo, Q.; Dong, Y. Characterization and virulence retention of viable but nonculturable Vibrio harveyi. FEMS Microbiol. Ecol. 2008, 64, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, T.; Papa, S.; Campana, R.; Manti, A.; Battistelli, M.; Baffone, W. State transitions of Vibrio parahaemolyticus VBNC cells evaluated by flow cytometry. Cytom. Part. B Clin. Cytom. 2008, 74, 272–281. [Google Scholar] [CrossRef]
- Wong, H.; Wang, P. Induction of viable but nonculturable state in Vibrio parahaemolyticus and its susceptibility to environmental stresses. J. Appl. Microbiol. 2004, 96, 359–366. [Google Scholar] [CrossRef]
- Nowakowska, J.; Oliver, J.D. Resistance to environmental stresses by Vibrio vulnificusin the viable but nonculturable state. FEMS Microbiol. Ecol. 2013, 84, 213–222. [Google Scholar] [CrossRef]
- Kaspar, C.W.; Tamplin, M.L. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl. Environ. Microbiol. 1993, 59, 2425–2429. [Google Scholar] [CrossRef]
- Randa, M.A.; Polz, M.F.; Lim, E. Effects of Temperature and Salinity on Vibrio vulnificus Population Dynamics as Assessed by Quantitative PCR. Appl. Environ. Microbiol. 2004, 70, 5469–5476. [Google Scholar] [CrossRef]
- Blackwell, K.D.; Oliver, J.D. The ecology of Vibrio vulnificus, Vibrio cholerae, and Vibrio parahaemolyticus in North Carolina Estuaries. J. Microbiol. 2008, 46, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.-I.; Jan, M.-S.; Chi, H.-J. Impacts of climatic variability on Vibrio parahaemolyticus outbreaks in Taiwan. Int. J. Environ. Res. Public Health 2016, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Brettar, I.; Pezzati, E.; Reid, P.C.; Colwell, R.R.; Höfle, M.G.; Pruzzo, C. Long-term effects of ocean warming on the prokaryotic community: Evidence from the vibrios. ISME J. 2011, 6, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pereira, P.R.; Hartmann, M.; Grob, C.; Tarran, G.A.; Martin, A.P.; Fuchs, B.M.; Scanlan, D.J.; Zubkov, M.V. Comparable light stimulation of organic nutrient uptake by SAR11 and Prochlorococcus in the North Atlantic subtropical gyre. ISME J. 2012, 7, 603–614. [Google Scholar] [CrossRef]
- Mary, I.; Tarran, G.A.; Warwick, P.E.; Terry, M.J.; Scanlan, D.J.; Burkill, P.H.; Zubkov, M.V. Light enhanced amino acid uptake by dominant bacterioplankton groups in surface waters of the Atlantic Ocean. FEMS Microbiol. Ecol. 2008, 63, 36–45. [Google Scholar] [CrossRef]
- Gin, K.Y.-H.; Goh, S.G. Modeling the effect of light and salinity on viable but non-culturable (VBNC) Enterococcus. Water Res. 2013, 47, 3315–3328. [Google Scholar] [CrossRef]
- Muela, A.; Seco, C.; Camafeita, E.; Arana, I.; Orruã, O.M.; la Pez, J.A.; Barcina, I.; Orruño, M.; Arana, I.; López, J.A. Changes in Escherichia coli outer membrane subproteome under environmental conditions inducing the viable but nonculturable state. FEMS Microbiol. Ecol. 2008, 64, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Davies-Colley, R.J.; Bell, R.G.; Donnison, A.M. Sunlight Inactivation of Enterococci and Fecal Coliforms in Sewage Effluent Diluted in Seawater. Appl. Environ. Microbiol. 1994, 60, 2049–2058. [Google Scholar] [CrossRef]
- Muela, A.; García-Bringas, J.; Seco, C.; Arana, I.; Barcina, I. Participation of Oxygen and Role of Exogenous and Endogenous Sensitizers in the Photoinactivation of Escherichia coli by Photosynthetically Active Radiation, UV-A and UV-B. Microb. Ecol. 2002, 44, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Banin, E.; Vassilakos, D.; Orr, E.; Martinez, R.J.; Rosenberg, E. Superoxide Dismutase Is a Virulence Factor Produced by the Coral Bleaching Pathogen Vibrio shiloi. Curr. Microbiol. 2003, 46, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Munn, C.B.; Marchant, H.K.; Moody, A.J. Defences against oxidative stress in vibrios associated with corals. FEMS Microbiol. Lett. 2008, 281, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Montánchez, I.; Arana, I.; Parada, C.; Garaizabal, I.; Orruño, M.; Barcina, I.; Kaberdin, V.R. Reprogramming of Vibrio harveyigene expression during adaptation in cold seawater. FEMS Microbiol. Ecol. 2013, 87, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kong, I.-S.; Bates, T.C.; Hülsmann, A.; Hassan, H.; Smith, B.E.; Oliver, J.D. Role of catalase and oxyR in the viable but nonculturable state of Vibrio vulnificus. FEMS Microbiol. Ecol. 2004, 50, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kaberdin, V.R.; Montánchez, I.; Parada, C.; Orruño, M.; Arana, I.; Barcina, I. Unveiling the Metabolic Pathways Associated with the Adaptive Reduction of Cell Size During Vibrio harveyi Persistence in Seawater Microcosms. Microb. Ecol. 2015, 70, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Abboudi, M.; Surget, S.M.; Rontani, J.-F.; Sempéré, R.; Joux, F. Physiological Alteration of the Marine Bacterium Vibrio angustum S14 Exposed to Simulated Sunlight During Growth. Curr. Microbiol. 2008, 57, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Takayama, K.; Kjelleberg, S. Role of spoT-dependent ppGpp accumulation in the survival of light-exposed starved bacteria. Microbiology 2002, 148, 559–570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kehoe, S.; Barer, M.; Devlin, L.; McGuigan, K. Batch process solar disinfection is an efficient means of disinfecting drinking water contaminated with Shigella dysenteriae type I. Lett. Appl. Microbiol. 2004, 38, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, S.; Ren, H.; Lin, X.; Wu, L.; Peng, X. Proteomic analysis on the expression of outer membrane proteins of Vibrio alginolyticus at different sodium concentrations. Proteomics 2005, 5, 3142–3152. [Google Scholar] [CrossRef]
- Hobbie, J.E.; Daley, R.J.; Jasper, S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 1977, 33, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Joux, F.; le Baron, P.; Troussellier, M. Succession of cellular states in a Salmonella typhimurium population during starvation in artificial seawater microcosms. FEMS Microbiol. Ecol. 1997, 22, 65–76. [Google Scholar] [CrossRef]
- Massana, R.; Gasol, J.M.; Bjørnsen, P.K.; Blackburn, N.; Hagström, A.; Hietanen, S.; Hygum, B.H.; Kuparinen, J.; Pedrós-Alió, C. Measurement of bacterial size via image analysis of epifluorescence preparations: Description of an inexpensive system and solutions to some of the most common problems. Sci. Mar. 1997, 61, 397–407. [Google Scholar]
- Gonzalez-Fernandez, R.; Aloria, K.; Arizmendi, J.M.; Jorrin-Novo, J.V. Application of Label-Free Shotgun nUPLC–MSEand 2-DE Approaches in the Study of Botrytis cinerea Mycelium. J. Proteome Res. 2013, 12, 3042–3056. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Denny, R.; Dorschel, C.A.; Gorenstein, M.; Kass, I.J.; Li, G.-Z.; McKenna, T.; Nold, M.J.; Richardson, K.; Young, A.P.; et al. Quantitative Proteomic Analysis by Accurate Mass Retention Time Pairs. Anal. Chem. 2005, 77, 2187–2200. [Google Scholar] [CrossRef]
- Li, G.-Z.; Vissers, J.P.C.; Silva, J.C.; Golick, D.; Gorenstein, M.V.; Geromanos, S.J. Database searching and accounting of multiplexed precursor and product ion spectra from the data independent analysis of simple and complex peptide mixtures. Proteom. 2009, 9, 1696–1719. [Google Scholar] [CrossRef]
- Silva, J.C.; Gorenstein, M.V.; Li, G.-Z.; Vissers, J.P.C.; Geromanos, S.J. Absolute Quantification of Proteins by LCMSE. Mol. Cell. Proteom. 2006, 5, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef]
- Wagley, S.; Morcrette, H.; Kovacs-Simon, A.; Yang, Z.R.; Power, A.; Tennant, R.K.; Love, J.; Murray, N.; Titball, R.W.; Butler, C.S. Bacterial dormancy: A subpopulation of viable but non-culturable cells demonstrates better fitness for revival. PLoS Pathog. 2021, 17, e1009194. [Google Scholar] [CrossRef] [PubMed]
- Montánchez, I.; Kaberdin, V.R. Vibrio harveyi: A brief survey of general characteristics and recent epidemiological traits associated with climate change. Mar. Environ. Res. 2020, 154, 104850. [Google Scholar] [CrossRef] [PubMed]
- Orruño, M.; Parada, C.; Ogayar, E.; Kaberdin, V.; Arana, I. Effects of abiotic and biotic factors on Vibrio harveyi ATCC 14126T survival dynamics in seawater microcosms. Aquat. Microb. Ecol. 2019, 83, 109–118. [Google Scholar] [CrossRef]
- Abia, A.L.K.; Ubomba-Jaswa, E.; Momba, M.N.B. Competitive Survival of Escherichia coli, Vibrio cholerae, Salmonella typhimurium and Shigella dysenteriae in Riverbed Sediments. Microb. Ecol. 2016, 72, 881–889. [Google Scholar] [CrossRef]
- Jia, J.; Li, Z.; Cao, J.; Jiang, Y.; Liang, C.; Liu, M. Proteomic Analysis of Protein Expression in the Induction of the Viable But Nonculturable State of Vibrio harveyi SF1. Curr. Microbiol. 2013, 67, 442–447. [Google Scholar] [CrossRef]
- Hernroth, B.; Lothigius, Ã.; Bãlin, I.; Bölin, I. Factors influencing survival of enterotoxigenic Escherichia coli, Salmonella enterica (serovar Typhimurium) and Vibrio parahaemolyticus in marine environments. FEMS Microbiol. Ecol. 2009, 71, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Marco-Noales, E.; Biosca, E.G.; Amaro, C. Effects of Salinity and Temperature on Long-Term Survival of the Eel Pathogen Vibrio vulnificus Biotype 2 (Serovar E). Appl. Environ. Microbiol. 1999, 65, 1117–1126. [Google Scholar] [CrossRef]
- Jiang, X.; Chai, T.J. Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable, nonculturable cells. Appl. Environ. Microbiol. 1996, 62, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.W.; Oliver, J.D. Temperature effects on the viable but non-culturable state of Vibrio vulnificus. FEMS Microbiol. Lett. 1992, 101, 33–39. [Google Scholar] [CrossRef]
- Barcina, I.; González, J.; Iriberri, J.; Egea, L. Survival strategy of Escherichia coli and Enterococcus faecalis in illuminated fresh and marine systems. J. Appl. Bacteriol. 1990, 68, 189–198. [Google Scholar] [CrossRef]
- Pommepuy, M.; Butin, M.; Derrien, A.; Gourmelon, M.; Colwell, R.R.; Cormier, M. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl. Environ. Microbiol. 1996, 62, 4621–4626. [Google Scholar] [CrossRef] [PubMed]
- Sassoubre, L.M.; Nelson, K.L.; Boehm, A.B. Mechanisms for Photoinactivation of Enterococcus faecalis in Seawater. Appl. Environ. Microbiol. 2012, 78, 7776–7785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giacobone, A.F.F.; Oppezzo, O.J. Survival of Pseudomonas aeruginosa exposed to sunlight resembles the phenom of persistence. J. Photochem. Photobiol. B Biol. 2015, 142, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.F.; de Wergifosse, B.; Noiset, O.; Dubuisson, M.; Janssens, B.; Thompson, E.M. The origins of marine bioluminescence: Turning oxygen defence mechanisms into deep-sea communication tools. J. Exp. Biol. 1998, 201, 1211–1221. [Google Scholar] [PubMed]
- Kozakiewicz, J.; Gajewska, M.; Łyzen, R.; Czyz, A.; Wegrzyn, G. Bioluminescence-mediated stimulation of photoreactivation in bacteria. FEMS Microbiol. Lett. 2005, 250, 105–110. [Google Scholar] [CrossRef]
- Lyzeń, R.; Wegrzyn, G. Sensitivity of dark mutants of various strains of luminescent bacteria to reactive oxygen species. Arch. Microbiol. 2005, 183, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Alifano, P.; Nassisi, V.; Siciliano, M.V.; Talà, A.; Tredici, S.M. Unexpected photoreactivation of Vibrio harveyi bacteria living in ionization environment. J. Appl. Phys. 2011, 109, 104703. [Google Scholar] [CrossRef]
- Czyz, A.; Wróbel, B.; Węgrzyn, G. Vibrio harveyi bioluminescence plays a role in stimulation of DNA repair We would like to dedicate this paper to the memory of Karol Taylor, who introduced V. harveyi projects to our laboratories. Microbiology 2000, 146, 283–288. [Google Scholar] [CrossRef]
- Lin, L.-C.; Lin, G.-H.; Wang, Z.-L.; Tseng, Y.-H.; Yu, M.-S. Differential expression of catalases in Vibrio parahaemolyticus under various stress conditions. Res. Microbiol. 2015, 166, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, S.; Zhang, J.; Rothenbacher, F.P.; Jiang, T.; Kan, B.; Zhong, Z.; Zhu, J. Catalases Promote Resistance of Oxidative Stress in Vibrio cholerae. PLoS ONE 2012, 7, e53383. [Google Scholar] [CrossRef]
- Yumoto, I.; Ichihashi, D.; Iwata, H.; Istokovics, A.; Ichise, N.; Matsuyama, H.; Okuyama, H.; Kawasaki, K. Purification and Characterization of a Catalase from the Facultatively Psychrophilic Bacterium Vibrio rumoiensis S-1T Exhibiting High Catalase Activity. J. Bacteriol. 2000, 182, 1903–1909. [Google Scholar] [CrossRef]
- Asakura, H.; Kawamoto, K.; Haishima, Y.; Igimi, S.; Yamamoto, S.; Makino, S.-I. Differential expression of the outer membrane protein W (OmpW) stress response in enterohemorrhagic Escherichia coli O157:H7 corresponds to the viable but non-culturable state. Res. Microbiol. 2008, 159, 709–717. [Google Scholar] [CrossRef]
- Govindarajan, S.; Amster-Choder, O. Where are things inside a bacterial cell? Curr. Opin. Microbiol. 2016, 33, 83–90. [Google Scholar] [CrossRef]
- Laddomada, F.; Miyachiro, M.M.; Dessen, A. Structural Insights into Protein-Protein Interactions Involved in Bacterial Cell Wall Biogenesis. Antibiotics 2016, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-W.; Chen, S.-Y.; Wong, H.-C. Dynamic Localization of MreB in Vibrio parahaemolyticus and in the Ectopic Host Bacterium Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 6739–6745. [Google Scholar] [CrossRef][Green Version]
- Al Dahouk, S.; Jubier-Maurin, V.; Neubauer, H.; Köhler, S. Quantitative analysis of the Brucella suis proteome reveals metabolic adaptation to long-term nutrient starvation. BMC Microbiol. 2013, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Bronowski, C.; Mustafa, K.; Goodhead, I.; James, C.E.; Nelson, C.; Lucaci, A.; Wigley, P.; Humphrey, T.J.; Williams, N.J.; Winstanley, C.; et al. Campylobacter jejuni transcriptome changes during loss of culturability in water. PLoS ONE 2017, 12, e0188936. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Inouye, S.; Inouye, M. Lipoprotein-28, a cytoplasmic membrane lipoprotein from Escherichia coli. Cloning, DNA sequence, and expression of its gene. J. Biol. Chem. 1986, 261, 2284–2288. [Google Scholar] [CrossRef]
- Wang, W.; Jeffery, C.J. An analysis of surface proteomics results reveals novel candidates for intracellular/surface moonlighting proteins in bacteria. Mol. BioSyst. 2016, 12, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Thofte, O.; Su, Y.-C.; Brant, M.; Littorin, N.; Duell, B.L.; Alvarado, V.; Jalalvand, F.; Riesbeck, K. EF-Tu From Non-typeable Haemophilus influenzae Is an Immunogenic Surface-Exposed Protein Targeted by Bactericidal Antibodies. Front. Immunol. 2018, 9, 2910. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-J.; Chen, S.-Y.; Lin, I.-H.; Chang, C.-H.; Wong, H.-C. Change of protein profiles in the induction of the viable but nonculturable state of Vibrio parahaemolyticus. Int. J. Food Microbiol. 2009, 135, 118–124. [Google Scholar] [CrossRef]
- Malmcrona-Friberg, K.; Goodman, A.; Kjelleberg, S. Chemotactic Responses of Marine Vibrio sp. Strain S14 (CCUG 15956) to Low-Molecular-Weight Substances under Starvation and Recovery Conditions. Appl. Environ. Microbiol. 1990, 56, 3699–3704. [Google Scholar] [CrossRef]
- Stretton, S.; Danon, S.J.; Kjelleberg, S.; Goodman, A.E. Changes in cell morphology and motility in the marine Vibrio sp. strain S14 during conditions of starvation and recovery. FEMS Microbiol. Lett. 1997, 146, 23–29. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Chen, C.-Y. Starvation Induces Phenotypic Diversification and Convergent Evolution in Vibrio vulnificus. PLoS ONE 2014, 9, e88658. [Google Scholar] [CrossRef]
- Dawson, M.P.; Humphrey, B.A.; Marshall, K.C. Adhesion: A tactic in the survival strategy of a marine vibrio during starvation. Curr. Microbiol. 1981, 6, 195–199. [Google Scholar] [CrossRef]
- Lutz, C.; Erken, M.; Noorian, P.; Sun, S.; McDougald, D. Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front. Microbiol. 2013, 4, 375. [Google Scholar] [CrossRef]
- Meng, L.; Alter, T.; Aho, T.; Huehn, S. Gene expression profiles of Vibrio parahaemolyticus in viable but non-culturable state. FEMS Microbiol. Ecol. 2015, 91, 035. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.W.; Ellwood, M.J. The biogeochemical cycle of iron in the ocean. Nat. Geosci. 2010, 3, 675–682. [Google Scholar] [CrossRef]
- Cornelis, P.; Wei, Q.; Andrews, S.C.; Vinckx, T. Iron homeostasis and management of oxidative stress response in bacteria. Metallomics 2011, 3, 540–549. [Google Scholar] [CrossRef]
- Miethke, M.; Pierik, A.J.; Peuckert, F.; Seubert, A.; Marahiel, M.A. Identification and Characterization of a Novel-type Ferric Siderophore Reductase from a Gram-positive Extremophile. J. Biol. Chem. 2011, 286, 2245–2260. [Google Scholar] [CrossRef] [PubMed]
- Koprowski, P.; Grajkowski, W.; Balcerzak, M.; Filipiuk, I.; Fabczak, H.; Kubalski, A. Cytoplasmic Domain of MscS Interacts with Cell Division Protein FtsZ: A Possible Non-Channel Function of the Mechanosensitive Channel in Escherichia coli. PLoS ONE 2015, 10, e0127029. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed]
 > 1.74 μm). The data are mean values from three independent experiments with errors bars representing the standard deviations calculated.
> 1.74 μm). The data are mean values from three independent experiments with errors bars representing the standard deviations calculated.
 > 1.74 μm). The data are mean values from three independent experiments with errors bars representing the standard deviations calculated.
> 1.74 μm). The data are mean values from three independent experiments with errors bars representing the standard deviations calculated.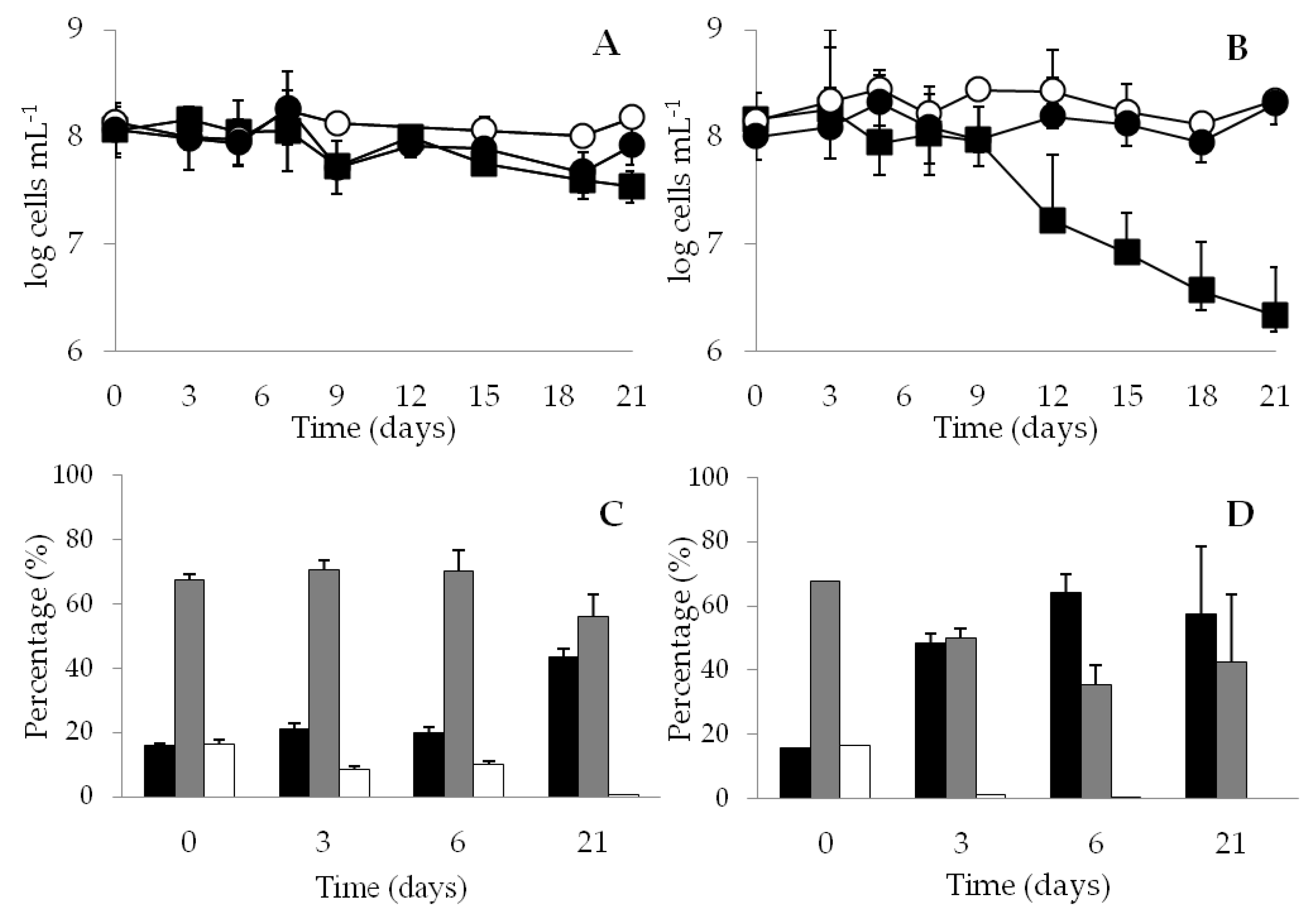
| Protein Accession Number | Location a | Category b | Protein Name (Uniprot) |
|---|---|---|---|
| D0XEL2_VIBH1 | OM | S | Major outer membrane lipoprotein |
| D0XD95_VIBH1 | CM | S | Lipoprotein |
| D0X8I2_VIBH1 | OM | S | Outer membrane protein assembly factor BamA |
| D0X8E2_VIBH1 | OM | S | Outer membrane protein assembly factor BamC |
| D0XDX1_VIBH1 | OM | S | Outer membrane protein assembly factor BamD |
| D0XEA2_VIBH1 | CM | S | Membrane protein insertase YidC |
| D0XAE4_VIBH1 | Cyt | S | Rod shape-determining protein MreB |
| D0X6I2_VIBH1 | OM | T | OmpU |
| D0X5G7_VIBH1 | OM | T | OmpW |
| D0XAK6_VIBH1 | OM | T | Gram-negative porin family protein |
| D0XAX8_VIBH1 | OM | T | Vitamin B12 transporter BtuB |
| D0XI69_VIBH1 | P | T | Maltose operon periplasmic protein (MalM) |
| D0XDJ8_VIBH1 | CM | T | Preprotein translocase subunit YajC |
| D0X7E4_VIBH1 | CM | T | Protein translocase subunit SecA |
| D0XDJ7_VIBH1 | CM | T | Protein translocase subunit SecD |
| D0X520_VIBH1 | CM | T | Type II secretion system core protein G |
| D0X596_VIBH1 | IM | T | Multidrug resistance protein MdtA |
| D0XI24_VIBH1 | OM | T | Type I secretion outer membrane, TolC family protein |
| D0X6W0_VIBH1 | CM | T | Na+/proline symporter |
| D0XAD2_VIBH1 | OM | T | MSHA biogenesis protein MshL |
| D0X9U0_VIBH1 | CM | T | LemA family protein |
| D0XE90_VIBH1 | Cyt | B | ATP synthase subunit alpha |
| D0X9V8_VIBH1 | Cyt | B | ATP synthase subunit alpha |
| D0XE88_VIBH1 | Cyt | B | ATP synthase subunit beta |
| D0XE92_VIBH1 | Cyt | B | ATP synthase subunit b |
| D0X7C0_VIBH1 | CM | B | Cytochrome b |
| D0X872_VIBH1 | CM | B | Na(+)- translocating NADH-quinone reductase subunit A |
| D0X873_VIBH1 | CM | B | Na(+)-translocating NADH-quinone reductase subunit B |
| D0X877_VIBH1 | CM | B | Na(+)-translocating NADH-quinone reductase subunit F |
| D0X602_VIBH1 | CM | B | NAD(P) transhydrogenase subunit alpha |
| D0X8Q1_VIBH1 | CM | B | Succinate dehydrogenase iron-sulfur subunit |
| D0XAW2_VIBH1 | CM | SDT | HflC |
| D0XAW1_VIBH1 | CM | SDT | HflK |
| D0X8K6_VIBH1 | CM | SDT | Modulator of FtsH protease HflK |
| D0XAQ5_VIBH1 | Cyt | O | Elongation factor Tu (EF-Tu) |
| D0XCD5_VIBH1 | Cyt | O | Elongation factor G (EF-G) |
| Protein Accession Number | Loc a | Cat b | Protein Name | P0 c | Illumination | |||
|---|---|---|---|---|---|---|---|---|
| (−) | (+) | |||||||
| P1 | P2 | P1 | P2 | |||||
| D0X8N6_VIBH1 | CM | T | PTS system N-acetylglucosamine-specific IIABC component | D | ND | ND | ND | ND |
| D0XD90_VIBH1 | CM | T | PTS system trehalose-specific IIBC component | D | ND | ND | ND | ND |
| D0X553_VIBH1 | CM | T | Sec-independent protein translocase protein TatA | D | ND | ND | ND | ND |
| D0X593_VIBH1 | ? | B | Cytochrome c5 | D | ND | ND | ND | ND |
| D0XEK4_VIBH1 D0XEY1_VIBH1 | CM | O | Methyl-accepting chemotaxis proteins | D | ND | ND | ND | ND |
| D0X9J1_VIBH1 D0XEC5_VIBH1 D0XHW4_VIBH1 D0XGG1_VIBH1 D0X5R4_VIBH1 D0X9F5_VIBH1 D0XCQ6_VIBH1 | CM | O | Methyl-accepting chemotaxis proteins | D | ND | ND | D | ND |
| D0XD78_VIBH1 | CM | S | Tail-specific protease | D | ND | ND | D | ND |
| D0XB91_VIBH1 | Ex | O | Flagellin | D | D | ND | ND | ND |
| D0X7B5_VIBH1 | CM | S | Protein YhcB | D | D | 0.49 | D | 0.22 |
| D0X6W2_VIBH1 | CM/Cyt | T | Bifunctional protein PutA | D | 0.17 | 0.18 | 0.26 | 0.11 |
| D0XFE0_VIBH1 | CM | B | Cytochrome c oxidase subunit CcoO | D | D | D | D | ND |
| D0X7B9_VIBH1 | CM | B | Ubiquinol-cytochrome c reductase iron sulfur subunit | D | D | D | D | ND |
| D0X7C7_VIBH1 | P | S | Penicillin-binding protein activator LpoA | D | D | D | D | ND |
| D0XAK5_VIBH1 | ? | SDT | Protease | D | D | D | D | ND |
| D0X545_VIBH1 | P | B | Cytochrome c4 | D | D | D | ND | ND |
| D0X955_VIBH1 | CM | T | Multidrug resistance protein MexA | D | D | D | ND | ND |
| D0XFH9_VIBH1 | ? | SDT | ATP-dependent Zn protease | D | D | D | ND | ND |
| D0XE93_VIBH1 | CM | B | ATP synthase subunit c | D | D | 5.50 | ND | ND |
| D0XFI8_VIBH1 | CM | B | Cytochrome d ubiquinol oxidase, subunit I | D | D | 4.29 | ND | ND |
| D0X601_VIBH1 | CM | B | NAD(P) transhydrogenase subunit beta | D | D | 2.65 | D | ND |
| D0X7C1_VIBH1 | CM | B | Ubiquinol-cytochrome c reductase cytochrome c1 | D | D | D | D | 0.11 |
| D0XFD9_VIBH1 | CM | B | cbb3-type cytochrome c oxidase subunit | D | D | D | D | 0.06 |
| D0XEK0_VIBH1 | CM | T | Phosphate import ATP binding protein PstB | D | D | ND | D | D |
| D0XEK3_VIBH1 | P | T | Phosphate ABC transporter periplasmic phosphate-binding protein | D | ND | ND | D | 3.36 |
| D0X9E5_VIBH1 | Cyt | SDT | ATP dependent Clp protease ATP binding subunit ClpA | D | ND | ND | D | D |
| D0X9N4_VIBH1 | Cyt | SDT | ATP dependent Clp protease ATP binding subunit ClpX | D | ND | ND | D | D |
| D0XCF5_VIBH1 | CM | T | PTS system glucose-specific IIBC component | D | D | 0.38 | D | D |
| D0X6J9_VIBH1 | OM | T | OmpA–like protein | D | D | 0.40 | D | D |
| D0X9V6_VIBH1 | CM | B | ATP synthase subunit beta | D | 0.07 | 0.18 | D | D |
| D0XCA0_VIBH1 | CM | SDT | ATP-dependent zinc metalloprotease FtsH | D | D | 0.48 | D | D |
| D0X861_VIBH1 | OM | O | Outer membrane protein OmpK | D | D | 0.32 | D | D |
| D0X8R9_VIBH1 | OM | S | Outer membrane protein slp | D | D | D | D | 2.06 |
| D0X9I8_VIBH1 | OM | S | Putative YcfL protein | D | D | D | D | 2.92 |
| D0XFJ5_VIBH1 | OM | S | Peptidoglycan-associated lipoprotein | D | D | D | D | 2.43 |
| D0X523_VIBH1 | OM | T | General secretion pathway protein D | D | D | D | D | 2.20 |
| D0X7A6_VIBH1 | OM | T | Outer membrane protein TolC | D | D | D | D | 2.75 |
| D0XI94_VIBH1 | OM | T | Agglutination protein | D | D | D | D | 4.12 |
| D0XCR3_VIBH1 | CM | T | Mechanosensitive ion channel protein MscS | ND | ND | D | D | D |
| D0XAQ6_VIBH1 | Cyt | O | Bacterioferritin | ND | ND | D | D | D |
| D0X8W1_VIBH1 | Cyt | O | Catalase peroxidase | ND | ND | D | D | D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orruño, M.; Parada, C.; Kaberdin, V.R.; Arana, I. The Effect of Visible Light on Cell Envelope Subproteome during Vibrio harveyi Survival at 20 °C in Seawater. Microorganisms 2021, 9, 594. https://doi.org/10.3390/microorganisms9030594
Orruño M, Parada C, Kaberdin VR, Arana I. The Effect of Visible Light on Cell Envelope Subproteome during Vibrio harveyi Survival at 20 °C in Seawater. Microorganisms. 2021; 9(3):594. https://doi.org/10.3390/microorganisms9030594
Chicago/Turabian StyleOrruño, Maite, Claudia Parada, Vladimir R. Kaberdin, and Inés Arana. 2021. "The Effect of Visible Light on Cell Envelope Subproteome during Vibrio harveyi Survival at 20 °C in Seawater" Microorganisms 9, no. 3: 594. https://doi.org/10.3390/microorganisms9030594
APA StyleOrruño, M., Parada, C., Kaberdin, V. R., & Arana, I. (2021). The Effect of Visible Light on Cell Envelope Subproteome during Vibrio harveyi Survival at 20 °C in Seawater. Microorganisms, 9(3), 594. https://doi.org/10.3390/microorganisms9030594







