Rapid Testing and Interventions to Control Legionella Proliferation following a Legionnaires’ Disease Outbreak Associated with Cooling Towers
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Culture
3.2. qPCR
3.2.1. Legionella spp. qPCR Results
3.2.2. Legionella pneumophila qPCR Results
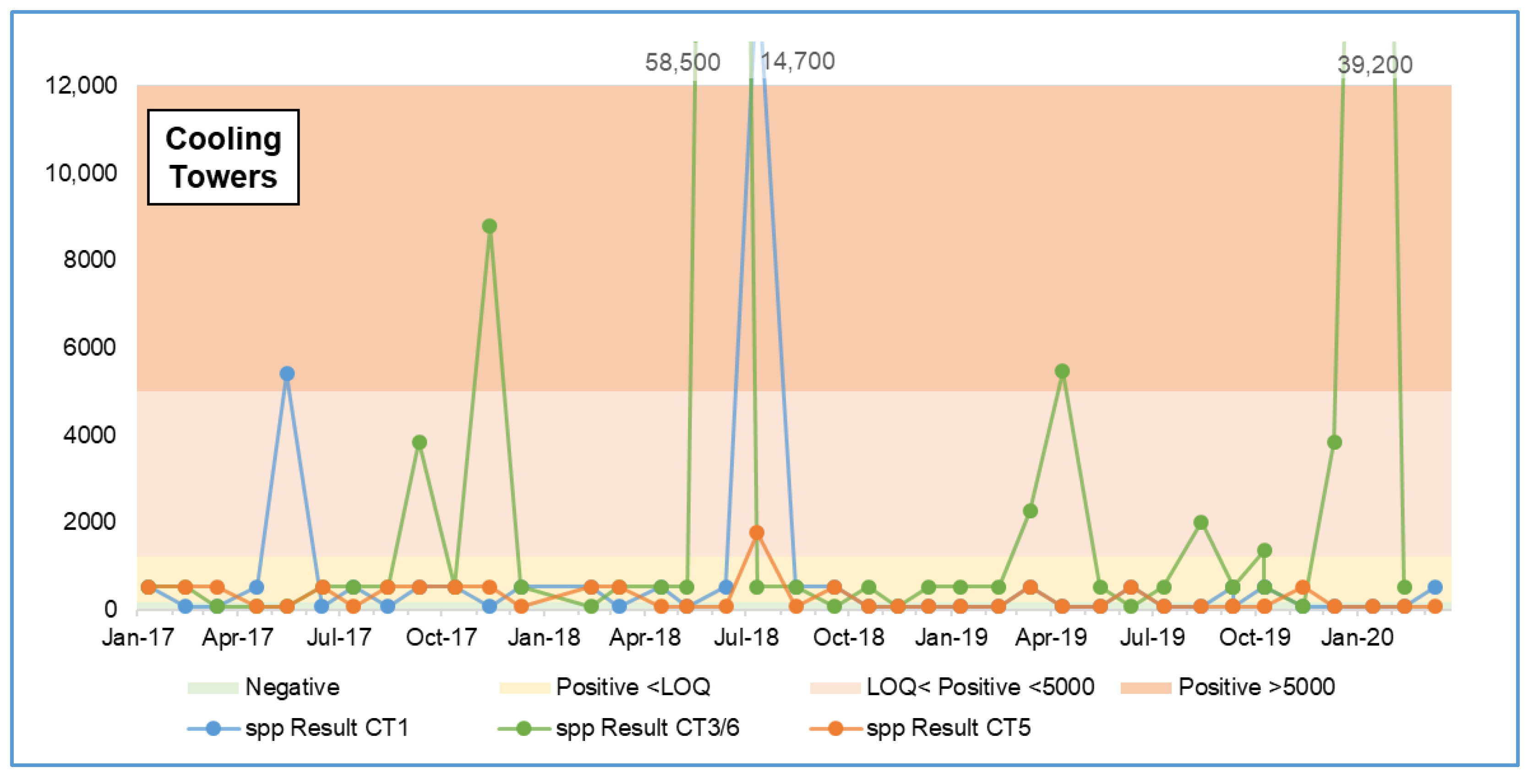
3.2.3. July 2018 Results Spike
3.2.4. March 2020 Results Spike
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glick, T.H.; Gregg, M.B.; Berman, B.; Mallison, G.; Rhodes, W.W., Jr.; Kassanoff, I. Pontiac Fever: An epidemic of unknown aetiology in a health department: I. Clinical and epidemiologic aspects. Am. J. Epidemiol. 1978, 107, 149–160. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Wrench, J.G.; Collier, P.W.; Emslie, J.A.; Fallon, R.J.; Forbes, G.I.; McKay, T.M.; Macpherson, A.C.; Markwick, T.A.; Reid, D. Lochgoilhead fever: Outbreak of non-pneumonic legionellosis due to Legionella micdadei. Lancet 1989, 1, 316–318. [Google Scholar] [CrossRef]
- McDade, J.E.; Shepard, C.C.; Fraser, D.W.; Tsai, T.R.; Redus, M.A.; Dowdle, W.R. Legionnaires’ disease: Isolation of a bacterium and demonstration of its role in other respiratory disease. N. Engl. J. Med. 1977, 297, 1197–1203. [Google Scholar] [CrossRef]
- Report of the Public Meetings into the Legionella Outbreak in Barrow-in-Furness, August 2002; Health and Safety Executive (HSE): Liverpool, UK, 2007.
- Völker, S.; Schreiber, C.; Kistemann, T. Modelling characteristics to predict Legionella contamination risk—Surveillance of drinking water plumbing systems and identification of risk areas. Int. J. Hyg. Environ. Health 2016, 219, 101–109. [Google Scholar] [CrossRef]
- Tsao, H.F.; Scheikl, U.; Herbold, C.; Indra, A.; Walochnik, J.; Horn, M. The cooling tower water microbiota: Seasonal dynamics and co-occurrence of bacterial and protist phylotypes. Water Res. 2019, 159, 464–479. [Google Scholar] [CrossRef]
- L8. Legionnaires’ disease—The control of legionella bacteria in water systems. In Approved Code of Practice and Guidance on Regulations, 4th ed.; HSE: Liverpool, UK, 2013. [Google Scholar]
- Legionella and the Prevention of Legionellosis; World Health Organisation (WHO): Geneva, Switzerland, 2007.
- Skaliy, P.; Thompson, T.A.; Gorman, G.W.; Morris, G.K.; McEachern, H.V.; Mackel, D.C. Laboratory studies of disinfectants against Legionella pneumophila. Appl. Environ. Microbiol. 1980, 40, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Crook, B.; Willerton, L.; Smith, D.; Wilson, L.; Poran, V.; Helps, J.; McDermott, P. Legionella risk in evaporative cooling systems and underlying causes of associated breaches in health and safety compliance. Int. J. Hyg. Environ. Health 2020, 224, 113425. [Google Scholar] [CrossRef]
- Sabria, M.; Alvarez, A.; Dominguez, A.; Pedrol, A.; Sauca, G.; Salleras, L.; Lopez, A.; Garcia-Nuñez, I.; Parron, I.; Barrufet, M.P. A community outbreak of Legionnaires’ disease: Evidence of a cooling tower as the source. Clin. Microbiol. Infect. 2006, 12, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Ferré, M.R.; Arias, C.; Oliva, J.M.; Pedrol, A.; García, M.; Pellicer, T.; Roura, P.; Domínguez, A. A community outbreak of Legionnaires’ disease associated with a cooling tower in Vic and Gurb, Catalonia (Spain) in 2005. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Ilef, D.; Jarraud, S.; Rouil, L.; Campese, C.; Che, D.; Haeghebaert, S.; Ganiayre, F.; Marcel, F.; Etienne, J.; et al. A community-wide outbreak of legionnaires disease linked to industrial cooling towers—How far can contaminated aerosols spread? J. Infect. Dis. 2016, 193, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, Á.; Montero, J.C.; Castro, F.J.; Alejandres, E.M.; Bayón, C.; Solís, I.; Fernández-Lafuente, R.; Rodríguez, G. Comparing methods of determining Legionella spp. in complex water matrices. BMC Microbiol. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.; Shaheed, R.A.; Al-Ali, H.H.; Al-Ghamdi, A.S.; Al-Hamaqi, G.M.; Maan, H.S.; Al-Mahfoodh, Z.A.; Al-Seba, H.Z. Legionella confirmation in cooling tower water—Comparison of culture, real-time PCR and next generation sequencing. SMJ 2018, 39, 137–141. [Google Scholar] [CrossRef]
- Yaradou, D.F.; Hallier-Soulier, S.; Moreau, S.; Poty, F.; Hillion, Y.; Reyrolle, M.; André, J.; Festoc, G.; Delabre, K.; Vandenesch, J.E.; et al. Integrated Real-Time PCR for Detection and Monitoring of Legionella pneumophila in Water Systems. Appl. Environ. Microbiol. 2007, 73, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Yamamoto, H.; Arima, K.; Fujii, J.; Maruta, K.; Izu, K.; Shiomori, T.; Yoshida, S.-I. Development of a New Seminested PCR Method for Detection of Legionella Species and Its Application to Surveillance of Legionellae in Hospital Cooling Tower Water. Appl. Environ. Microbiol. 1997, 63, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Falconnet, P.-A.; André, J.; Weill, N.; Reyrolle, M.; Vandenesch, F.; Maurin, M.; Etienne, J.; Jarraud, S. Quantitative Real-Time Legionella PCR for Environmental Water Samples: Data Interpretation. Appl. Environ. Microbiol. 2006, 74, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Krøjgaard, L.H.; Krogfelt, K.A.; Albrechtsen, H.-J.; Uldum, S.A. Detection of Legionella by quantitative-polymerase chain reaction (qPCR) for monitoring and risk assessment. BMC Microbiol. 2011, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Touron-Bodilis, A.; Pougnard, C.; Frenkiel-Lebossé, H.; Hallier-Soulier, S. Usefulness of real-time PCR as a complementary tool to the monitoring of Legionella spp. and Legionella pneumophila by culture in industrial cooling systems. J. Appl. Microbiol. 2011, 111, 499–510. [Google Scholar] [CrossRef]
- ISO11731:2017. Water Quality—Enumeration of Legionella; International Standards Organisation (ISO): Geneva, Switzerland, 2017.
- ISO/TS12869:2019. Water Quality—Detection and Quantification of Legionella spp. and/or Legionella Pneumophila by Concentration and Genic Amplification and Quantitative Polymerase Chain Reaction (qPCR); ISO: Geneva, Switzerland, 2019.
- HSG274 Part 1. Legionnaires’ Disease: Technical Guidance: The Control of Legionella Bacteria in Evaporative Cooling Systems; HSE: Liverpool, UK, 2013.
- European Technical Guidelines for the Prevention, Control and Investigation, of Infections Caused by Legionella Species; European Centre for Disease Prevention and Control (ECDC): Solna, Sweden, 2017.
- Llewellyn, A.C.; Lucas, C.E.; Roberts, S.E.; Brown, E.W.; Nayak, B.S.; Raphael, B.H.; Winchell, J.M. Distribution of Legionella and bacterial community composition among regionally diverse US cooling towers. PLoS ONE 2017, 12, e018993. [Google Scholar] [CrossRef]
- Lee, J.V.; Lai, S.; Exner, M.; Lenz, J.; Gaia, V.; Casati, S.; Hartemann, P.; Lück, C.; Pangon, B.; Ricci, M.L.; et al. An international trial of quantitative PCR for monitoring Legionella in artificial water systems. J. Appl. Microbiol. 2011, 110, 1032–1044. [Google Scholar] [CrossRef]
- Whiley, H.; Taylor, M. Legionella detection by culture and qPCR: Comparing apples and oranges. Crit. Rev. Microbiol. 2016, 42, 65–71. [Google Scholar] [CrossRef]
- Collins, S.; Stevenson, J.; Walker, J.; Bennett, A. Evaluation of Legionella real-time PCR against traditional culture for routine and public health testing of water samples. J. Appl. Microbiol. 2017, 122, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Levi, K.; Smedley, J.; Towner, K.J. Evaluation of a real-time PCR hybridization assay for rapid detection of Legionella pneumophila in hospital and environmental water samples. Clin. Microbiol. Infect. 2003, 9, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Jorgensen, F.; Willis, C.; Walker, J. Real-time PCR to supplement gold-standard culture-based detection of Legionella in environmental samples. J. Appl. Microbiol. 2015, 119, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- BS 7592:2008. Sampling for Legionella Bacteria in Water Systems. Code of Practice; British Standards Institute: London, UK, 2008.
- Shih, H.-Y.; Lin, Y.E. Caution on interpretation of Legionella results obtained using real-time PCR for environmental water samples. Appl. Environ. Microbiol. 2006, 72, 6859. [Google Scholar] [CrossRef] [PubMed]
- Opinion of the French Agency for Food, Environmental and Occupational Health and Safety on “Methods of Detection and Enumeration of Legionella in Water”; ANSES—Request No. 2009-SA-330; Agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail (ANSES): Paris, France, 2011.
- MD-15161-2013. Control of Legionella in Mechanical Systems. Standard for Building Owners, Design Professionals, and Maintenance Personnel; Public Works and Government Services Canada (PWGCS): Quebec City, QC, Canada, March 2016.



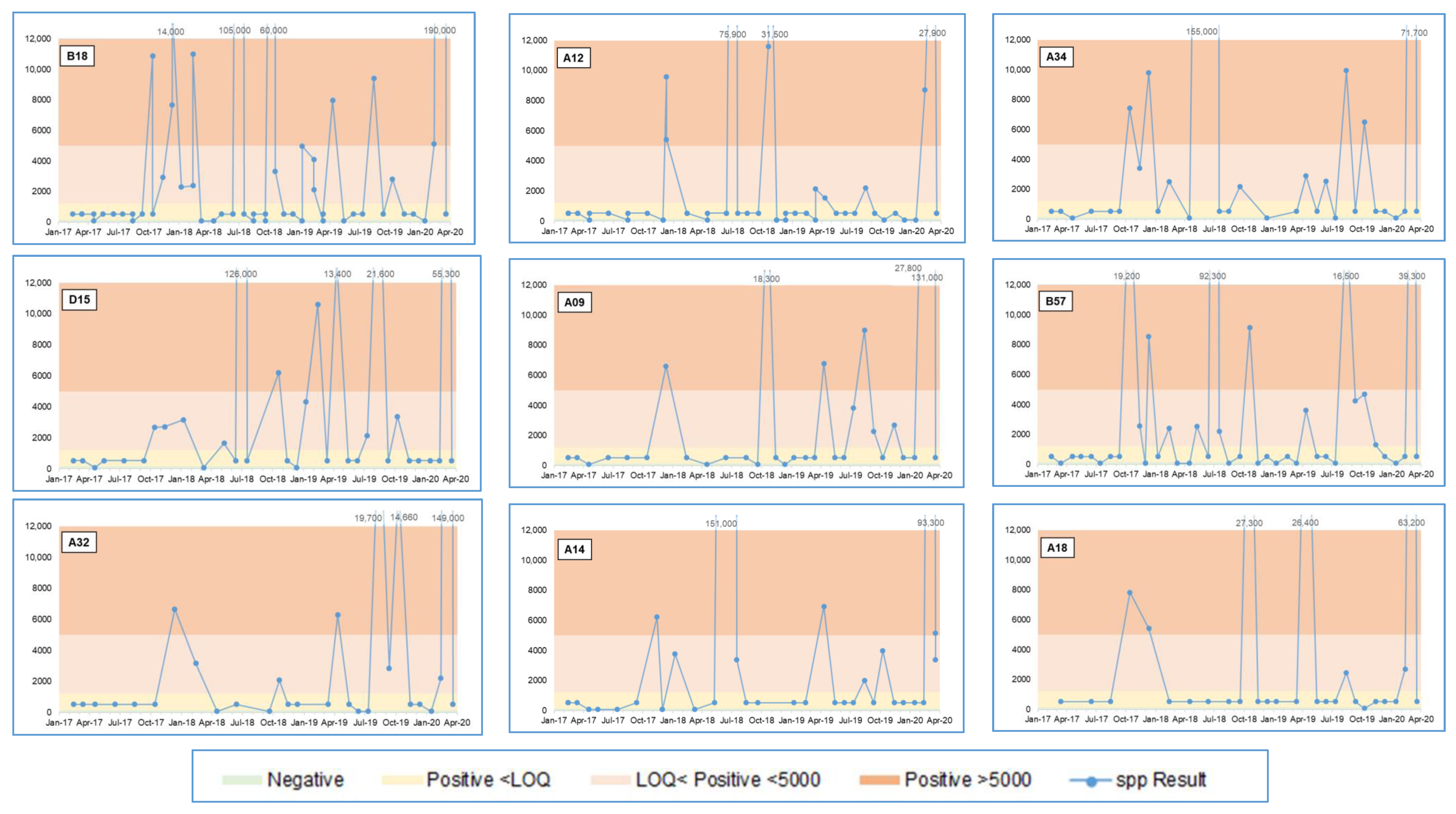
| Sample Location | Factory | Negative | Positive <LOQ | Positive <5000 | Positive >5000 | Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |||
| B18 | Factory 2 | 11 | 19% | 29 | 50% | 8 | 14% | 10 | 17% | 58 |
| A12 | Factory 2 | 12 | 24% | 29 | 57% | 3 | 6% | 7 | 14% | 51 |
| A34 | Factory 2 | 5 | 16% | 15 | 48% | 5 | 16% | 6 | 19% | 31 |
| D15 | Factory 2 | 3 | 9% | 17 | 52% | 7 | 21% | 6 | 18% | 33 |
| A09 | Factory 2 | 4 | 13% | 18 | 58% | 3 | 10% | 6 | 19% | 31 |
| B57 | Factory 2 | 13 | 28% | 19 | 41% | 8 | 17% | 6 | 13% | 46 |
| A32 | Factory 2 | 5 | 18% | 14 | 50% | 4 | 14% | 5 | 18% | 28 |
| A14 | Factory 2 | 7 | 21% | 17 | 50% | 5 | 15% | 5 | 15% | 34 |
| A18 | Factory 2 | 1 | 4% | 20 | 71% | 2 | 7% | 5 | 18% | 28 |
| Sample Location | Factory | Negative | Positive <LOQ | Positive <5000 | Positive >5000 | Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |||
| CT1 | Factory 1 | 21 | 53% | 17 | 43% | - | 0% | 2 | 5% | 40 |
| CT5 | Factory 1 | 27 | 64% | 14 | 33% | 1 | 2% | - | 0% | 42 |
| CT3/6 | Factory 2 | 8 | 21% | 22 | 56% | 5 | 13% | 4 | 10% | 39 |
| Sample Location | Test Month before | Result before | Month High | High Result | Test Month after | Result after |
|---|---|---|---|---|---|---|
| A01 | 9 May 2018 | Positive <LOQ | 11 July 2018 | 258,000 | 17 July 2018 | 1433 |
| A08 | 9 May 2018 | Positive <LOQ | 11 July 2018 | 113,000 | 17 July 2018 | Positive <LOQ |
| A08 | 12 February 2020 | 5715 | 11 March 2020 | 476,000 | 19 March 2020 | Positive <LOQ |
| A09 | 12 February 2020 | 27,800 | 11 March 2020 | 131,000 | 19 March 2020 | Positive <LOQ |
| A14 | 9 May 2018 | Positive <LOQ | 11 July 2018 | 151,000 | 17 July 2018 | 3374 |
| A15 | 14 August 2017 | Negative | 13 October 2018 | 219,000 | 11 December 2017 | 1918 |
| A16 | 9 May 2018 | Positive <LOQ | 11 July 2018 | 113,000 | 17 July 2018 | Positive <LOQ |
| A32 | 12 February 2020 | 2208 | 11 March 2020 | 149,000 | 19 March 2020 | Positive <LOQ |
| A34 | 14 April 2018 | Negative | 11 July 2018 | 155,000 | 17 July 2018 | 1148 |
| B18 | 13 June 2018 | Positive <LOQ | 11 July 2018 | 105,000 | 17 July 2018 | 1185 |
| B18 | 12 February 2020 | 5114 | 11 March 2020 | 190,000 | 19 March 2020 | Positive <LOQ |
| D12 | 13 June 2018 | Positive <LOQ | 11 July 2018 | 139,000 | 17 July 2018 | Positive <LOQ |
| D15 | 13 June 2018 | Positive <LOQ | 11 July 2018 | 126,000 | 17 July 2018 | Positive <LOQ |
| Test Date | Factory | Sample Location | Legionella spp. Result (GU/L) | Legionella pneumophila Result (GU/L) |
|---|---|---|---|---|
| 14 September 2017 | 1 | B22 | 9427 | 3614 |
| 11 July 2018 | 5 | A01 | 258,000 | 142,700 |
| 11 July 2018 | 2 | A14 | 151,000 | 4021 |
| 11 July 2018 | 2 | A34 | 155,000 | 11,790 |
| 11 July 2018 | 1 | B31 | 11,300 | 6962 |
| 11 July 2018 | 1 | B37 | 2819 | 1738 |
| 11 July 2018 | 1 | B62 | 5701 | 4188 |
| 11 July 2018 | 1 | CT1 | 14,700 | 1780 |
| Source | Parameter | Alert Level (GU/L) | Action Level (GU/L) |
|---|---|---|---|
| Lee et al. (2011) [26] | Legionella pneumophila | 5 × 103 | 5 × 104 |
| Legionella spp. | 1 × 105 | 1 × 106 | |
| ANSES [33] | Legionella pneumophila | 5 × 103 | 5 × 105 |
| Legionella spp. | - | - | |
| Collins et al. (2017) [28] | Legionella pneumophila | - | - |
| Legionella spp. | 1 × 103 | 1 × 104 | |
| PWGSC [34] | Legionella pneumophila | 1 × 104 | 1 × 105 |
| Legionella spp. | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, C.; Smith, D.; Wafer, T.; Crook, B. Rapid Testing and Interventions to Control Legionella Proliferation following a Legionnaires’ Disease Outbreak Associated with Cooling Towers. Microorganisms 2021, 9, 615. https://doi.org/10.3390/microorganisms9030615
Young C, Smith D, Wafer T, Crook B. Rapid Testing and Interventions to Control Legionella Proliferation following a Legionnaires’ Disease Outbreak Associated with Cooling Towers. Microorganisms. 2021; 9(3):615. https://doi.org/10.3390/microorganisms9030615
Chicago/Turabian StyleYoung, Charlotte, Duncan Smith, Tim Wafer, and Brian Crook. 2021. "Rapid Testing and Interventions to Control Legionella Proliferation following a Legionnaires’ Disease Outbreak Associated with Cooling Towers" Microorganisms 9, no. 3: 615. https://doi.org/10.3390/microorganisms9030615
APA StyleYoung, C., Smith, D., Wafer, T., & Crook, B. (2021). Rapid Testing and Interventions to Control Legionella Proliferation following a Legionnaires’ Disease Outbreak Associated with Cooling Towers. Microorganisms, 9(3), 615. https://doi.org/10.3390/microorganisms9030615







