Epidemiology of Mucormycosis in India
Abstract
1. Introduction
2. Mucormycosis Prevalence and Incidence in India
3. Underlying Disease and Risk Factors
4. Clinical Forms of Mucormycosis
5. Causative Agents of Mucormycosis
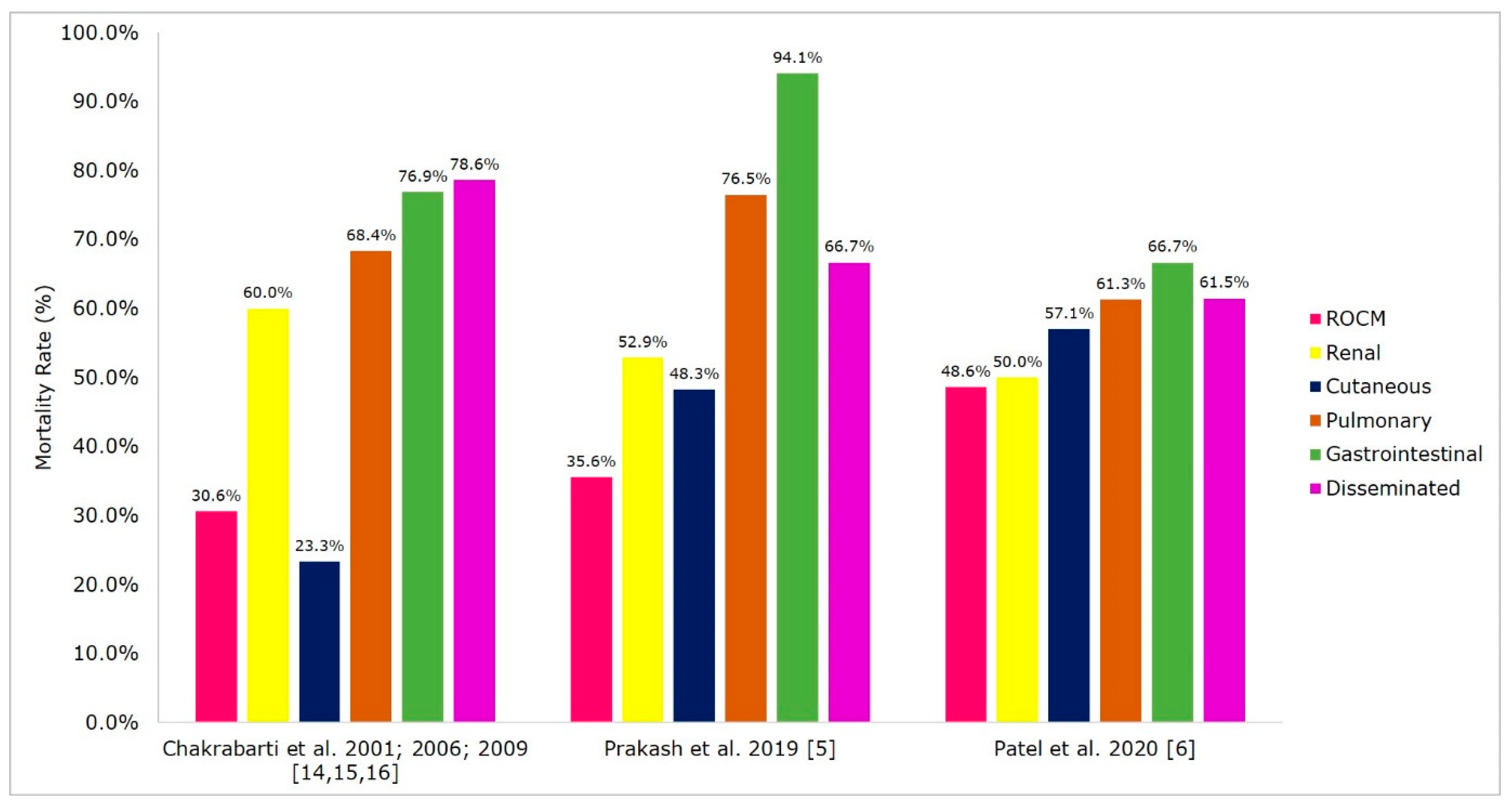
6. Treatment and Outcome of Mucormycosis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frater, J.L.; Hall, G.S.; Procop, G.W. Histologic features of zygomycosis: Emphasis on perineural invasion and fungal morphology. Arch. Pathol. Lab. Med. 2001, 125, 375–378. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef]
- Reid, G.; Lynch, J.P.; Fishbein, M.C.; Clark, N.M. Mucormycosis. Semin. Respir. Crit. Care Med. 2020, 41, 099–114. [Google Scholar] [CrossRef]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.M.; Singh, P.; Xess, I.; Savio, J.; Pamidimukkala, U.; Jillwin, J.; Varma, S.; Das, A.; et al. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med. Mycol. 2019, 57, 395–402. [Google Scholar] [CrossRef]
- Patel, A.; Kaur, H.; Xess, I.; Michael, J.S.; Savio, J.; Rudramurthy, S.; Singh, R.; Shastri, P.; Umabala, P.; Sardana, R.; et al. A multi-centre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin. Microbiol. Infect. 2020, 26, 944.e9–944.e15. [Google Scholar] [CrossRef]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef]
- Patel, A.K.; Patel, K.K.; Patel, K.; Gohel, S.; Chakrabarti, A. Mucormycosis at a tertiary care centre in Gujarat, India. Mycoses 2017, 60, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Yang, H.; Song, J.; Kelkar, S.S.; Yang, X.; Azie, N.; Harrington, R.; Fan, A.; Lee, E.; Spalding, J.R. Prevalence, clinical and economic burden of mucormycosis-related hospitalisations in the United States: A retrospective study. BMC Infect. Dis. 2016, 16, 730. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Dhaliwal, M. Epidemiology of Mucormycosis in India. Curr. Fungal Infect. Rep. 2013, 7, 287–292. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Singh, R. Mucormycosis in India: Unique features. Mycoses 2014, 57, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Chakrabarti, A. Global Epidemiology of Mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Singh, G.; Agarwal, R.; Dabas, Y.; Jyotsna, V.P.; Kumar, R.; Xess, I. Emerging Rhizopus microsporus Infections in India. J. Clin. Microbiol. 2018, 56, 1–5. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Das, A.; Sharma, A.; Panda, N.; Das, S.; Gupta, K.L.; Sakhuja, V. Ten Years’ Experience in Zygomycosis at a Tertiary Care Centre in India. J. Infect. 2001, 42, 261–266. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Das, A.; Mandal, J.; Shivaprakash, M.R.; George, V.K.; Tarai, B.; Rao, P.; Panda, N.; Verma, S.C.; Sakhuja, V. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med. Mycol. 2006, 44, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Panda, N.; Shivaprakash, M.R.; Kaur, A.; Varma, S.C.; Singhi, S.; Bhansali, A.; Sakhuja, V. Invasive zygomycosis in India: Experience in a tertiary care hospital. Postgrad. Med. J. 2009, 85, 573–581. [Google Scholar] [CrossRef]
- Manesh, A.; Rupali, P.; Sullivan, M.O.; Mohanraj, P.; Rupa, V.; George, B.; Michael, J.S. Mucormycosis-A clinicoepidemiological review of cases over 10 years. Mycoses 2019, 62, 391–398. [Google Scholar] [CrossRef]
- Priya, P.; Ganesan, V.; Rajendran, T.; Geni, V.G. Mucormycosis in a Tertiary Care Center in South India: A 4-Year Experience. Indian J. Crit. Care Med. 2020, 24, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Kaur, H.; Savio, J.; Rudramurthy, S.M.; Patel, A.; Shastri, P.; Pamidimukkala, U.; Karthik, R.; Bhattacharya, S.; Kindo, A.J.; et al. Epidemiology and clinical outcomes of invasive mould infections in Indian intensive care units (FISF study). J. Crit. Care 2019, 51, 64–70. [Google Scholar] [CrossRef]
- Sindhu, D.; Jorwal, P.; Gupta, N.; Xess, I.; Singh, G.; Soneja, M.; Nischal, N.; Sethi, P.; Ray, A.; Biswas, A.; et al. Clinical spectrum and outcome of hospitalised patients with invasive fungal infections: A prospective study from a medical ward/intensive care unit of a teaching hospital in North India. Le Infez. Med. 2019, 27, 398–402. [Google Scholar]
- Chakrabarti, A.; Sood, P.; Denning, D. Estimating Fungal Infection Burden in India: Mucormycosis Burden as a Case Study. Available online: https://www.gaffi.org/wp-content/uploads/P1044.pdf (accessed on 1 December 2020).
- Chander, J.; Kaur, M.; Singla, N.; Punia, R.; Singhal, S.; Attri, A.; Alastruey-Izquierdo, A.; Stchigel, A.; Cano-Lira, J.; Guarro, J. Mucormycosis: Battle with the Deadly Enemy over a Five-Year Period in India. J. Fungi 2018, 4, 46. [Google Scholar] [CrossRef]
- Bhansali, A. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad. Med. J. 2004, 80, 670–674. [Google Scholar] [CrossRef]
- Dayal, D.; Jain, P.; Kumar, R.; Bakshi, J.; Menon, P.; Das, A.; Singhi, S.; Singh, M. Clinical spectrum and outcome of invasive filamentous fungal infections in children with Type 1 diabetes: North Indian experience. Clin. Pediatr. Endocrinol. 2015, 24, 51–57. [Google Scholar] [CrossRef]
- Skiada, A.; Pavleas, I.; Drogari-Apiranthitou, M. Epidemiology and Diagnosis of Mucormycosis: An Update. J. Fungi 2020, 6, 265. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Colagiuri, S.; Almutairi, R.; Montoya, P.A.; Basit, A.; Beran, D.; Besançon, S.; Bommer, C.; Borgnakke, W.; Boyko, E.; et al. International Diabetes Federation Diabetes Atlas. Ninth Edition. 2019. Available online: https://diabetesatlas.org/en/sections/worldwide-toll-of-diabetes.html (accessed on 5 December 2020).
- Das, A.; Oberoi, S.; Trehan, A.; Chakrabarti, A.; Bansal, D.; Saxena, A.K.; Sodhi, K.S.; Kakkar, N.; Srinivasan, R. Invasive Fungal Disease in Pediatric Acute Leukemia in the Nontransplant Setting: 8 Years’ Experience From a Tertiary Care Center in North India. J. Pediatr. Hematol. Oncol. 2018, 40, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Korula, A.; Abraham, A.; Abubacker, F.N.; Viswabandya, A.; Lakshmi, K.M.; Abraham, O.C.; Rupali, P.; Varghese, G.M.; Michael, J.S.; Srivastava, A.; et al. Invasive fungal infection following chemotherapy for acute myeloid leukaemia-Experience from a developing country. Mycoses 2017, 60, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.H.; Patel, R.D.; Vanikar, A.V.; Kanodia, K.V.; Suthar, K.S.; Nigam, L.K.; Patel, H.V.; Patel, A.H.; Kute, V.B.; Trivedi, H.L. Invasive fungal infections in renal transplant patients: A single center study. Ren. Fail. 2017, 39, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Godara, S.M.; Kute, V.B.; Goplani, K.R.; Gumber, M.R.; Gera, D.N.; Shah, P.R.; Vanikar, A.V.; Trivedi, H.L. Mucormycosis in renal transplant recipients: Predictors and outcome. Saudi J. Kidney Dis. Transpl. 2011, 22, 751–756. [Google Scholar] [PubMed]
- Shekar, M.; Elumalai, R.; Elayaperumal, I.; Yelahanka, R.P.; Anandkumar, D.G.; Bandi, V.K.; Matcha, J. Prevalence and outcome of systemic fungal infections in renal transplant recipients—A tertiary care experience. Saudi J. Kidney Dis. Transpl. 2019, 30, 1137–1143. [Google Scholar] [CrossRef]
- Jayakumar, M.; Gopalakrishnan, N.; Vijayakumar, R.; Rajendran, S.; Muthusethupathi, M.A. Systemic fungal infections in renal transplant recipients at Chennai, India. Transplant. Proc. 1998, 30, 3135. [Google Scholar] [CrossRef]
- Gupta, K. Fungal infections and the kidney. Indian J. Nephrol. 2001, 11, 147–154. [Google Scholar]
- Gupta, K.L.; Bagai, S.; Ramachandran, R.; Kumar, V.; Rathi, M.; Kohli, H.S.; Sharma, A.; Chakrabarti, A. Fungal infection in post-renal transplant patient: Single-center experience. Indian J. Pathol. Microbiol. 2020, 63, 587–592. [Google Scholar] [CrossRef]
- Almyroudis, N.G.; Sutton, D.A.; Linden, P.; Rinaldi, M.G.; Fung, J.; Kusne, S. Zygomycosis in Solid Organ Transplant Recipients in a Tertiary Transplant Center and Review of the Literature. Am. J. Transplant. 2006, 6, 2365–2374. [Google Scholar] [CrossRef]
- Kumar, C.; Jain, P.; Wadhwa, N.; Diwaker, P.; Khan, N. Nosocomial Jejunal Mucormycosis—An Unusual Cause of Perforation Peritonitis. Iran. J. Pathol. 2017, 12, 295–300. [Google Scholar] [CrossRef]
- Garg, J.; Sujatha, S.; Garg, A.; Parija, S.C. Nosocomial cutaneous zygomycosis in a patient with diabetic ketoacidosis. Int. J. Infect. Dis. 2009, 13, 508–510. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhadauria, D.; Etta, P.; Chelappan, A.; Gurjar, M.; Kaul, A.; Sharma, R.K.; Gupta, A.; Prasad, N.; Marak, R.S.; Jain, M.; et al. Isolated bilateral renal mucormycosis in apparently immunocompetent patients—A case series from India and review of the literature. Clin. Kidney J. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Devana, S.K.; Gupta, V.G.; Mavuduru, R.S.; Bora, G.S.; Sharma, A.P.; Parmar, K.M.; Kumar, S.; Mete, U.K.; Singh, S.K.; Mandal, A.K.; et al. Isolated Renal Mucormycosis in Immunocompetent Hosts: Clinical Spectrum and Management Approach. Am. J. Trop. Med. Hyg. 2019, 100, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.L.; Radotra, B.D.; Sakhuja, V.; Banerjee, A.K.; Chugh, K.S. Mucormycosis in patients with renal failure. Ren. Fail. 1989, 11, 195–199. [Google Scholar] [CrossRef]
- Kursun, E.; Turunc, T.; Demiroglu, Y.Z.; Alışkan, H.E.; Arslan, A.H. Evaluation of 28 cases of mucormycosis. Mycoses 2015, 58, 82–87. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, A.; Singh, G.; Ratnakar, G.; Vinod, K.S.; Wig, N. Breakthrough mucormycosis after voriconazole use in a case of invasive fungal rhinosinusitis due to Curvularia lunata. Drug Discov. Ther. 2017, 11, 349–352. [Google Scholar] [CrossRef][Green Version]
- Mandhaniya, S.; Swaroop, C.; Thulkar, S.; Vishnubhatla, S.; Kabra, S.K.; Xess, I.; Bakhshi, S. Oral Voriconazole Versus Intravenous Low Dose Amphotericin B for Primary Antifungal Prophylaxis in Pediatric Acute Leukemia Induction. J. Pediatr. Hematol. Oncol. 2011, 33, e333–e341. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.P.; Sharma, J.; Singh, G.; Kumar, S.; Malik, S.; Kumar, V. Primary breast mucormycosis: FNAC diagnosis of a rare entity. Diagn. Cytopathol. 2016, 44, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Hadgaonkar, S.; Shah, K.; Bhojraj, S.; Nene, A.; Shyam, A. Isolated Mucormycotic Spondylodiscitis of Lumbar Spine-A Rare Case Report. J. Orthop. Case Rep. 2015, 5, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Nene, A. Spinal mucormycosis. J. Glob. Infect. Dis. 2017, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R. Sclerosing Mediastinitis Presenting as Complete Heart Block. J. Clin. Diagn. Res. 2017, 11, ED12–ED14. [Google Scholar] [CrossRef]
- Krishnappa, D.; Naganur, S.; Palanisamy, D.; Kasinadhuni, G. Cardiac mucormycosis: A case report. Eur. Hear. J. Case Rep. 2019, 3, 1–5. [Google Scholar] [CrossRef]
- Bhatt, M.; Soneja, M.; Fazal, F.; Vyas, S.; Kumar, P.; Jorwal, P.; Raj, U.; Sachdev, J.; Singh, G.; Xess, I.; et al. Two cases of Osteoarticular Mucor menace: A diagnostic and management conundrum. Drug Discov. Ther. 2018, 12, 374–378. [Google Scholar] [CrossRef]
- Urs, A.; Singh, H.; Mohanty, S.; Sharma, P. Fungal osteomyelitis of maxillofacial bones: Rare presentation. J. Oral Maxillofac. Pathol. 2016, 20, 546. [Google Scholar] [CrossRef]
- Nithyanandam, S.; Jacob, M.S.; Battu, R.R.; Thomas, R.K.; Correa, M.A.; D’Souza, O. Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J. Ophthalmol. 2003, 51, 231–236. [Google Scholar]
- Kolekar, J.S. Rhinocerebral Mucormycosis: A Retrospective Study. Indian J. Otolaryngol. Head Neck Surg. 2015, 67, 93–96. [Google Scholar] [CrossRef]
- Bakshi, S.S.; Das, S.; Ramesh, S.; Gopalakrishnan, S. Nasal Mucormycosis: Our experience with 24 cases. Otolaryngol. Pol. 2020, 74, 37–40. [Google Scholar] [CrossRef]
- Ramadorai, A.; Ravi, P.; Narayanan, V. Rhinocerebral Mucormycosis: A Prospective Analysis of an Effective Treatment Protocol. Ann. Maxillofac. Surg. 2018, 9, 192–196. [Google Scholar] [CrossRef]
- Shah, K.; Dave, V.; Bradoo, R.; Shinde, C.; Prathibha, M. Orbital Exenteration in Rhino-Orbito-Cerebral Mucormycosis: A Prospective Analytical Study with Scoring System. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bansal, C.; Kaintura, M. Sinonasal Mucormycosis: A to Z. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 1962–1971. [Google Scholar] [CrossRef]
- Nilesh, K.; Vande, A. Mucormycosis of maxilla following tooth extraction in immunocompetent patients: Reports and review. J. Clin. Exp. Dent. 2018, 10, e300. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Anand, A.; Ranjan, P.; Meena, V.P.; Ray, A.; Dutta, R.; Jadon, R.S.; Vikram, N.K. Case of mucormycosis of mandible after self-extraction of teeth incidentally detected to have chronic granulomatous disease: Case report and literature review. Med. Mycol. Case Rep. 2020, 28, 55–59. [Google Scholar] [CrossRef]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O. French Mycosis Study Group A global analysis of mucormycosis in France: The RetroZygo Study (2005-2007). Clin. Infect. Dis. 2012, 54 (Suppl. 1), S35–S43. [Google Scholar] [CrossRef]
- Feng, J.; Sun, X. Characteristics of pulmonary mucormycosis and predictive risk factors for the outcome. Infection 2018, 46, 503–512. [Google Scholar] [CrossRef]
- Kaushik, R. Primary Cutaneous Zygomycosis in India. Indian J. Surg. 2012, 74, 468–475. [Google Scholar] [CrossRef]
- Skiada, A.; Rigopoulos, D.; Larios, G.; Petrikkos, G.; Katsambas, A. Global epidemiology of cutaneous zygomycosis. Clin. Dermatol. 2012, 30, 628–632. [Google Scholar] [CrossRef]
- Kaur, H.; Ghosh, A.; Rudramurthy, S.M.; Chakrabarti, A. Gastrointestinal mucormycosis in apparently immunocompetent hosts-A review. Mycoses 2018, 61, 898–908. [Google Scholar] [CrossRef]
- Patra, S.; Chirla, D.; Kumar, N.; Vij, M.; Samal, S. Unsuspected invasive neonatal gastrointestinal mucormycosis: A clinicopathological study of six cases from a tertiary care hospital. J. Indian Assoc. Pediatr. Surg. 2012, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Dioverti, M.V.; Cawcutt, K.A.; Abidi, M.; Sohail, M.R.; Walker, R.C.; Osmon, D.R. Gastrointestinal mucormycosis in immunocompromised hosts. Mycoses 2015, 58, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.M.; Paul, R.A.; Gupta, S.; Negi, V.; Chakrabarti, A. The environmental source of emerging Apophysomyces variabilis infection in India. Med. Mycol. 2016, 54, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Singh, S.; Rudramurthy, S.M.; Singh, P.; Mehta, N.; Shaw, D.; Ghosh, A.K. An aero mycological analysis of Mucormycetes in indoor and outdoor environments of northern India. Med. Mycol. 2020, 58, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.; Pawłowska, J.; Alastruey-Izquierdo, A.; Wrzosek, M.; Rodriguez-Tudela, J.L.; Dolatabadi, S.; Chakrabarti, A.; de Hoog, G.S. DNA barcoding in Mucorales: An inventory of biodiversity. Persoonia Mol. Phylogeny Evol. Fungi 2013, 30, 11–47. [Google Scholar] [CrossRef] [PubMed]
- Kokkayil, P.; Pandey, M.; Agarwal, R.; Kale, P.; Singh, G.; Xess, I. Rhizopus homothallicus Causing Invasive Infections: Series of Three Cases from a Single Centre in North India. Mycopathologia 2017, 182, 921–926. [Google Scholar] [CrossRef]
- Pamidimukkala, U.; Sudhaharan, S.; Kancharla, A.; Vemu, L.; Challa, S.; Karanam, S.D.; Chavali, P.; Prakash, H.; Ghosh, A.K.; Gupta, S.; et al. Mucormycosis due to Apophysomyces species complex- 25 years’ experience at a tertiary care hospital in southern India. Med. Mycol. 2020, 58, 425–433. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Ghosh, A.; Prasad, G.S.; David, J.K.; Gupta, S.; Das, A.; Sakhuja, V.; Panda, N.K.; Singh, S.K.; Das, S.; et al. Apophysomyces elegans: An emerging zygomycete in India. J. Clin. Microbiol. 2003, 41, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Hemashettar, B.M.; Patil, R.N.; O’Donnell, K.; Chaturvedi, V.; Ren, P.; Padhye, A.A. Chronic rhinofacial mucormycosis caused by Mucor irregularis (Rhizomucor variabilis) in India. J. Clin. Microbiol. 2011, 49, 2372–2375. [Google Scholar] [CrossRef]
- Xess, I.; Mohapatra, S.; Shivaprakash, M.R.; Chakrabarti, A.; Benny, G.L.; O’Donnell, K.; Padhye, A.A. Evidence implicating Thamnostylum lucknowense as an etiological agent of rhino-orbital mucormycosis. J. Clin. Microbiol. 2012, 50, 1491–1494. [Google Scholar] [CrossRef]
- Chander, J.; Singla, N.; Kaur, M.; Punia, R.S.; Attri, A.; Alastruey-Izquierdo, A.; Cano-Lira, J.F.; Stchigel, A.M.; Guarro, J. Saksenaea erythrospora, an emerging mucoralean fungus causing severe necrotising skin and soft tissue infections—A study from a tertiary care hospital in north India. Infect. Dis. (Lond. Engl.). 2017, 49, 170–177. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Chen, S.C.A.; Kong, D.C.M. Contemporary management and clinical outcomes of mucormycosis: A systematic review and meta-analysis of case reports. Int. J. Antimicrob. Agents 2019, 53, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Manesh, A.; John, A.O.; Mathew, B.; Varghese, L.; Rupa, V.; Zachariah, A.; Varghese, G.M. Posaconazole: An emerging therapeutic option for invasive rhino-orbito-cerebral mucormycosis. Mycoses 2016, 59, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
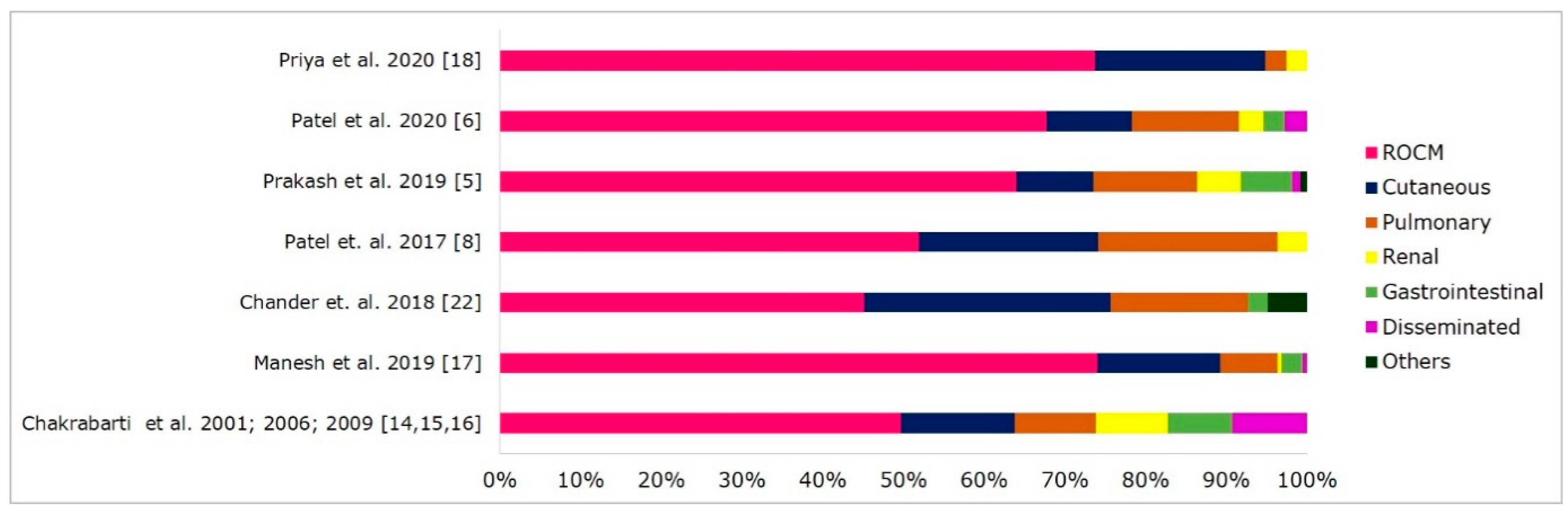
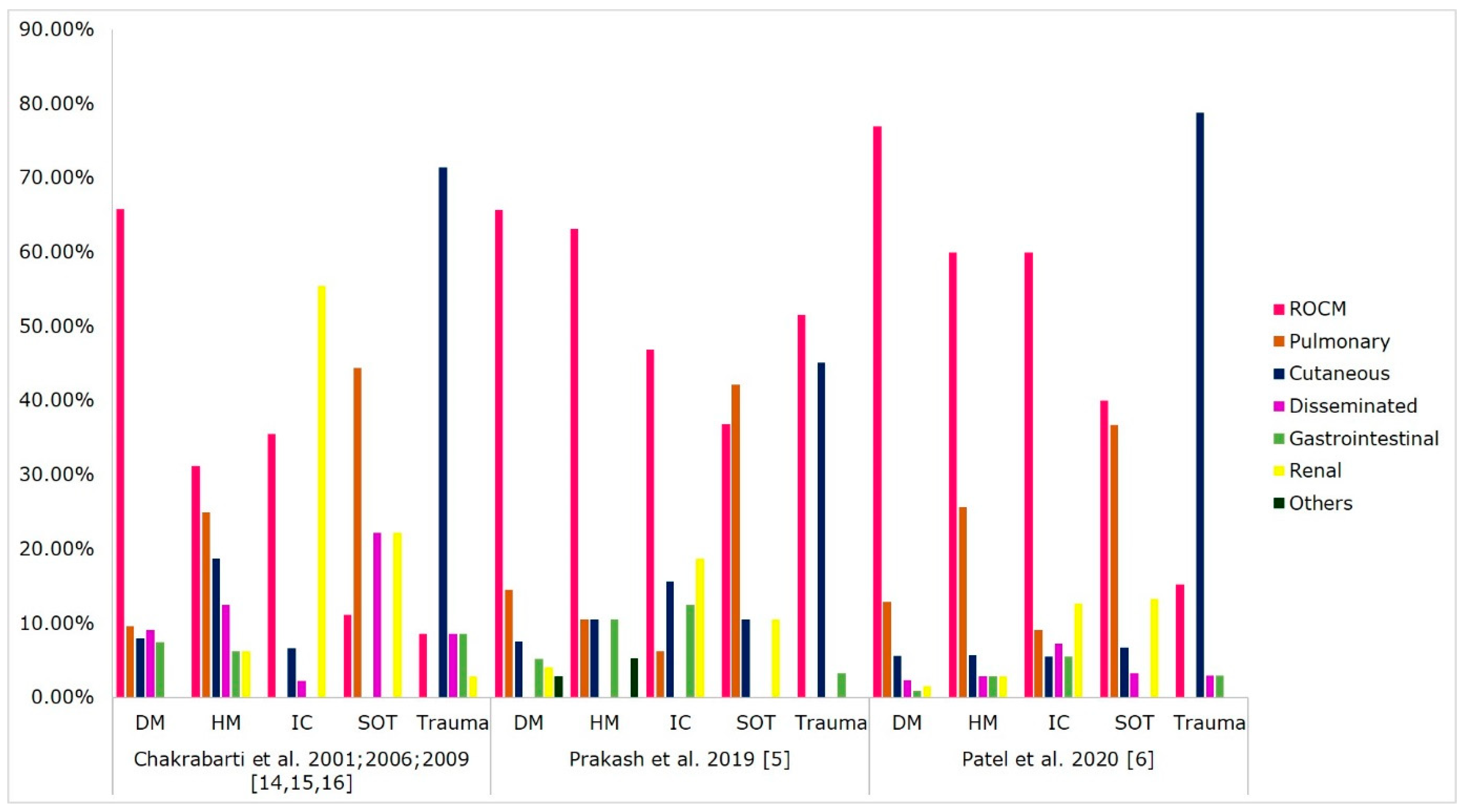
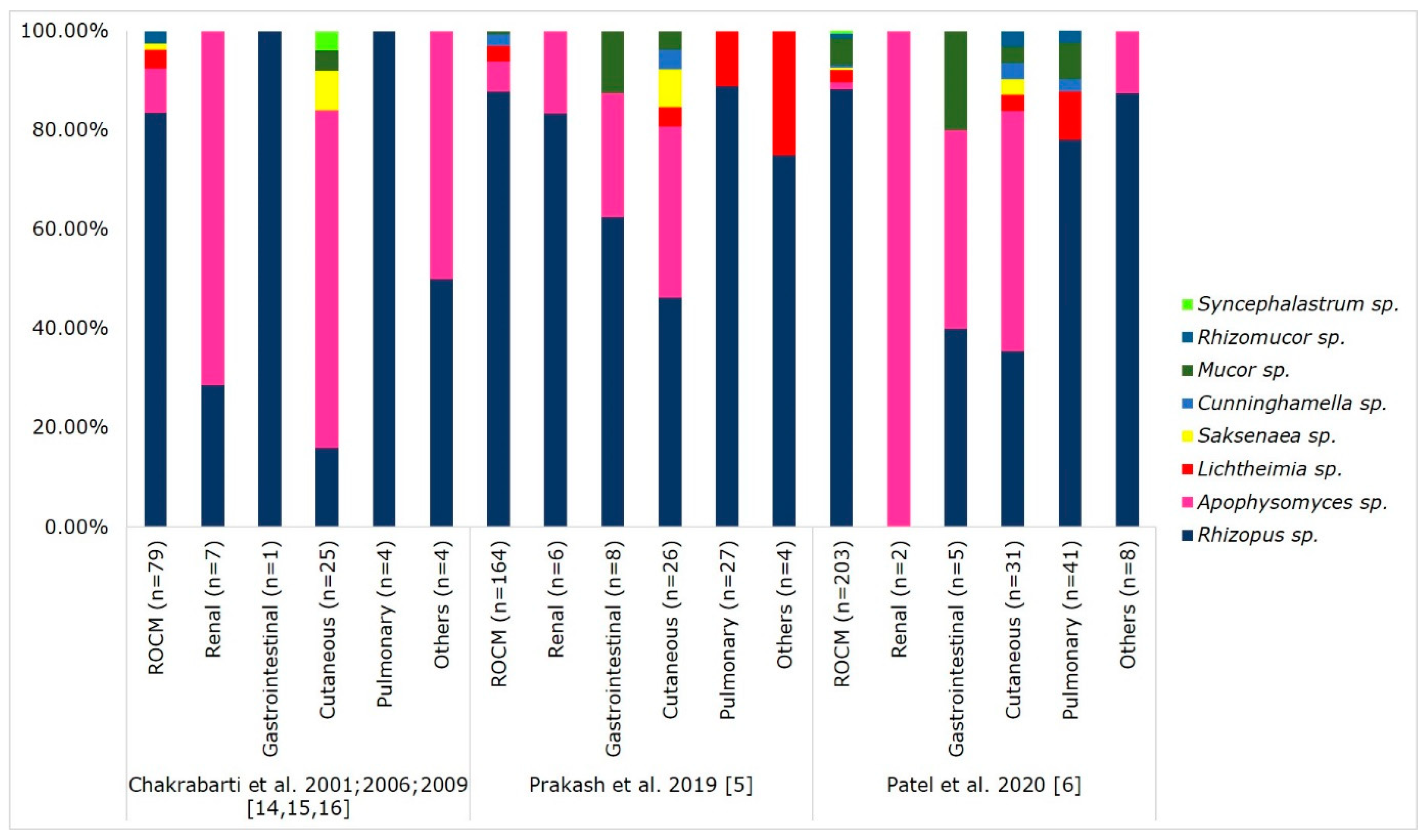
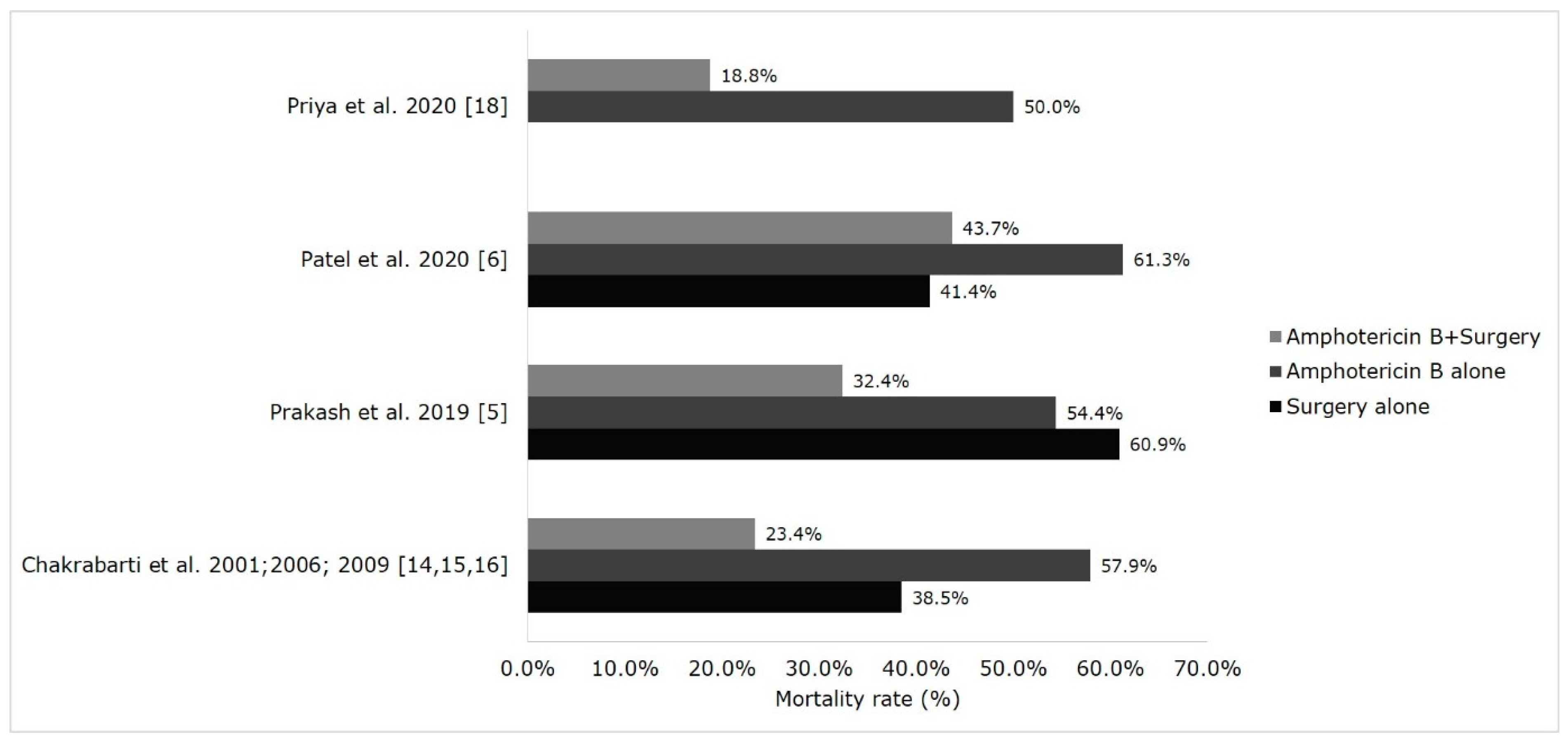
| Parameters | Chakrabarti et al., 2001; 2006; 2009 [14,15,16] | Manesh et al., 2019 [17] | Chander et al., 2018 [22] | Patel et al., 2017 [8] | Prakash et al., 2019 [5] | Patel et al., 2020 [6] | Priya et al., 2020 [18] |
|---|---|---|---|---|---|---|---|
| Study centre | 1 | 1 | 1 | 2 | 4 | 12 | 1 |
| Study period | 1990–2004; 2006–2007 | 2005–2015 | 2010–2014 | January 2013– May 2015 | 2013–2015 | January 2016–September 2017 | 2015–2019 |
| Study duration | 15 years 6 months | 10 years | 5 years | 2 years 5 months | 3 years | 1 year and 9 months | 4 years |
| Place of study | Chandigarh (North India) | Tamilnadu (South India) | Chandigarh (North India) | Gujarat (West of India) | North and South India | Across India | Tamilnadu (South India) |
| Total cases | 382 | 184 | 82 | 27 | 388 | 465 | 38 |
| Mean annual incidence | 24.5 | 18.4 | 16.4 | - | - | - | 9.5 |
| Male: female ratio | 2.4:1 | 2.5:1 | 2.04:1 | 2.3:1 | 2.3:1 | 2.3:1 | 2.8:1 |
| *Paediatric (10–16 years) (n (%)) | 30 (7.9) | 7 (3.8) | 4 (4.9) | - | 46 (11.9) | 27 (5.81) | 1 (2.6) |
| Adults (n (%)) | 352 (92.1) | 177 (96.2) | 78 (95.1) | - | 342 (89.1) | 438 (94.2) | 37 (97.4) |
| Underlying disease and risk factors (n (%)) | |||||||
| Total number of patients with underlying disease or risk factors | 349 $ | 184 | 82 | 27 | 303 | 465 | 38 |
| Diabetes mellitus | 187 (53.6) a | 120 (65.2) | 51 (62.2) | 15 (55.6) | 172 (56.8) | 342 (73.5) | 29 (76.3) |
| Diabetic ketoacidosis | 21 (21.6) b | 16.9% g | - | - | 31 (10.2) | 50 (14.6) | 3 (7.9) |
| Solid-organ transplant | 9 (2.6) a | - | - | 3 (11.1) | 19 (6.3) | 30 (6.5) | - |
| HSCT | - | 4 (2.2) | - | - | 1 (0.3) | 6 (1.3) | - |
| Haematological and solid organ malignancy | 16 (4.6) a | 14 (7.6) | - | 1 (3.7) | 23 (7.6) | 42 (9) | 2 (5.3) |
| Brach of skin (trauma due to accidents, burns, injection site) | 35 (10) a | 20 (10.9) | 12 (14.6) | 6 (22.2) | 31 (10.2) | 35 (7.5) | 8 (21.1) |
| Pulmonary disease (tuberculosis, COPD, asthma) | 1 (0.6) c | - | - | 2 (7.4) | 21 (6.9) | 30 (6.5) | - |
| Neutropenia | 11 (14.6) d | - | - | - | 18 (5.9) | 12 (2.6) | - |
| Steroid therapy | 28 (8) a | - | - | 6 (22.2) | 30 (9.9) | 17 (3.7) | - |
| Chronic alcoholism | 15 (5.9) e | - | - | - | 28 (9.2) | - | - |
| Chronic kidney disease | 24 (32) d | 1 (0.5) | 1 (1.2) | 1 (3.7) | 27 (8.9) | 93 (20) | 2 (5.3) |
| Human immunodeficiency virus | 2 (0.8) e | - | - | - | 3 (1) | 7 (1.5) | - |
| Immunocompetent host | 45 (12.9) a | 10 (5.4) | 16 (19.5) | 7 (25.9) | 32 (10.6) | 55 (11.8) | 1 (2.6) |
| # Miscellaneous | 53 (31) f | 15 (8.2) | 8 (9.8) | 6 (22.2) | 6 (2.0) | 143 (30.8) | 4 (10.5) |
| ^ Causative Agents | Chakrabarti et al., 2001; 2006; 2009 [14,15,16] | Manesh et al., 2019 [17] | Chander et. al., 2018 [22] | Prakash et al., 2019 [5] | Patel et al., 2020 [6] | Priya et al., 2020 [18] |
|---|---|---|---|---|---|---|
| Total number of isolated Mucorales | 120 $ | 184 | 60 | 239 | 290 | 25 |
| Rhizopus species | 79 (65.8) a | 143 (77.7) | 28 (46.7) | 193 (80.8) | 231 (79.7) | 14 (56) |
| Rhizopus arrhizus | 74 (61.7) a | 91 (49.5) | 17 (28.3) | 124 (51.9) | 176 (60.7) | - |
| Rhizopus microsporus | 4 (4.2) b | 32 (17.4) | 9 (15) | 30 (12.6) | 32 (11) | - |
| Rhizopus homothallicus | 1 (3.1) c | - | 2 (3.3) | 6 (2.5) | 22 (7.6) | - |
| Apophysomyces species | 31 (25.8) a | 20 (10.9) | 13 (21.7) | 22 (9.2) | 23 (7.9) | 5 (20) |
| Lichtheimia species | 3 (5.3) d | 1 (0.5) | 8 (13.3) | 10 (4.2) | 10 (3.5) | 1 (4) |
| Saksenaea species | 3 (3.4) e | 1 (0.5) | 5 (8.3) | 2 (0.8) | 2 (0.7) | - |
| Cunninghamella species | - | 1 (0.5) | - | 5 (2.1) | 3 (1) | - |
| Mucor species | 1 (4) f | 4 (2.2) | 1 (1.7) | 3 (1.3) | 16 (5.5) | 3 (12) |
| Rhizomucor species | 2 (2.3) e | 1 (0.5) | 1 (1.7) | - | 4 (1.4) | - |
| Syncephalastrum species | 1 (3.1) c | 1 (0.5) | 4 (6.7) | - | 1 (0.4) | - |
| Nonsporulating Mucorales/other fungi | - | 12 (6.5) | - | 4 (1.7) | - | 2 (8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, H.; Chakrabarti, A. Epidemiology of Mucormycosis in India. Microorganisms 2021, 9, 523. https://doi.org/10.3390/microorganisms9030523
Prakash H, Chakrabarti A. Epidemiology of Mucormycosis in India. Microorganisms. 2021; 9(3):523. https://doi.org/10.3390/microorganisms9030523
Chicago/Turabian StylePrakash, Hariprasath, and Arunaloke Chakrabarti. 2021. "Epidemiology of Mucormycosis in India" Microorganisms 9, no. 3: 523. https://doi.org/10.3390/microorganisms9030523
APA StylePrakash, H., & Chakrabarti, A. (2021). Epidemiology of Mucormycosis in India. Microorganisms, 9(3), 523. https://doi.org/10.3390/microorganisms9030523






