Environmental Management of Legionella in Domestic Water Systems: Consolidated and Innovative Approaches for Disinfection Methods and Risk Assessment
Abstract
1. Introduction
2. Methods
3. Factors Related to the Growth and Survival of Legionellae and the Management of Environmental Prevention
3.1. Water Temperature
3.2. Protozoa and Biofilm
3.3. Organic and Inorganic Sediment Accumulation
4. Disinfection Strategies
4.1. Oxidizing Agents
4.2. Non-Oxidizing Agents
4.3. Point-of-Use (POU) Filtration
5. Emerging Treatment Technologies
5.1. Essential Oils (Eos)
5.2. Biosurfactants
5.3. Smart Surfaces
6. Water Sampling
7. Viable but Non Culturable (VBNC) State
7.1. VBNC-Legionella Isolation Methods
7.2. Emerging Detection Methods
8. Water Systems Management
Water Safety Plan (WSP)
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Legionnaires’ Disease associated with potting soil—California, Oregon, and Washington, May–June 2000. MMWR Morb. Mortal. Wkly Rep. 2000, 49, 777–778. [Google Scholar]
- Katz, S.M.; Hammel, J.M. The effect of drying, heat, and pH on the survival of Legionella pneumophila. Ann. Clin. Lab. Sci. 1987, 17, 150–156. [Google Scholar]
- Sehulster, L.; Chinn, R.Y.W.; Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Morb. Mortal. Wkly Rep. 2003, 52, 1–42. [Google Scholar]
- European Technical Guidelines for the Prevention, Control and Investigation, of Infections Caused by Legionella Species. ECDC. 2017. Available online: https://ecdc.europa.eu/sites/portal/files/documents/Legionella%20GuidelinesFinal%20updated%20for%20ECDC%20corrections.pdf (accessed on 9 February 2021).
- Ministero Della Salute. Linee Guida per la Prevenzione ed il Controllo Della Legionellosi. 2015. Available online: http://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?id=2362 (accessed on 9 February 2021).
- Sathasivan, A.; Chiang, J.; Nolan, P. Temperature dependence of chemical and microbiological chloramine decay in bulk waters of distribution system. Water Sci. Technol. Water Supply 2009, 9, 493–499. [Google Scholar] [CrossRef]
- García-Ávila, F.; Sánchez-Alvarracín, C.; Cadme-Galabay, M.; Conchado-Martínez, J.; García-Mera, G.; Zhindón-Arévalo, C. Relationship between chlorine decay and temperature in the drinking water. MethodsX 2020, 7, 101002. [Google Scholar] [CrossRef]
- Pan, J.; Rhoads, W.J.; Edwards, M.A.; Pruden, A. Effect of heat shock on hot water plumbing microbiota and Legionella pneumophila control. Microbiome 2018, 6, 30. [Google Scholar]
- Health and Safe Executive (HSE). Legionnaires’ Disease. Part 2: The Control of Legionella Bacteria in Hot and Cold Water Systems. 2014. Available online: www.hse.gov.uk/pubns/priced/hsg274part2.pdf (accessed on 9 February 2021).
- Erdoğan, H. Legionnaires’ Disease. Mediterr. J. Infect. Microb. Antimicrob. 2018, 7, 2. [Google Scholar] [CrossRef]
- Lesnik, R.; Brettar, I.; Höfle, M.G. Legionella species diversity and dynamics from surface reservoir to tap water: From cold adaptation to thermophily. ISME J. 2016, 10, 1064–1080. [Google Scholar] [CrossRef]
- Dilger, T.; Melzl, H.; Gessner, A. Legionella contamination in warm water systems: A species-level survey. Int. J. Hyg. Environ. Health 2018, 221, 199–210. [Google Scholar] [CrossRef]
- Gomez-Valero, L.; Rusniok, C.; Jarraud, S.; Vacherie, B.; Rouy, Z.; Barbe, V.; Medigue, C.; Etienne, J.; Buchrieser, C. Extensive recombination events and horizontal gene transfer shaped the Legionella pneumophila genomes. BMC Genom. 2011, 12, 536. [Google Scholar] [CrossRef]
- Pierre, D.; Baron, J.L.; Ma, X.; Sidari, F.P.; Wagener, M.M.; Stout, J.E. Water quality as a predictor of Legionella positivity of building water systems. Pathogens 2019, 8, 295. [Google Scholar] [CrossRef]
- Ohno, A.; Kato, N.; Sakamoto, R.; Kimura, S.; Yamaguchi, K. Temperature-Dependent Parasitic Relationship between Legionella pneumophila and a Free-Living Amoeba (Acanthamoeba castellanii). Appl. Environ. Microbiol. 2008, 74, 4585–4588. [Google Scholar] [CrossRef] [PubMed]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [PubMed]
- Sriram, R.; Shoff, M.; Booton, G.; Fuerst, P.; Visvesvara, G.S. Survival of Acanthamoeba cysts after desiccation for more than 20 years. J. Clin. Microbiol. 2008, 46, 4045–4048. [Google Scholar] [CrossRef]
- Mazur, T.; Hadaś, E.; Iwanicka, I. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop. Med. Parasitol. 1995, 46, 106–108. [Google Scholar] [PubMed]
- Kilvington, S. Moist-heat disinfection of Acanthamoeba cysts. Rev. Infect. Dis. 1991, 5, 418. [Google Scholar] [CrossRef]
- Storey, M.V.; Winiecka-Krusnell, J.; Ashbolt, N.J.; Stenström, T.A. The efficacy of heat and chlorine treatment against thermotolerant Acanthamoebae and Legionellae. Scand. J. Infect. Dis. 2004, 36, 656–662. [Google Scholar] [CrossRef]
- Thomas, V.; McDonnell, G.; Denyer, S.P.; Maillard, J.Y. Free-living amoebae and their intracellular pathogenic microorganisms: Risks for water quality. FEMS Microbiol. Rev. 2010, 34, 231–259. [Google Scholar] [CrossRef] [PubMed]
- Mampel, J.; Spirig, T.; Weber, S.S.; Haagensen, J.A.; Molin, S.; Hilbi, H. Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl. Environ. Microbiol. 2006, 72, 2885–2895. [Google Scholar] [CrossRef]
- Hindré, T.; Brüggemann, H.; Buchrieser, C.; Héchard, Y. Transcriptional profiling of Legionella pneumophila biofilm cells and the influence of iron on biofilm formation. Microbiology 2008, 154, 30–41. [Google Scholar] [CrossRef]
- Pécastaings, S.; Bergé, M.; Dubourg, K.M.; Roques, C. Sessile Legionella pneumophila is able to grow on surfaces and generate structured monospecies biofilms. Biofouling 2010, 26, 809–819. [Google Scholar] [CrossRef]
- Stewart, C.R.; Muthye, V.; Cianciotto, N.P. Legionella pneumophila persists within biofilms formed by Klebsiella pneumoniae, Flavobacterium sp., and Pseudomonas fluorescens under dynamic flow conditions. PLoS ONE 2012, 7, 50560. [Google Scholar] [CrossRef]
- Kives, J.; Orgaz, B.; Sanjosé, C. Polysaccharide differences between planktonic and biofilm-associated EPS from Pseudomonas fluorescens B52. Colloids Surf. B Biointerfaces 2006, 52, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.; Flemming, L.A.; Chenia, H.Y. Evaluation of adherence, hydrophobicity, aggregation, and biofilm development of Flavobacterium johnsoniae-like isolates. Microb. Ecol. 2008, 55, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Lin, T.L.; Hsieh, P.F.; Yang, H.C.; Wang, J.T. Isolation of genes involved in biofilm formation of a Klebsiella pneumoniae strain causing pyogenic liver abscess. PLoS ONE 2011, 6, e23500. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, E.; Bondi, M.; Sabia, C.; de Niederhäusern, S.; Borella, P.; Messi, P. Effect of bacterial interference on biofilm development by Legionella pneumophila. Curr. Microbiol. 2008, 57, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Mallegol, J.; Duncan, C.; Prashar, A.; So, J.; Low, D.E.; Terebeznik, M.; Guyard, C. Essential roles and regulation of the Legionella pneumophila collagen-like adhesin during biofilm formation. PLoS ONE 2012, 7, e46462. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.B.; Ruseska, I.; Costerton, J.W. Decreased biocide susceptibility of adherent Legionella pneumophila. J. Appl. Bacteriol. 1991, 71, 531–538. [Google Scholar] [CrossRef]
- Lin, Y.E.; Stout, J.E.; Yu, V.L. Controlling Legionella in hospital drinking water: An evidence-based review of disinfection methods. Infect. Control Hosp. Epidemiol. 2011, 32, 166–173. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Liu, T.; Kong, W.; He, X.; Jin, Y.; Zhang, B. Effects of Assimilable Organic Carbon and free chlorine on bacterial growth in drinking water. PLoS ONE 2015, 10, e0128825. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Pruden, A.; Falkinham, J.O.; Edwards, M. Relationship between Organic Carbon and Opportunistic Pathogens in Simulated Glass Water Heaters. Pathogens 2015, 4, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooij, D. Assimilable organic carbon as an indicator of bacterial regrowth. J. Am. Water Work. Assoc. 1992, 84, 57–65. [Google Scholar] [CrossRef]
- Hammes, F.; Berger, C.; Köster, O.; Egli, T. Assessing biological stability of drinking water without disinfectant residuals in a full-scale water supply system. J. Water Supply Res. Technol. Aqua 2010, 59, 31–40. [Google Scholar] [CrossRef]
- Volk, C.J.; LeChevallier, M.W. Assessing biodegradable organic matter. J. Am. Water Work. Assoc. AWWA 2000, 92, 64–76. [Google Scholar] [CrossRef]
- Reeves, M.W.; Pine, L.; Hutner, S.H.; George, J.R.; Harrell, W.K. Metal requirements of Legionella pneumophila. J. Clin. Microbiol. 1981, 13, 688–695. [Google Scholar] [CrossRef]
- States, S.J.; Conley, L.F.; Ceraso, M.; Stephenson, T.E.; Wolford, R.S.; Wadowsky, R.M.; McNamara, A.M.; Yee, R.B. Effects of metals on Legionella pneumophila growth in drinking water plumbing systems. Appl. Environ. Microbiol. 1985, 50, 1149–1154. [Google Scholar] [CrossRef]
- Shang, C.; Blatchley, E.R. Differentiation and quantification of free chlorine and inorganic chloramines in aqueous solution by MIMS. Environ. Sci. Technol. 1999, 33, 2218–2223. [Google Scholar] [CrossRef]
- Virto, R.; Mañas, P.; Álvarez, I.; Condon, S.; Raso, J. Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Appl. Environ. Microbiol. 2005, 71, 5022–5028. [Google Scholar] [CrossRef]
- World Health Organization. Chlorine in Drinking-Water; World Health Organization: Geneva, Switzerland, 2003; p. 11. Available online: https://www.who.int/water_sanitation_health/dwq/chlorine.pdf (accessed on 10 February 2021).
- Cooper, I.R.; White, J.; Mahenthiralingam, E.; Hanlon, G.W. Long-term persistence of a single Legionella pneumophila strain possessing the mip gene in a municipal shower de-spite repeated cycles of chlorination. J. Hosp. Infect. 2008, 70, 154–159. [Google Scholar] [CrossRef] [PubMed]
- García-Ávila, F.; Del Pino, L.F.; Bonifaz, G.A.; Zhindòn-Arévalo, C.; Ramos-Fernàndez, L.; Garcìa-Altamirano, D.; Sanchez, C. Effect of chlorine residual on copper pipes in drinking water systems. J. Eng. Sci. Technol. Rev. 2019, 12, 119–126. [Google Scholar] [CrossRef]
- Cantor, A.F.; Park, J.K.; Vaiyavatjamai, P. The effect of chlorine on corrosion in drinking water systems. J. Am. Water Work. Assoc. AWWA 2003, 95, 112–123. [Google Scholar] [CrossRef]
- Holm, T.R.; Schock, M.R. Potential effects of polyphosphate products on lead solubility in plumbing systems. J. Am. Water Work. Assoc. AWWA 1991, 83, 76. [Google Scholar] [CrossRef]
- US EPA. EPA Drinking Water Guidance on Disinfection By-Products Advice Note No. 4. Version 2. Disinfection By-Products in Drinking Water. 2001. Available online: https://www.epa.ie/pubs/advice/drinkingwater/DrinkingWaterGuide4_v8.pdf (accessed on 10 February 2021).
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. 1998. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31998L0083 (accessed on 10 February 2021).
- Nikolau, A.D.; Golfinopoulos, S.K.; Arhonditsis, G.B.; Kolo-voyiannis, V.; Lekkas, T.D. Modeling the formation of chlorination by-products in river waters with different quality. Chemosphere 2004, 55, 409–420. [Google Scholar] [CrossRef]
- Teksoy, A.; Alkan, U.; Başkaya, H.S. Influence of the treatment process combinations on the formation of THM species in water. Sep. Purif. Technol. 2008, 61, 447–454. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Ma, X.; Ni, J.; Zhang, H. THMs precursor removal by an integrated process of ozonation and biological granular activated carbon for typical Northern China water. Sep. Purif. Technol. 2010, 72, 263–268. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Luo, Z.; Huo, M.; Wu, J.; Huo, H.; Yang, W. Removing of disinfection-by-products precursors from surface water by using magnetic graphene oxide. PLoS ONE 2015, 10, e0143819. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, M.; Mazoua, S.; Berne, F.; Bodet, C.; Garrec, N.; Herbelin, P.; Menard-Szczebara, F.; Oberti, S.; Rodier, M.H.; Soreau, S.; et al. Efficiency of water disinfectants against Legionella pneumophila and Acanthamoeba. Water Res. 2011, 45, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mccann, C.; Hanrahan, J.; Jencson, A.; Joyce, D.; Fyffe, S.; Piesczynski, S.; Hawks, R.; Stout, J.E. Legionella control by chlorine dioxide in hospital water systems. J. Am. Water Work. Assoc. AWWA 2009, 101, 117–127. [Google Scholar] [CrossRef]
- Marchesi, I.; Marchegiano, P.; Bargellini, A.; Cencetti, S.; Frezza, G.; Miselli, M.; Borella, P. Effectiveness of different methods to control legionella in the water supply: Ten-year experience in an Italian university hospital. J. Hosp. Infect. 2011, 77, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Casini, B.; Buzzigoli, A.; Cristina, M.L.; Spagnolo, A.M.; DelGiudice, P.; Brusaferro, S.; Poscia, A.; Moscato, U.; Valentini, P.; Baggiani, A.; et al. Long-term effects of hospital water network disinfection on Legionella and other waterborne bacteria in an Italian University Hospital. Infect. Control Hosp. Epidemiol. 2014, 35, 293–299. [Google Scholar] [CrossRef]
- Chord, F.; Fascia, P.; Mallaval, F.; Cornillon, J.; Roesch, L.; Pozzetto, B.; Grattard, F.; Berthelot, P. Chlorine dioxide for Legionella spp. disinfection: A danger for cross-linked polyethylene pipes? J. Hosp. Infect. 2011, 78, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Azhdar, B.; Anderson, D.; Reitberger, T.; Hassinen, J.; Hjertberg, T.; Gedde, U.W. Deterioration of polyethylene pipes exposed to water containing chlorine dioxide. Polym. Degrad. Stabil. 2011, 96, 790–797. [Google Scholar] [CrossRef]
- Gan, W.; Huang, H.; Yang, X.; Peng, Z.; Chen, G. Emerging investigators series: Disinfection-by-products in mixed chlorine dioxide and chlorine water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 838. [Google Scholar] [CrossRef]
- World Health Organization. Monochloramine in Drinking-Water. 2004. Available online: https://www.who.int/water_sanitation_health/water-quality/guidelines/chemicals/chloramine-background.pdf (accessed on 10 February 2021).
- Alexander, M.T.; Waters, T.E.; Smith, G.A.; Wahman, D.G. New EPA Method to Determine Monochloramine Concentration in Drinking Water. In Proceedings of the 17th Annual EPA Drinking Water Workshop: Small System Challenges and Solutions, Covington, KY, USA, 1–3 September 2020. [Google Scholar]
- Coniglio, M.A.; Ferrante, M.; Yassin, M.H. Preventing Healthcare-Associated Legionellosis: Results after 3 Years of Continuous Disinfection of Hot Water with Monochloramine and an Effective Water Safety Plan. Int. J. Environ. Res. Public Health 2018, 15, 1594. [Google Scholar] [CrossRef] [PubMed]
- Jacangelo, J.G.; Olivieri, V.P.; Kawata, K. Investigating the mechanism of inactivation of Escherichia coli B by monochloramine. J. Am. Water Work. Assoc. 1991, 83, 80–87. [Google Scholar] [CrossRef]
- Buse, H.Y.; Morris, B.J.; Struewing, I.T.; Szaboa, J.G. Chlorine and Monochloramine Disinfection of Legionella pneumophila Colonizing Copper and Polyvinyl Chloride Drinking Water Biofilms. Appl. Environ. Microbiol. 2019, 85, 02956–03018. [Google Scholar] [CrossRef]
- Marchesi, I.; Paduano, S.; Frezza, G.; Sircana, L.; Vecchi, E.; Zuccarello, P.; Conti, G.; Ferrante, M.; Borella, P.; Bargellini, A. Safety and Effectiveness of Monochloramine Treatment for Disinfecting Hospital Water Networks. Int. J. Environ. Res. Public Health 2020, 17, 6116. [Google Scholar] [CrossRef]
- Schramm, A.; Beer, D.; Gieseke, A.; Amann, R. Microenvironments and distribution of nitrifying bacteria in a membrane-bound biofilm. Environ. Microbiol. 2000, 2, 680–686. [Google Scholar] [CrossRef]
- Lipponen, M.; Martikainen, P.; Vasara, R.; Servomaa, K.; Zacheus, O.; Kontro, M. Occurrence of nitrifiers and diversity of ammonia-oxidizing bacteria in developing drinking water biofilms. Water Res. 2004, 38, 4424–4434. [Google Scholar] [CrossRef]
- Lieu, N.; Wolfe, R.; Means, E., III. Optimizing Chloramine Disinfection for the Control of Nitrification. J. Am. Water Work. Assoc. 1993, 85, 84–90. [Google Scholar] [CrossRef]
- Nguyen, C.; Elfland, C.; Edwards, M. Impact of advanced water conservation features and new copper pipe on rapid chloramine decay and microbial regrowth. Water Res. 2012, 46, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Senevirathna, L.; Sathasivan, A.; Kastl, G. Forecasting the chloramine residual in service reservoirs from online measurement. Desalin. Water Treat. 2015, 57, 7943–7950. [Google Scholar] [CrossRef]
- McGuire, M.J.; Wu, X.; Blute, N.K.; Askenaizer, D.; Qin, G. Prevention of nitrification using chlorite iron: Results of a demonstration project in Glendale, Calif. J. Am. Water Work. Assoc. AWWA 2009, 101, 47–59. [Google Scholar] [CrossRef]
- Vikesland, P.; Ozekin, K.; Valentine, R. Effect of Natural Organic Matter on Monochloramine decomposition: Pathway Elucidation through the Use of Mass and Redox Balances. Environ. Sci. Technol. 1998, 32, 1409–1416. [Google Scholar] [CrossRef]
- Proctor, C.R.; Dai, D.; Edwards, M.A.; Pruden, A. Interactive effects of temperature, organic carbon, and pipe material on microbiota composition and Legionella pneumophila in hot water plumbing systems. Microbiome 2017, 5, 130. [Google Scholar] [CrossRef]
- Loveless, J.; Painter, H. The Influence of Metal Ion Concentrations and pH Value on the Growth of a Nitrosomonas Strain Isolated from Activated Sludge. Microbiology 1968, 52, 1–14. [Google Scholar] [CrossRef][Green Version]
- Wilczak, A. Chloramine Decay Rate: Factors and Research Needs. In Proceedings of the 2001 AWWA Annual Conference Proceedings, Washington, DC, USA, 17–21 June 2001. [Google Scholar]
- Domingue, E.L.; Tyndall, R.L.; Mayberry, W.R.; Pancorbo, O.C. Effects of three oxidizing biocides on Legionella pneumophila serogroup 1. Appl. Environ. Microbiol. 1988, 54, 741–747. [Google Scholar] [CrossRef] [PubMed]
- McGrane, W.K. Ozone, a study of the effects of biocides on Legionella pneumophila. Ind. Water Treat. 1995, 27, 28–32. [Google Scholar]
- Edelstein, P.H.; Whittaker, R.E.; Kreiling, R.L.; Howell, C.L. Efficacy of ozone in eradication of Legionella pneumophila from hospital plumbing fixtures. Appl. Environ. Microbiol. 1982, 44, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Blanc, D.S.; Carrara, P.; Zanetti, G.; Francioli, P. Water disinfection with ozone, copper and silver ions, and temperature increase to control Legionella: Seven years of experience in a university teaching hospital. J. Hosp. Infect. 2005, 60, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.; Utecht, K.U.; Exner, M.; Verstraete, W.; Rosenwinkel, K.H. Strategies for the reduction of Legionella in biological treatment systems. Water Sci. Technol. 2016, 4, 816–823. [Google Scholar] [CrossRef]
- Richardson, S.D.; Thruston, A.D., Jr.; Caughran, T.V.; Chen, P.H.; Collette, T.W.; Floyd, T.L.; Schenck, K.M.; Lykins, B.W., Jr.; Sun, G.R.; Majetich, G. Identification of new ozone disinfection by products in drinking water. Environ. Sci. Technol. 1999, 33, 3368–3377. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, X.; Yang, H.; Wang, H.; Xie, Y.F. Effects of ozonation on disinfection byproduct formation and speciation during subsequent chlorination. Chemosphere 2014, 117, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 528/2012 Concerning the Making Available on the Market and Use of Biocidal Products Evaluation of Active Substances Assessment. Report Hydrogen Peroxide. 2015. Available online: https://echa.europa.eu/documents/10162/cad256b7-8716-80f4-d091-c7bce0305d89 (accessed on 12 February 2021).
- USEPA. Alternative Disinfectants and Oxidants Guidance Manual. Office of Water 815-R-99-014. 1999. Available online: https://nepis.epa.gov/Exe/tiff2png.exe/2000229L.PNG?-r+75+-g+7+D%3A%5CZYFILES%5CINDEX%20DATA%5C95THRU99%5CTIFF%5C00001303%5C2000229L.TIF (accessed on 12 February 2021).
- Ditommaso, S.; Giacomuzzi, M.; Ricciardi, E.; Zotti, C.M. Efficacy of a Low Dose of Hydrogen Peroxide (Peroxy Ag+) for Continuous Treatment of Dental Unit Water Lines: Challenge Test with Legionella pneumophila Serogroup 1 in a Simulated Dental Unit Waterline. Int. J. Environ. Res. Public Health 2016, 13, 745. [Google Scholar] [CrossRef] [PubMed]
- Casini, B.; Aquino, F.; Totaro, M.; Miccoli, M.; Galli, I.; Manfredini, L.; Giustarini, C.; Costa, A.L.; Tuvo, B.; Valentini, P.; et al. Application of Hydrogen Peroxide as an Innovative Method of Treatment for Legionella Control in a Hospital Water Network. Pathogens 2017, 6, 15. [Google Scholar] [CrossRef]
- Landeen, L.K.; Moyasar, Y.; Gerba, C. Efficacy of copper and silver ions and reduced levels of free chlorine in inactivation of Legionella pneumophila. Appl. Environ. Microbiol. 1989, 55, 3045–3050. [Google Scholar] [CrossRef] [PubMed]
- Rohr, U.; Senger, M.; Selenka, F.; Turley, R.; Wilhelm, M. Four years of experience with silver-copper ionization for control of Legionella in German University Hospital hot water plumbing system. Clin. Infect. Dis. 1999, 29, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Vidic, R.D.; Stout, J.E.; Yu, V.L. Negative effect of high pH on biocidal efficacy of copper and silver ions in controlling Legionella pneumophila. Appl. Environ. Microbiol. 2002, 68, 2711–2715. [Google Scholar] [CrossRef]
- Pérez-Cachafeiro, S.; Mato, I.; Gonzàles Garcìa, I. Is copper-silver ionization safe and effective in controlling Legionella? J. Hosp. Infect. 2007, 67, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Andrews, S.A. Catalysis of copper corrosion products on chlorine decay and HAA formation in simulated distribution systems. Water Res. 2012, 46, 2665–2673. [Google Scholar] [CrossRef]
- Liu, Z.; Stout, J.E.; Tedesco, L.; Boldin, M.; Hwang, C.; Yu, V.L. Efficacy of ultraviolet light in preventing Legionella colonization of a hospital water distribution system. Water Res. 1995, 29, 2275–2280. [Google Scholar] [CrossRef]
- Kim, T.; Silva, J.L.; Chen, T.C. Effects of UV irradiation on selected pathogens in peptone water and on stainless steel and chicken meat. J. Food Prot. 2002, 65, 1142–1145. [Google Scholar] [CrossRef]
- Charpentier, X.; Kay, E.; Schneider, D.; Shuman, H.A. Antibiotics and UV radiation induce competence for natural transformation in Legionella pneumophila. J. Bacteriol. 2011, 193, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Vilhunen, S.; Sarkka, J.; Silanpaa, M. Ultraviolet Light-Emitting Diodes in Water Disinfection. Environ. Sci. Pollut. Res. 2009, 16, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Chevremont, A.C.; Farnet, A.M.; Coulomb, B.; Boudenne, J.L. Effect of Coupled UV-A and UV-C LEDs on Both Microbiological and Chemical Pollution of Urban Wastewaters. Sci. Total Environ. 2012, 426, 304–310. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R. Biofilms: Microbial Strategies for Surviving UV Exposure. In Ultraviolet Light in Human Health, Diseases and Environment. Advances in Experimental Medicine and Biology; Ahmad, S., Ed.; Springer: Cham, Switzerland, 2017; p. 996. [Google Scholar]
- Parkinson, J.; Baron, J.L.; Hall, B.; Bos, H.; Racine, P.; Wagener, M.M.; Stout, J.E. Point-of-use filters for prevention of health care—Acquired Legionnaires’ disease: Field evaluation of a new filter product and literature review. Am. J. Infect. Control 2020, 48, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S.L.; Ives, R.; Hoyt, A.; Taylor, R.; Rose, J.B. The use of copper and silver in carbon point-of-use filters for the suppression of Legionella throughput in domestic water systems. Appl. Microbiol. 2008, 104, 998–1007. [Google Scholar] [CrossRef]
- Wu, C.C.; Ghosh, S.; Martin, K.J.; Pinto, A.J.; Denef, V.J.; Olson, T.M.; Love, N.G. The microbial colonization of activated carbon block point-of-use (PoU) filters with and without chlorinated phenol disinfection by-products. Environ. Sci. Water Res. Technol. 2017, 3, 830–843. [Google Scholar] [CrossRef]
- Sheffer, P.J.; Stout, J.E.; Wagener, M.M.; Muder, R.R. Efficacy of new point-of-use water filter for preventing exposure to Legionella and waterborne bacteria. Am. J. Infect. Control 2005, 33, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.L.; Peters, T.; Shafer, R.; MacMurray, B.; Stout, J.E. Field evaluation of a new point-of-use faucet filter for preventing exposure to Legionella and other waterborne pathogens in health care facilities. Am. J. Infect. Control 2014, 42, 1193–1196. [Google Scholar] [CrossRef]
- Vonberg, R.P.; Sohr, D.; Bruderek, J.; Gastmeier, P. Impact of a silver layer on the membrane of tap water filters on the microbiological quality of filtered water. BMC Infect. Dis. 2008, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Noga, R.; Shanov, V.; Ryu, H.; Chandra, H.; Yadav, B.; Yadav, J.; Chae, S. Electrically heatable carbon nanotube point-of-use filters for effective separation and in-situ inactivation of Legionella pneumophila. Chem. Eng. J. 2019, 366, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Oyanedel-Craver, V.A.; Smith, J.A. Sustainable Colloidal-Silver-Impregnated Ceramic Filter for Point-of-Use Water Treatment. Environ. Sci. Technol. 2008, 42, 927–933. [Google Scholar] [CrossRef]
- Chaftar, N.; Girardot, M.; Quellard, N.; Labanowski, J.; Ghrairi, T.; Hani, K.; Frère, J.; Imbert, C. Activity of six essential oils extracted from tunisian plants against Legionella pneumophila. Chem. Biodivers. 2015, 12, 1565–1574. [Google Scholar] [CrossRef]
- Chang, C.W.; Chang, W.L.; Chang, S.T.; Cheng, S.S. Antibacterial activities of plant essential oils against Legionella pneumophila. Water Res. 2008, 42, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Chang, W.L.; Chang, S.T. Influence of pH on bioactivity of cinnamon oil against Legionella pneumophila and its disinfection efficacy in hot springs. Water Res. 2008, 42, 5022–5030. [Google Scholar] [CrossRef]
- Ceylan, O.; Turasay, B. Removing Legionella pneumophila and biofilms from water supply systems using plant essential oils. J. Water Sanit. Hyg. Dev. 2017, 7, 67–73. [Google Scholar] [CrossRef][Green Version]
- Mondello, F.; Girolamo, A.; Scaturro, M.; Ricci, M.L. Determination of Legionella pneumophila susceptibility to Melaleuca alternifolia Cheel (tea tree) oil by an improved broth micro-dilution method under vapour controlled conditions. J. Microbiol. Methods 2009, 77, 243–248. [Google Scholar] [CrossRef]
- Buchoux, S.; Lai-Kee-Him, J.; Garnier, M.; Tsan, P.; Besson, F.; Brisson, A.; Dufourc, E.J. Surfactin-Triggered Small Vesicle Formation of Negatively Charged Membranes: A Novel Membrane-Lysis Mechanism. Biophys J. 2008, 95, 3840–3849. [Google Scholar] [CrossRef]
- Loiseau, C.; Portier, E.; Corre, M.H.; Schlusselhuber, M.; Depayras, S.; Berjeaud, J.M.; Verdon, J. Highlighting the Potency of Biosurfactants Produced by Pseudomonas Strains as Anti-Legionella Agents. Biomed. Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Mora, I.; Cabrefiga, J.; Montesinos, E. Cyclic lipopeptide biosynthetic genes and products, and inhibitory activity of plant-associated Bacillus against phytopathogenic bacteria. PLoS ONE 2015, 10, e0127738. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, C.; Schlusselhuber, M.; Bigot, R.; Bertaux, J.; Berjeaud, J.M.; Verdon, J. Surfactin from Bacillus subtilis displays an unexpected anti-Legionella activity. Appl. Microbiol. Biotechnol. 2015, 99, 5083–5093. [Google Scholar] [CrossRef] [PubMed]
- Fuochi, V.; Coniglio, M.A.; Laghi, L.; Rescifina, A.; Caruso, M.; Stivala, A.; Furneri, P.M. Metabolic Characterization of Supernatants Produced by Lactobacillus spp. With in vitro Anti-Legionella Activity. Front. Microbiol. 2019, 10, 1403. [Google Scholar] [CrossRef]
- Cavallaro, A.; Taheri, S.; Vasilev, K. Responsive and “smart” antibacterial surfaces: Common approaches and new developments. Biointerphases 2014, 9, 029005. [Google Scholar] [CrossRef]
- Susarrey-Arce, A.; Sorzabal-Bellido, I.; Oknianska, A.; McBride, F.; Beckett, A.J.; Gardeniers, J.G.E.; Raval, R.; Tiggelaar, R.M.; Diaz Fernandez, Y.A. Bacterial viability on chemically modified silicon nanowire arrays. J. Mater. Chem. B 2016, 4, 3104–3112. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, E.L.; Filice, S.; Coniglio, M.A.; Faro, G.; Gradon, L.; Galati, C.; Spinella, N.; Libertino, S.; Scalese, S. Antimicrobial s-PBC Coatings for Innovative Multifunctional Water Filters. Molecules 2020, 25, 5196. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Sampling Procedure and Potential Sampling Sites. Protocol for Collecting Environmental Samples for Legionella Culture during a Cluster or Outbreak Investigation or When Cases of Disease May Ba Associated with a Facility. 2015. Available online: https://www.cdc.gov/legionella/downloads/cdc-sampling-procedure.pdf (accessed on 12 February 2021).
- U.K. Environmental Agency. The Determination of Legionella Bacteria in Waters and Other Environmental Samples—Part 1—Rationale of Surveying and Sampling—Methods for the Examination of Waters and Associated Materials. 2005. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/316814/book_200_1028650.pdf (accessed on 12 February 2021).
- Bartram, J.; Chartier, Y.; Lee, J.V.; Pond, K.; Surman-Lee, S. Legionella and the Prevention of Legionellosis; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Delia, S.; Laganà, P.; Minutoli, E. Occurrence of Legionella in beach shower facilities. J. Prev. Med. Hyg. 2007, 48, 114–117. [Google Scholar] [PubMed]
- Laganà, P.; Gambuzza, M.E.; Delia, S. Legionella risk assessment in cruise ships and ferries. Ann. Agric. Environ. Med. 2017, 24, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Coniglio, M.A.; Andolfi, N.; Faro, G.; Pellegrino, M.B.; Sgalambro, A.; D’Aquila, G.; Spina, A.; Melada, S. Continuous Disinfection by Monochloramine on Domestic Hot Water System of Health-care Facilities for the Control of Legionella Contamination in Italy. J. Health Sci. 2015, 3, 11–17. [Google Scholar]
- Coniglio, M.A.; Strano, V.; D’Angelo, S.; Guercio, M.A.; Spada, R.; Melada, S. Effectiveness of in-Situ Generated Monochloramine for the Control of Legionella in a Real Industrial Cooling Tower. Glob. J. Med. Res. K Interdiscip. 2015, 15, 9–15. [Google Scholar]
- Coniglio, M.A. Evaluation of the Effectiveness of a 4-Months Continuous Injection of a Gas Mixture (CO2 and Inert Gases) on Legionella Contamination of a Hot Water Distribution System. Health 2015, 7, 819–823. [Google Scholar] [CrossRef][Green Version]
- Kool, J.L.; Bergmire-Sweat, D.; Butler, J.C.; Brown, E.W.; Peabody, D.J.; Massi, D.S.; Carpenter, J.C.; Pruckler, J.M.; Benson, R.F.; Fields, B.S. Hospital characteristics associated with colonization of water systems by Legionella and risk of nosocomial legionnaires’ disease: A cohort study of 15 hospitals. Infect. Control Hosp. Epidemiol. 1999, 20, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Bentham, R. Risk assessment for Legionella in building water systems. Environ. Health 2003, 3, 20. [Google Scholar]
- Napoli, C.; Iatta, R.; Fasano, F.; Marsico, T.; Montagna, M.T. Variable bacterial load of Legionella spp. in a hospital water system. Sci. Total Environ. 2009, 408, 242–244. [Google Scholar] [CrossRef]
- Casini, B.; Baggiani, A.; Totaro, M.; Mansi, A.; Costa, A.L.; Aquino, F.; Miccoli, M.; Valentini, P.; Bruschi, F.; Lopalco, P.L.; et al. Detection of viable but non-culturable legionella in hospital water network following monochloramine disinfection. J. Hosp. Infect. 2018, 98, 46–52. [Google Scholar] [CrossRef]
- Ducret, A.; Chabalier, M.; Dukan, S. Characterization and resuscitation of ‘non-culturable’ cells of Legionella pneumophila. BMC Microbiol. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Dietersdorfer, E.; Kirschner, A.; Schrammel, B.; Ohradanova-Repic, A.; Stockinger, H.; Sommer, R.; Walochnik, J.; Cervero-Aragó, S. Starved viable but non-culturable (VBNC) Legionella strains can infect and replicate in amoebae and human macrophages. Water Res. 2018, 141, 428–438. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Tudela, J.A.; Allende, A.; Gil, M.I. Microbial and chemical characterization of commercial washing lines of fresh produce highlights the need for process water control. Innov. Food Sci. Emerg. Technol. 2019, 51, 211–219. [Google Scholar] [CrossRef]
- Kirschner, A.K.T. Determination of viable legionellae in engineered water systems: Do we find what we are looking for? Water Res. 2016, 93, 276–288. [Google Scholar] [CrossRef]
- Baudart, J.; Guillaume, C.; Mercier, A.; Lebaron, P.; Binet, M. Rapid quantification of viable Legionella in nuclear cooling tower waters using filter cultivation, fluorescent in situ hybridization and solidphase cytometry. J. Appl. Microbiol. 2015, 118, 1238–1249. [Google Scholar] [CrossRef][Green Version]
- Allegra, S.; Girardot, F.; Grattard, F.; Berthelot, P.; Helbig, J.H.; Pozzetto, B.; Riffard, S. Evaluation of an immunomagnetic separation assay in combination with cultivation to improve Legionella pneumophila serogroup 1 recovery from environmental samples. J. Appl. Microbiol. 2011, 110, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.; Santos, M.A.; Chambel, L. Thirty years of viable but nonculturable state research: Unsolved molecular mechanisms. Crit. Rev. Microbiol. 2015, 41, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Strauber, H.; Muller, S. Viability states of bacteria–specific mechanisms of selected probes. Cytom. A 2010, 77, 623–634. [Google Scholar] [CrossRef]
- Stocks, S.M. Mechanism and use of the commercially available viability stain, BacLight. Cytom. A 2004, 61, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Jepras, R.I.; Carter, J.; Pearson, S.C.; Paul, F.E.; Wilkinson, M.J. Development of a robust flow cytometric assay for determining numbers of viable bacteria. Appl. Environ. Microbiol. 1995, 61, 2696–2701. [Google Scholar] [CrossRef]
- Tholozan, J.L.; Cappelier, J.M.; Tissier, J.P.; Delattre, G.; Federighi, M. Physiological Characterization of Viable-but-Nonculturable Campylobacter jejuni Cells. Appl. Environ. Microbiol. 1999, 65, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C. Tips and tricks for high quality MAR-FISH preparations: Focus on bacterioplankton analysis. Syst. Appl. Microbiol. 2012, 35, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.P.; Rottenberg, H.; Caplan, S.R. An estimation of the light-induced electrochemical potential difference of protons across the membrane of Halobacterium halobium. Biochim. Biophys. Acta 1976, 440, 557–572. [Google Scholar] [CrossRef]
- Wang, Y.; Claeys, L.; van der Ha, D.; Verstraete, W.; Boon, N. Effects of chemically and electrochemically dosed chlorine on Escherichia coli and Legionella beliardensis assessed by flow cytometry. Appl. Microbiol. Biotechnol. 2010, 87, 331–334. [Google Scholar] [CrossRef]
- Su, X.; Sun, F.; Wang, Y.; Hashmi, M.Z.; Guo, L.; Ding, L.; Shen, C. Identification, characterization and molecular analysis of the viable but nonculturable Rhodococcus biphenylivorans. Sci. Rep. 2015, 5, 18590. [Google Scholar] [CrossRef]
- Kirschner, A.K.; Rameder, A.; Schrammel, B.; Indra, A.; Farnleitner, A.H.; Sommer, R. Development of a new CARD-FISH protocol for quantification of Legionella pneumophila and its application in two hospital cooling towers. J. Appl. Microbiol. 2012, 112, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Karner, M.; Fuhrman, J.A. Determination of active Marine bacterioplankton: A comparison of universal 16S rRNA probes, autoradiography, and nucleoid staining. Appl. Environ. Microbiol. 1997, 63, 1208–1213. [Google Scholar] [CrossRef]
- Moreno, Y.; Moreno-Mesonero, L.; García-Hernández, J. DVC-FISH to identify potentially pathogenic Legionella inside free-living amoebae from water sources. Environ. Res. 2019, 176, 108521. [Google Scholar] [CrossRef]
- Delgado-Viscogliosi, P.; Solignac, L.; Delattre, J. Viability PCR, a Culture-Independent Method for Rapid and Selective Quantification of Viable Legionella pneumophila Cells in Environmental Water Samples. Appl. Environ. Microbiol. 2009, 75, 3502–3512. [Google Scholar] [CrossRef]
- Boss, R.; Baumgartner, A.; Kroos, S.; Blattner, M.; Fretz, R.; Moor, D. Rapid detection of viable Legionella pneumophila in tap water by a qPCR and RT-PCR-based method. J. Appl. Microbiol. 2018, 125, 1216–1225. [Google Scholar] [CrossRef]
- Dusserre, E.; Ginevra, C.; Hallier-Soulier, S.; Vandenesch, F.; Festoc, G.; Etienne, J.; Jarraud, S.; Molmeret, M. A PCR-Based Method for Monitoring Legionella pneumophila in Water Samples Detects Viable but Noncultivable Legionellae That Can Recover Their Cultivability. Appl. Environ. Microbiol. 2008, 74, 4817–4824. [Google Scholar] [CrossRef]
- Truchado, P.; Gil, M.I.; Larrosa, M.; Allende, A. Detection and Quantification Methods for Viable but Non-culturable (VBNC) Cells in Process Wash Water of Fresh-Cut Produce: Industrial Validation. Front. Microbiol. 2020, 11, 673. [Google Scholar] [CrossRef]
- Zhao, L.; Lv, X.; Cao, X.; Zhang, J.; Gu, X.; Zeng, H.; Wang, L. Improved quantitative detection of VBNC Vibrio parahaemolyticus using immunomagnetic separation and PMAxx-qPCR. Food Control 2020, 110, 106962. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Huertas, C.S.; Calvo-Lozano, O.; Mitchell, A.; Lechuga, L.M. Advanced Evanescent-Wave Optical Biosensors for the Detection of Nucleic Acids: An Analytic Perspective. Front. Chem. 2019, 7, 724. [Google Scholar] [CrossRef]
- Pardoux, É.; Boturyn, D.; Roupioz, Y. Antimicrobial Peptides as Probes in Biosensors Detecting Whole Bacteria: A Review. Molecules 2020, 25, 1998. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Zhang, S.; Link, A.J.; McAlpine, M.C. Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2010, 107, 19207–19212. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Santangelo, M.F.; Villaggio, G.; Sinatra, F.; Bongiorno, C.; Nicotra, G.; Libertino, S. Photo-physical characterization of fluorophore Ru(bpy)32+ for optical biosensing applications. Sens. Biosens. Res. 2015, 6, 65–71. [Google Scholar]
- Sciuto, E.L.; Villaggio, G.; Santangelo, M.F.; Laudani, S.; Federico, C.; Saccone, S.; Sinatra, F.; Libertino, S. Study of a miniaturizable system for optical sensing application to human cells. Appl. Sci. 2019, 9, 975. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Petralia, S.; Calabrese, G.; Conoci, S. An integrated biosensor platform for extraction and detection of nucleic acids. Biotechnol. Bioeng. 2020, 117, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, M.F.; Pagano, R.; Lombardo, S.; Sciuto, E.; Sanfilippo, D.; Fallica, G.; Sinatra, F.; Libertino, S. Silicon photomultipliers applications to biosensors. In Proceedings of the Silicon Photonics IX, San Francisco, CA, USA, 3–5 February 2014. [Google Scholar]
- Petralia, S.; Sciuto, E.L.; Mirabella, S.; Di Pietro, M.L.; Zimbone, M.; Conoci, S. An Innovative Chemical Strategy for PCR-free Genetic Detection of Pathogens by an Integrated Electrochemical Biosensor. Analyst 2017, 142, 2090–2093. [Google Scholar] [CrossRef]
- Petralia, S.; Sciuto, E.L.; Messina, M.A.; Mirabella, S.; Scandurra, A.; Priolo, F.; Conoci, S. Miniaturized and Multi-Purpose Electrochemical Sensing Device based on thin Ni Oxides. Sens. Actuators B Chem. 2018, 263, 10–19. [Google Scholar] [CrossRef]
- Massad-Ivanir, N.; Shtenberg, G.; Raz, N.; Gazenbeek, C.; Budding, D.; Bos, M.P.; Segal, E. Porous Silicon-Based Biosensors: Towards Real-Time Optical Detection of Target Bacteria in the Food Industry. Sci. Rep. 2016, 6, 38099. [Google Scholar] [CrossRef]
- Bartram, J.; Corrales, L.; Davison, A.; Deere, D.; Drury, D.; Gordon, B.; Howard, G.; Rinehold, A.; Stevens, M. Water Safety Plan Manual: Step-by-Step Risk Management for Drinking-Water Suppliers; World Health Organization: Geneva, Switzerland, 2009; Available online: https://apps.who.int/iris/bitstream/handle/10665/75141/9789241562638_eng.pdf?sequence=1&isAllowed=y (accessed on 12 February 2021).
- ASHRAE. Legionellosis: Risk Management for Building Water Systems; ASHRAE Standard 188; ASHRAE: Atlanta, GA, USA, 2015; Available online: https://www.ashrae.org/technical-resources/bookstore/ansi-ashrae-standard-188-2018-legionellosis-risk-management-for-building-water-systems (accessed on 12 February 2021).
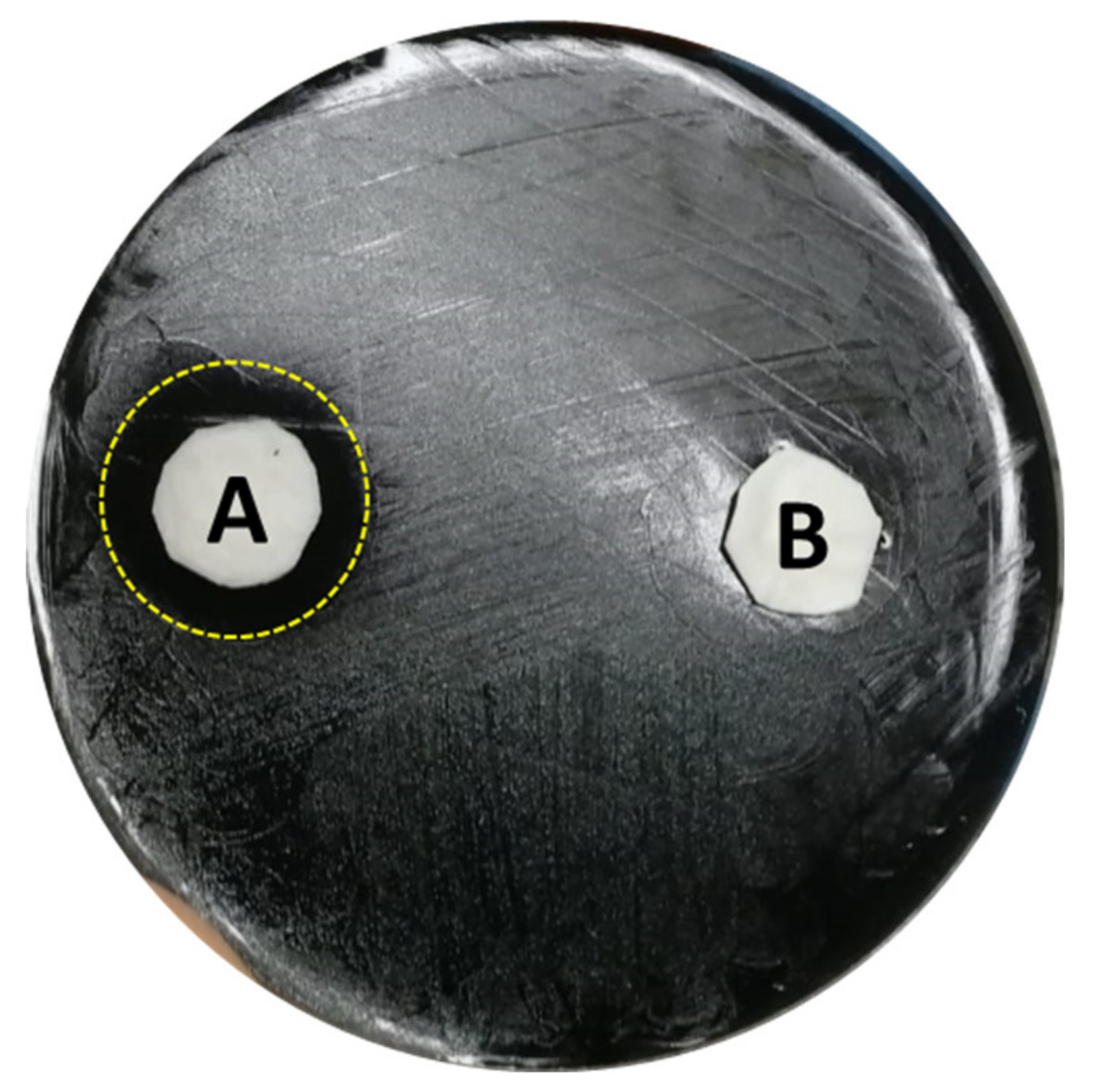
| Type of Facility | Total Samples | Positives (%) | SG 2-16 (%) |
|---|---|---|---|
| Hospitals | 2610 | 1193 (45.7) | 823 (69) |
| Marine | 1133 | 246 (21.7) | 184 (74.8) |
| Touristic | 582 | 146 (25.2) | 83 (56.8) |
| Corporate | 196 | 69 (35.2) | 61 (88.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciuto, E.L.; Laganà, P.; Filice, S.; Scalese, S.; Libertino, S.; Corso, D.; Faro, G.; Coniglio, M.A. Environmental Management of Legionella in Domestic Water Systems: Consolidated and Innovative Approaches for Disinfection Methods and Risk Assessment. Microorganisms 2021, 9, 577. https://doi.org/10.3390/microorganisms9030577
Sciuto EL, Laganà P, Filice S, Scalese S, Libertino S, Corso D, Faro G, Coniglio MA. Environmental Management of Legionella in Domestic Water Systems: Consolidated and Innovative Approaches for Disinfection Methods and Risk Assessment. Microorganisms. 2021; 9(3):577. https://doi.org/10.3390/microorganisms9030577
Chicago/Turabian StyleSciuto, Emanuele Luigi, Pasqualina Laganà, Simona Filice, Silvia Scalese, Sebania Libertino, Domenico Corso, Giuseppina Faro, and Maria Anna Coniglio. 2021. "Environmental Management of Legionella in Domestic Water Systems: Consolidated and Innovative Approaches for Disinfection Methods and Risk Assessment" Microorganisms 9, no. 3: 577. https://doi.org/10.3390/microorganisms9030577
APA StyleSciuto, E. L., Laganà, P., Filice, S., Scalese, S., Libertino, S., Corso, D., Faro, G., & Coniglio, M. A. (2021). Environmental Management of Legionella in Domestic Water Systems: Consolidated and Innovative Approaches for Disinfection Methods and Risk Assessment. Microorganisms, 9(3), 577. https://doi.org/10.3390/microorganisms9030577










