A Narrative Review of the W, X, Y, E, and NG of Meningococcal Disease: Emerging Capsular Groups, Pathotypes, and Global Control
Abstract
1. Introduction
2. Minor N. meningitidis Capsular Groups
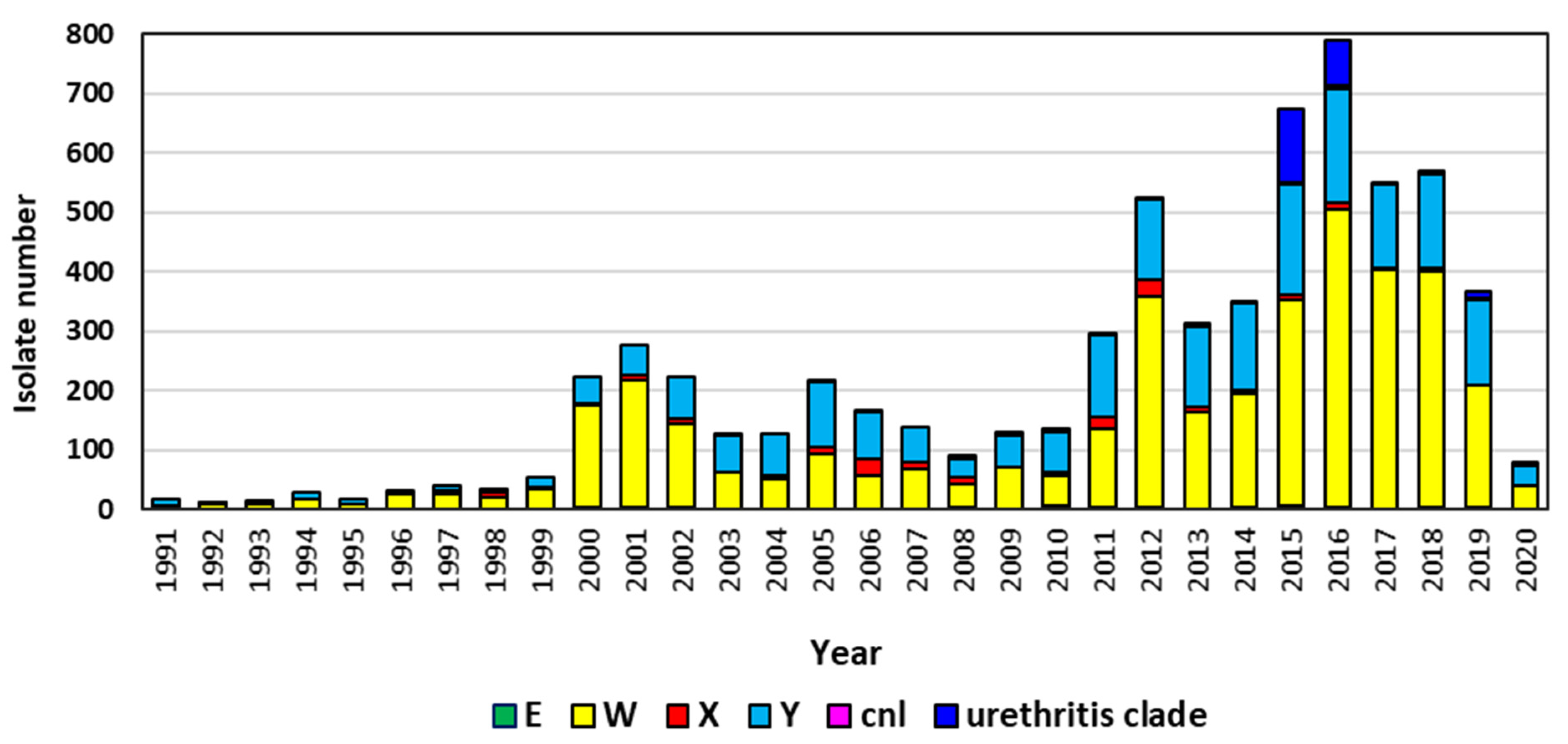
2.1. Group W
2.2. Group X
2.3. Group Y
2.4. Group E
2.5. Nongroupable
3. Population Structure of Invasive “Minor” Capsular Groups and Nongroupable N. meningitidis
4. Meningococcal Vaccines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gold, R.; Goldschneider, I.; Lepow, M.L.; Draper, T.F.; Randolph, M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J. Infect. Dis. 1978, 137, 112–121. [Google Scholar] [CrossRef]
- Cartwright, K.A.; Stuart, J.M.; Jones, D.M.; Noah, N.D. The Stonehouse survey: Nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 1987, 99, 591–601. [Google Scholar] [CrossRef]
- Harrison, O.B.; Claus, H.; Jiang, Y.; Bennett, J.S.; Bratcher, H.B.; Jolley, K.A.; Corton, C.; Care, R.; Poolman, J.T.; Zollinger, W.D.; et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg. Infect. Dis. 2013, 19, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Bundle, D.R.; Jennings, H.J.; Kenny, C.P. Studies on the group-specific polysaccharide of Neisseria meningitidis serogroup X and an improved procedure for its isolation. J. Biol. Chem. 1974, 249, 4797–4801. [Google Scholar] [CrossRef]
- Bhattacharjee, A.K.; Jennings, H.J.; Kenny, C.P. Structural elucidation of the 3-deoxy-D-manno-octulosonic acid containing meningococcal 29-e capsular polysaccharide antigen using carbon-13 nuclear magnetic resonance. Biochemistry 1978, 17, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Retchless, A.C.; Kretz, C.B.; Chang, H.Y.; Bazan, J.A.; Abrams, A.J.; Norris Turner, A.; Jenkins, L.T.; Trees, D.L.; Tzeng, Y.L.; Stephens, D.S.; et al. Expansion of a urethritis-associated Neisseria meningitidis clade in the United States with concurrent acquisition of N. gonorrhoeae alleles. BMC Genom. 2018, 19, 176. [Google Scholar] [CrossRef]
- Tzeng, Y.L.; Bazan, J.A.; Turner, A.N.; Wang, X.; Retchless, A.C.; Read, T.D.; Toh, E.; Nelson, D.E.; Del Rio, C.; Stephens, D.S. Emergence of a new Neisseria meningitidis clonal complex 11 lineage 11.2 clade as an effective urogenital pathogen. Proc. Natl. Acad. Sci. USA 2017, 114, 4237–4242. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.A.; Turner, A.N.; Kirkcaldy, R.D.; Retchless, A.C.; Kretz, C.B.; Briere, E.; Tzeng, Y.L.; Stephens, D.S.; Maierhofer, C.; Del Rio, C.; et al. Large Cluster of Neisseria meningitidis Urethritis in Columbus, Ohio, 2015. Clin. Infect. Dis. 2017, 65, 92–99. [Google Scholar] [CrossRef]
- Chang, Q.; Tzeng, Y.L.; Stephens, D.S. Meningococcal disease: Changes in epidemiology and prevention. Clin. Epidemiol. 2012, 4, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Borrow, R.; Bukovski, S.; Caugant, D.A.; Culic, D.; Delic, S.; Dinleyici, E.C.; Eloshvili, M.; Erdosi, T.; Galajeva, J.; et al. Prevention and control of meningococcal disease: Updates from the Global Meningococcal Initiative in Eastern Europe. J. Infect. 2019, 79, 528–541. [Google Scholar] [CrossRef]
- Taha, M.K.; Deghmane, A.E. Impact of COVID-19 pandemic and the lockdown on invasive meningococcal disease. BMC Res. Notes 2020, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.K.; Achtman, M.; Alonso, J.M.; Greenwood, B.; Ramsay, M.; Fox, A.; Gray, S.; Kaczmarski, E. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet 2000, 356, 2159. [Google Scholar] [CrossRef]
- Mayer, L.W.; Reeves, M.W.; Al-Hamdan, N.; Sacchi, C.T.; Taha, M.K.; Ajello, G.W.; Schmink, S.E.; Noble, C.A.; Tondella, M.L.; Whitney, A.M.; et al. Outbreak of W135 meningococcal disease in 2000: Not emergence of a new W135 strain but clonal expansion within the electophoretic type-37 complex. J. Infect. Dis. 2002, 185, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Lucidarme, J.; Hill, D.M.; Bratcher, H.B.; Gray, S.J.; du Plessis, M.; Tsang, R.S.; Vazquez, J.A.; Taha, M.K.; Ceyhan, M.; Efron, A.M.; et al. Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage. J. Infect. 2015, 71, 544–552. [Google Scholar] [CrossRef]
- Abad, R.; Lopez, E.L.; Debbag, R.; Vazquez, J.A. Serogroup W meningococcal disease: Global spread and current affect on the Southern Cone in Latin America. Epidemiol. Infect. 2014, 142, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Lucidarme, J.; Scott, K.J.; Ure, R.; Smith, A.; Lindsay, D.; Stenmark, B.; Jacobsson, S.; Fredlund, H.; Cameron, J.C.; Smith-Palmer, A.; et al. An international invasive meningococcal disease outbreak due to a novel and rapidly expanding serogroup W strain, Scotland and Sweden, July to August 2015. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef]
- Krone, M.; Gray, S.; Abad, R.; Skoczynska, A.; Stefanelli, P.; van der Ende, A.; Tzanakaki, G.; Molling, P.; Joao Simoes, M.; Krizova, P.; et al. Increase of invasive meningococcal serogroup W disease in Europe, 2013 to 2017. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef]
- Mulhall, R.M.; Bennett, D.E.; Bratcher, H.B.; Jolley, K.A.; Bray, J.E.; O’Lorcain, P.P.; Cotter, S.M.; Maiden, M.C.J.; Cunney, R.J. cgMLST characterisation of invasive Neisseria meningitidis serogroup C and W strains associated with increasing disease incidence in the Republic of Ireland. PLoS ONE 2019, 14, e0216771. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Hedberg, S.T.; Jacobsson, S.; Fredlund, H.; Molling, P.; Stenmark, B. Whole-Genome Sequencing of Emerging Invasive Neisseria meningitidis Serogroup W in Sweden. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Martin, N.V.; Ong, K.S.; Howden, B.P.; Lahra, M.M.; Lambert, S.B.; Beard, F.H.; Dowse, G.K.; Saul, N.; Communicable Diseases Network Australia Men, W.W.G. Rise in invasive serogroup W meningococcal disease in Australia 2013–2015. Commun. Dis. Intell. Q. Rep. 2016, 40, E454–E459. [Google Scholar] [PubMed]
- Tsang, R.S.W.; Ahmad, T.; Tyler, S.; Lefebvre, B.; Deeks, S.L.; Gilca, R.; Hoang, L.; Tyrrell, G.; Van Caeseele, P.; Van Domselaar, G.; et al. Whole genome typing of the recently emerged Canadian serogroup W Neisseria meningitidis sequence type 11 clonal complex isolates associated with invasive meningococcal disease. Int. J. Infect. Dis. 2018, 69, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sudbury, E.L.; O’Sullivan, S.; Lister, D.; Varghese, D.; Satharasinghe, K. Case Manifestations and Public Health Response for Outbreak of Meningococcal W Disease, Central Australia, 2017. Emerg. Infect. Dis. 2020, 26, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Aye, A.M.M.; Bai, X.; Borrow, R.; Bory, S.; Carlos, J.; Caugant, D.A.; Chiou, C.S.; Dai, V.T.T.; Dinleyici, E.C.; Ghimire, P.; et al. Meningococcal disease surveillance in the Asia-Pacific region (2020): The global meningococcal initiative. J. Infect. 2020, 81, 698–711. [Google Scholar] [CrossRef]
- Collard, J.M.; Maman, Z.; Yacouba, H.; Djibo, S.; Nicolas, P.; Jusot, J.F.; Rocourt, J.; Maitournam, R. Increase in Neisseria meningitidis serogroup W135, Niger, 2010. Emerg. Infect. Dis. 2010, 16, 1496–1498. [Google Scholar] [CrossRef] [PubMed]
- Mounkoro, D.; Nikiema, C.S.; Maman, I.; Sakande, S.; Bozio, C.H.; Tall, H.; Sadji, A.Y.; Njanpop-Lafourcade, B.M.; Sibabe, A.; Landoh, D.E.; et al. Neisseria meningitidis Serogroup W Meningitis Epidemic in Togo, 2016. J. Infect. Dis. 2019, 220, S216–S224. [Google Scholar] [CrossRef] [PubMed]
- Retchless, A.C.; Hu, F.; Ouedraogo, A.S.; Diarra, S.; Knipe, K.; Sheth, M.; Rowe, L.A.; Sangare, L.; Ky Ba, A.; Ouangraoua, S.; et al. The Establishment and Diversification of Epidemic-Associated Serogroup W Meningococcus in the African Meningitis Belt, 1994 to 2012. mSphere 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Kretz, C.B.; Retchless, A.C.; Sidikou, F.; Issaka, B.; Ousmane, S.; Schwartz, S.; Tate, A.H.; Pana, A.; Njanpop-Lafourcade, B.M.; Nzeyimana, I.; et al. Whole-Genome Characterization of Epidemic Neisseria meningitidis Serogroup C and Resurgence of Serogroup W, Niger, 2015. Emerg. Infect. Dis. 2016, 22, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Retchless, A.C.; Congo-Ouedraogo, M.; Kambire, D.; Vuong, J.; Chen, A.; Hu, F.; Ba, A.K.; Ouedraogo, A.S.; Hema-Ouangraoua, S.; Patel, J.C.; et al. Molecular characterization of invasive meningococcal isolates in Burkina Faso as the relative importance of serogroups X and W increases, 2008–2012. BMC Infect. Dis. 2018, 18, 337. [Google Scholar] [CrossRef]
- Meiring, S.; Cohen, C.; de Gouveia, L.; du Plessis, M.; Kularatne, R.; Hoosen, A.; Lekalakala, R.; Lengana, S.; Seetharam, S.; Naicker, P.; et al. Declining Incidence of Invasive Meningococcal Disease in South Africa: 2003–2016. Clin. Infect. Dis. 2019, 69, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Hansman, D. Meningococcal disease in South Australia: Incidence and serogroup distribution 1971–1980. J. Hyg. 1983, 90, 49–54. [Google Scholar] [CrossRef]
- Ryan, N.J.; Hogan, G.R. Severe meningococcal disease caused by serogroups X and Z. Am. J. Dis. Child. 1980, 134, 1173. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.M.; Fe, A.; Gomis, M.; Gil, D. Meningococcal meningitis caused by Neisseria meningitidis of the X serogroup. Med. Clin. 1985, 85, 208–209. [Google Scholar]
- Gagneux, S.P.; Hodgson, A.; Smith, T.A.; Wirth, T.; Ehrhard, I.; Morelli, G.; Genton, B.; Binka, F.N.; Achtman, M.; Pluschke, G. Prospective study of a serogroup X Neisseria meningitidis outbreak in northern Ghana. J. Infect. Dis. 2002, 185, 618–626. [Google Scholar] [CrossRef]
- Djibo, S.; Nicolas, P.; Alonso, J.M.; Djibo, A.; Couret, D.; Riou, J.Y.; Chippaux, J.P. Outbreaks of serogroup X meningococcal meningitis in Niger 1995–2000. Trop. Med. Int. Health 2003, 8, 1118–1123. [Google Scholar] [CrossRef]
- Boisier, P.; Nicolas, P.; Djibo, S.; Taha, M.K.; Jeanne, I.; Mainassara, H.B.; Tenebray, B.; Kairo, K.K.; Giorgini, D.; Chanteau, S. Meningococcal meningitis: Unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin. Infect. Dis. 2007, 44, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Materu, S.; Cox, H.S.; Isaakidis, P.; Baruani, B.; Ogaro, T.; Caugant, D.A. Serogroup X in meningococcal disease, Western Kenya. Emerg. Infect. Dis. 2007, 13, 944–945. [Google Scholar] [CrossRef]
- Mutonga, D.M.; Pimentel, G.; Muindi, J.; Nzioka, C.; Mutiso, J.; Klena, J.D.; Morcos, M.; Ogaro, T.; Materu, S.; Tetteh, C.; et al. Epidemiology and risk factors for serogroup X meningococcal meningitis during an outbreak in western Kenya, 2005–2006. Am. J. Trop. Med. Hyg. 2009, 80, 619–624. [Google Scholar] [CrossRef]
- Delrieu, I.; Yaro, S.; Tamekloe, T.A.; Njanpop-Lafourcade, B.M.; Tall, H.; Jaillard, P.; Ouedraogo, M.S.; Badziklou, K.; Sanou, O.; Drabo, A.; et al. Emergence of epidemic Neisseria meningitidis serogroup X meningitis in Togo and Burkina Faso. PLoS ONE 2011, 6, e19513. [Google Scholar] [CrossRef]
- Xie, O.; Pollard, A.J.; Mueller, J.E.; Norheim, G. Emergence of serogroup X meningococcal disease in Africa: Need for a vaccine. Vaccine 2013, 31, 2852–2861. [Google Scholar] [CrossRef]
- Chen, W.H.; Neuzil, K.M.; Boyce, C.R.; Pasetti, M.F.; Reymann, M.K.; Martellet, L.; Hosken, N.; LaForce, F.M.; Dhere, R.M.; Pisal, S.S.; et al. Safety and immunogenicity of a pentavalent meningococcal conjugate vaccine containing serogroups A, C, Y, W, and X in healthy adults: A phase 1, single-centre, double-blind, randomised, controlled study. Lancet Infect. Dis. 2018, 18, 1088–1096. [Google Scholar] [CrossRef]
- Jones, G.R.; Christodoulides, M.; Brooks, J.L.; Miller, A.R.; Cartwright, K.A.; Heckels, J.E. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: Subtype stability and specificity of the immune response following colonization. J. Infect. Dis. 1998, 178, 451–459. [Google Scholar] [CrossRef][Green Version]
- Leimkugel, J.; Hodgson, A.; Forgor, A.A.; Pfluger, V.; Dangy, J.P.; Smith, T.; Achtman, M.; Gagneux, S.; Pluschke, G. Clonal waves of Neisseria colonisation and disease in the African meningitis belt: Eight year longitudinal study in northern Ghana. PLoS Med. 2007, 4, e101. [Google Scholar] [CrossRef]
- Gagneux, S.; Wirth, T.; Hodgson, A.; Ehrhard, I.; Morelli, G.; Kriz, P.; Genton, B.; Smith, T.; Binka, F.; Pluschke, G.; et al. Clonal groupings in serogroup X Neisseria meningitidis. Emerg. Infect. Dis. 2002, 8, 462–466. [Google Scholar] [CrossRef]
- Stephens, D.S.; Greenwood, B.; Brandtzaeg, P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 2007, 369, 2196–2210. [Google Scholar] [CrossRef]
- Cohn, A.C.; MacNeil, J.R.; Harrison, L.H.; Hatcher, C.; Theodore, J.; Schmidt, M.; Pondo, T.; Arnold, K.E.; Baumbach, J.; Bennett, N.; et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: Implications for prevention of meningococcal disease. Clin. Infect. Dis. 2010, 50, 184–191. [Google Scholar] [CrossRef]
- Broker, M.; Bukovski, S.; Culic, D.; Jacobsson, S.; Koliou, M.; Kuusi, M.; Simoes, M.J.; Skoczynska, A.; Toropainen, M.; Taha, M.K.; et al. Meningococcal serogroup Y emergence in Europe: High importance in some European regions in 2012. Hum. Vaccines Immunother. 2014, 10, 1725–1728. [Google Scholar] [CrossRef][Green Version]
- Toros, B.; Hedberg, S.T.; Unemo, M.; Jacobsson, S.; Hill, D.M.; Olcen, P.; Fredlund, H.; Bratcher, H.B.; Jolley, K.A.; Maiden, M.C.; et al. Genome-Based Characterization of Emergent Invasive Neisseria meningitidis Serogroup Y Isolates in Sweden from 1995 to 2012. J. Clin. Microbiol. 2015, 53, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, S.T.; Toros, B.; Fredlund, H.; Olcen, P.; Molling, P. Genetic characterisation of the emerging invasive Neisseria meningitidis serogroup Y in Sweden, 2000 to 2010. Eurosurveillance 2011, 16, 19885. [Google Scholar] [PubMed]
- Ladhani, S.N.; Lucidarme, J.; Newbold, L.S.; Gray, S.J.; Carr, A.D.; Findlow, J.; Ramsay, M.E.; Kaczmarski, E.B.; Borrow, R. Invasive meningococcal capsular group Y disease, England and Wales, 2007–2009. Emerg. Infect. Dis. 2012, 18, 63–70. [Google Scholar] [CrossRef]
- Jackson, L.A.; Wenger, J.D. Laboratory-based surveillance for meningococcal disease in selected areas, United States, 1989–1991. Morb. Mortal. Wkly. Rep. CDC Surveill. Summ. 1993, 42, 21–30. [Google Scholar]
- Rosenstein, N.E.; Perkins, B.A.; Stephens, D.S.; Lefkowitz, L.; Cartter, M.L.; Danila, R.; Cieslak, P.; Shutt, K.A.; Popovic, T.; Schuchat, A.; et al. The changing epidemiology of meningococcal disease in the United States, 1992–1996. J. Infect. Dis. 1999, 180, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Baccarini, C.; Ternouth, A.; Wieffer, H.; Vyse, A. The changing epidemiology of meningococcal disease in North America 1945–2010. Hum. Vaccines Immunother. 2013, 9, 162–171. [Google Scholar] [CrossRef][Green Version]
- Kellerman, S.E.; McCombs, K.; Ray, M.; Baughman, W.; Reeves, M.W.; Popovic, T.; Rosenstein, N.E.; Farley, M.M.; Blake, P.; Stephens, D.S.; et al. Genotype-specific carriage of Neisseria meningitidis in Georgia counties with hyper- and hyposporadic rates of meningococcal disease. J. Infect. Dis. 2002, 186, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.H.; Shutt, K.A.; Arnold, K.E.; Stern, E.J.; Pondo, T.; Kiehlbauch, J.A.; Myers, R.A.; Hollick, R.A.; Schmink, S.; Vello, M.; et al. Meningococcal Carriage Among Georgia and Maryland High School Students. J. Infect. Dis. 2014. [Google Scholar] [CrossRef]
- Wang, X.; Shutt, K.A.; Vuong, J.T.; Cohn, A.; MacNeil, J.; Schmink, S.; Plikaytis, B.; Messonnier, N.E.; Harrison, L.H.; Clark, T.A.; et al. Changes in the Population Structure of Invasive Neisseria meningitidis in the United States After Quadrivalent Meningococcal Conjugate Vaccine Licensure. J. Infect. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Potts, C.C.; Joseph, S.J.; Chang, H.Y.; Chen, A.; Vuong, J.; Hu, F.; Jenkins, L.T.; Schmink, S.; Blain, A.; MacNeil, J.R.; et al. Population structure of invasive Neisseria meningitidis in the United States, 2011–2015. J. Infect. 2018, 77, 427–434. [Google Scholar] [CrossRef]
- Tsang, R.S.; Henderson, A.M.; Cameron, M.L.; Tyler, S.D.; Tyson, S.; Law, D.K.; Stoltz, J.; Zollinger, W.D. Genetic and antigenic analysis of invasive serogroup Y Neisseria meningitidis isolates collected from 1999 to 2003 in Canada. J. Clin. Microbiol. 2007, 45, 1753–1758. [Google Scholar] [CrossRef]
- Whitney, A.M.; Coulson, G.B.; von Gottberg, A.; Block, C.; Keller, N.; Mayer, L.W.; Messonnier, N.E.; Klugman, K.P. Genotypic comparison of invasive Neisseria meningitidis serogroup Y isolates from the United States, South Africa, and Israel, isolated from 1999 through 2002. J. Clin. Microbiol. 2009, 47, 2787–2793. [Google Scholar] [CrossRef]
- Safadi, M.A.; Cintra, O.A. Epidemiology of meningococcal disease in Latin America: Current situation and opportunities for prevention. Neurol. Res. 2010, 32, 263–271. [Google Scholar] [CrossRef]
- Ines Agudelo, C.; Sanabria, O.M.; Ovalle, M.V. Serogroup Y meningococcal disease, Colombia. Emerg. Infect. Dis. 2008, 14, 990–991. [Google Scholar] [CrossRef] [PubMed]
- Abad, R.; Agudelo, C.I.; Brandileone, M.C.; Chanto, G.; Gabastou, J.M.; Hormazabal, J.C.; MC, O.G.; Maldonado, A.; Moreno, J.; Muros-Le Rouzic, E.; et al. Molecular characterization of invasive serogroup Y Neisseria meningitidis strains isolated in the Latin America region. J. Infect. 2009, 59, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.E.O.; Villatoro, E.; Luna, M.J.; Barrientos, A.M.; Mendoza, E.; Lemos, A.P.S.; Camargo, C.H.; Sacchi, C.T.; Cunha, M.P.V.; Galas, M.; et al. Emergence of MDR invasive Neisseria meningitidis in El Salvador, 2017–2019. J. Antimicrob. Chemother. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.B.; Diggle, M.A.; Davies, R.L.; Clarke, S.C. Clonal analysis of meningococci during a 26 year period prior to the introduction of meningococcal serogroup C vaccines. PLoS ONE 2015, 10, e115741. [Google Scholar] [CrossRef]
- Broker, M.; Jacobsson, S.; DeTora, L.; Pace, D.; Taha, M.K. Increase of meningococcal serogroup Y cases in Europe: A reason for concern? Hum. Vaccines Immunother. 2012, 8, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Broker, M.; Emonet, S.; Fazio, C.; Jacobsson, S.; Koliou, M.; Kuusi, M.; Pace, D.; Paragi, M.; Pysik, A.; Simoes, M.J.; et al. Meningococcal serogroup Y disease in Europe: Continuation of high importance in some European regions in 2013. Hum. Vaccines Immunother. 2015, 11, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Ala’Aldeen, D.A.; Neal, K.R.; Ait-Tahar, K.; Nguyen-Van-Tam, J.S.; English, A.; Falla, T.J.; Hawkey, P.M.; Slack, R.C. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J. Clin. Microbiol. 2000, 38, 2311–2316. [Google Scholar] [CrossRef]
- Bidmos, F.A.; Neal, K.R.; Oldfield, N.J.; Turner, D.P.; Ala’Aldeen, D.A.; Bayliss, C.D. Persistence, replacement, and rapid clonal expansion of meningococcal carriage isolates in a 2008 university student cohort. J. Clin. Microbiol. 2011, 49, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Ala’aldeen, D.A.; Oldfield, N.J.; Bidmos, F.A.; Abouseada, N.M.; Ahmed, N.W.; Turner, D.P.; Neal, K.R.; Bayliss, C.D. Carriage of meningococci by university students, United Kingdom. Emerg. Infect. Dis. 2011, 17, 1762–1763. [Google Scholar] [CrossRef]
- Jeppesen, C.A.; Snape, M.D.; Robinson, H.; Gossger, N.; John, T.M.; Voysey, M.; Ladhani, S.; Okike, I.O.; Oeser, C.; Kent, A.; et al. Meningococcal carriage in adolescents in the United Kingdom to inform timing of an adolescent vaccination strategy. J. Infect. 2015, 71, 43–52. [Google Scholar] [CrossRef]
- Maiden, M.C.; Ibarz-Pavon, A.B.; Urwin, R.; Gray, S.J.; Andrews, N.J.; Clarke, S.C.; Walker, A.M.; Evans, M.R.; Kroll, J.S.; Neal, K.R.; et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J. Infect. Dis. 2008, 197, 737–743. [Google Scholar] [CrossRef]
- Evans, J.R.; Artenstein, M.S.; Hunter, D.H. Prevalence of meningococcal serogroups and description of three new groups. Am. J. Epidemiol. 1968, 87, 643–646. [Google Scholar] [CrossRef]
- Wachter, E.; Brown, A.E.; Kiehn, T.E.; Lee, B.J.; Armstrong, D. Neisseria meningitidis serogroup 29E (Z’) septicemia in a patient with far advanced multiple myeloma (plasma cell leukemia). J. Clin. Microbiol. 1985, 21, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Thangarajah, D.; Guglielmino, C.J.D.; Lambert, S.B.; Khandaker, G.; Vasant, B.R.; Malo, J.A.; Smith, H.V.; Jennison, A.V. Genomic Characterization of Recent and Historic Meningococcal Serogroup E Invasive Disease in Australia: A Case Series. Clin. Infect. Dis. 2020, 70, 1761–1763. [Google Scholar] [CrossRef]
- Jolley, K.A.; Kalmusova, J.; Feil, E.J.; Gupta, S.; Musilek, M.; Kriz, P.; Maiden, M.C. Carried meningococci in the Czech Republic: A diverse recombining population. J. Clin. Microbiol. 2000, 38, 4492–4498. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.; Walters, L.; Mark, T.; Lawrence, A.; Leong, L.E.X.; Sullivan, T.; Rogers, G.B.; Andrews, R.M.; Marshall, H.S. B Part of It study: A longitudinal study to assess carriage of Neisseria meningitidis in first year university students in South Australia. Hum. Vaccines Immunother. 2019, 15, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.L.; Thomas, J.; Stephens, D.S. Regulation of capsule in Neisseria meningitidis. Crit. Rev. Microbiol. 2016, 42, 759–772. [Google Scholar] [CrossRef]
- Dolan-Livengood, J.M.; Miller, Y.K.; Martin, L.E.; Urwin, R.; Stephens, D.S. Genetic basis for nongroupable Neisseria meningitidis. J. Infect. Dis. 2003, 187, 1616–1628. [Google Scholar] [CrossRef]
- Claus, H.; Maiden, M.C.J.; Maag, R.; Frosch, M.; Vogel, U. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology 2002, 148, 1813–1819. [Google Scholar] [CrossRef]
- Barnes, G.K.; Kristiansen, P.A.; Beyene, D.; Workalemahu, B.; Fissiha, P.; Merdekios, B.; Bohlin, J.; Preziosi, M.P.; Aseffa, A.; Caugant, D.A. Prevalence and epidemiology of meningococcal carriage in Southern Ethiopia prior to implementation of MenAfriVac, a conjugate vaccine. BMC Infect. Dis. 2016, 16, 639. [Google Scholar] [CrossRef]
- Neri, A.; Fazio, C.; Ambrosio, L.; Vacca, P.; Barbui, A.; Daprai, L.; Vocale, C.; Santino, I.; Conte, M.; Rossi, L.; et al. Carriage meningococcal isolates with capsule null locus dominate among high school students in a non-endemic period, Italy, 2012–2013. Int. J. Med. Microbiol. 2019, 309, 182–188. [Google Scholar] [CrossRef]
- Hoang, L.M.; Thomas, E.; Tyler, S.; Pollard, A.J.; Stephens, G.; Gustafson, L.; McNabb, A.; Pocock, I.; Tsang, R.; Tan, R. Rapid and fatal meningococcal disease due to a strain of Neisseria meningitidis containing the capsule null locus. Clin. Infect. Dis. 2005, 40, e38–42. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhu, B.; Xu, L.; Gao, Y.; Shao, Z. First case of Neisseria meningitidis capsule null locus infection in China. Infect. Dis. 2015, 47, 591–592. [Google Scholar] [CrossRef]
- Johswich, K.O.; Zhou, J.; Law, D.K.; St Michael, F.; McCaw, S.E.; Jamieson, F.B.; Cox, A.D.; Tsang, R.S.; Gray-Owen, S.D. Invasive potential of nonencapsulated disease isolates of Neisseria meningitidis. Infect. Immun. 2012, 80, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Findlow, H.; Vogel, U.; Mueller, J.E.; Curry, A.; Njanpop-Lafourcade, B.M.; Claus, H.; Gray, S.J.; Yaro, S.; Traore, Y.; Sangare, L.; et al. Three cases of invasive meningococcal disease caused by a capsule null locus strain circulating among healthy carriers in Burkina Faso. J. Infect. Dis. 2007, 195, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Allam, M.; Wolter, N.; Bratcher, H.B.; Harrison, O.B.; Lucidarme, J.; Borrow, R.; de Gouveia, L.; Meiring, S.; Birkhead, M.; et al. Molecular characterization of invasive capsule null Neisseria meningitidis in South Africa. BMC Microbiol. 2017, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Itoda, I.; Shimuta, K.; Takahashi, H.; Ohnishi, M. Urethritis caused by novel Neisseria meningitidis serogroup W in man who has sex with men, Japan. Emerg. Infect. Dis. 2014, 20, 1585–1587. [Google Scholar] [CrossRef]
- Khemees, T.A.; Porshinsky, B.S.; Patel, A.P.; McClung, C.D. Fournier’s Gangrene in a Heterosexual Man: A Complication of Neisseria meningitidis Urethritis. Case Rep. Urol. 2012, 2012, 312365. [Google Scholar] [CrossRef]
- Maini, M.; French, P.; Prince, M.; Bingham, J.S. Urethritis due to Neisseria meningitidis in a London genitourinary medicine clinic population. Int. J. STD AIDS 1992, 3, 423–425. [Google Scholar] [CrossRef]
- Leoni, A.F.; Littvik, A.; Moreno, S.B. Pelvic inflammatory disease associated with Neisseria meningitis bacteremia. Rev. Fac. Cienc. Med. 2003, 60, 77–81. [Google Scholar]
- Harrison, O.B.; Cole, K.; Peters, J.; Cresswell, F.; Dean, G.; Eyre, D.W.; Paul, J.; Maiden, M.C. Genomic analysis of urogenital and rectal Neisseria meningitidis isolates reveals encapsulated hyperinvasive meningococci and coincident multidrug-resistant gonococci. Sex. Transm. Infect. 2017, 93, 445–451. [Google Scholar] [CrossRef]
- Ma, K.C.; Unemo, M.; Jeverica, S.; Kirkcaldy, R.D.; Takahashi, H.; Ohnishi, M.; Grad, Y.H. Genomic Characterization of Urethritis-Associated Neisseria meningitidis Shows that a Wide Range of N. meningitidis Strains Can Cause Urethritis. J. Clin. Microbiol. 2017, 55, 3374–3383. [Google Scholar] [CrossRef]
- Bazan, J.A.; Peterson, A.S.; Kirkcaldy, R.D.; Briere, E.C.; Maierhofer, C.; Turner, A.N.; Licon, D.B.; Parker, N.; Dennison, A.; Ervin, M.; et al. Notes from the Field: Increase in Neisseria meningitidis-Associated Urethritis Among Men at Two Sentinel Clinics—Columbus, Ohio, and Oakland County, Michigan, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Kretz, C.B.; Bergeron, G.; Aldrich, M.; Bloch, D.; Del Rosso, P.E.; Halse, T.A.; Ostrowsky, B.; Liu, Q.; Gonzalez, E.; Omoregie, E.; et al. Neonatal Conjunctivitis Caused by Neisseria meningitidis US Urethritis Clade, New York, USA, August 2017. Emerg. Infect. Dis. 2019, 25, 972–975. [Google Scholar] [CrossRef]
- Taha, M.K.; Claus, H.; Lappann, M.; Veyrier, F.J.; Otto, A.; Becher, D.; Deghmane, A.E.; Frosch, M.; Hellenbrand, W.; Hong, E.; et al. Evolutionary Events Associated with an Outbreak of Meningococcal Disease in Men Who Have Sex with Men. PLoS ONE 2016, 11, e0154047. [Google Scholar] [CrossRef]
- Tzeng, Y.L.; Giuntini, S.; Berman, Z.; Sannigrahi, S.; Granoff, D.M.; Stephens, D.S. Neisseria meningitidis Urethritis Outbreak Isolates Express a Novel Factor H Binding Protein Variant That Is a Potential Target of Group B-Directed Meningococcal (MenB) Vaccines. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef]
- Bartley, S.N.; Tzeng, Y.L.; Heel, K.; Lee, C.W.; Mowlaboccus, S.; Seemann, T.; Lu, W.; Lin, Y.H.; Ryan, C.S.; Peacock, C.; et al. Attachment and Invasion of Neisseria meningitidis to Host Cells Is Related to Surface Hydrophobicity, Bacterial Cell Size and Capsule. PLoS ONE 2013, 8, e55798. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Rasmussen, A.W.; Gudlavalleti, S.K.; Stephens, D.S.; Stojiljkovic, I. Biofilm Formation by Neisseria meningitidis. Infect. Immun. 2004, 72, 6132–6138. [Google Scholar] [CrossRef] [PubMed]
- Neil, R.B.; Shao, J.Q.; Apicella, M.A. Biofilm formation on human airway epithelia by encapsulated Neisseria meningitidis serogroup B. Microbes Infect. 2009, 11, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Sukhum, K.V.; Jean, S.; Wallace, M.; Anderson, N.; Burnham, C.A.; Dantas, G. Genomic characterization of emerging bacterial uropathogen Neisseria meningitidis misidentified as Neisseria gonorrhoeae by nucleic acid amplification testing. J. Clin. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.; Lucidarme, J.; Campbell, H.; Campbell, L.; Fifer, H.; Gray, S.; Hughes, G.; Lekshmi, A.; Schembri, G.; Rayment, M.; et al. Detection of the United States Neisseria meningitidis urethritis clade in the United Kingdom, August and December 2019—Emergence of multiple antibiotic resistance calls for vigilance. Eurosurveillance 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; de la Fuente, L.; Berron, S.; O’Rourke, M.; Smith, N.H.; Zhou, J.; Spratt, B.G. Ecological separation and genetic isolation of Neisseria gonorrhoeae and Neisseria meningitidis. Curr. Biol. 1993, 3, 567–572. [Google Scholar] [CrossRef]
- Bratcher, H.B.; Corton, C.; Jolley, K.A.; Parkhill, J.; Maiden, M.C. A gene-by-gene population Genom. platform: De novo assembly, annotation and genealogical analysis of 108 representative Neisseria meningitidis genomes. BMC Genom. 2014, 15, 1138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Alikhan, N.F.; Sergeant, M.J.; Luhmann, N.; Vaz, C.; Francisco, A.P.; Carrico, J.A.; Achtman, M. GrapeTree: Visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018, 28, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Maiden, M.C. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.; Keshavan, P.; Welsch, J.A.; Han, L.; Smolenov, I. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum. Vaccines Immunother. 2016, 12, 1300–1310. [Google Scholar] [CrossRef]
- Trotter, C.L.; Andrews, N.J.; Kaczmarski, E.B.; Miller, E.; Ramsay, M.E. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 2004, 364, 365–367. [Google Scholar] [CrossRef]
- Ramsay, M.E.; Andrews, N.J.; Trotter, C.L.; Kaczmarski, E.B.; Miller, E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: Database analysis. BMJ 2003, 326, 365–366. [Google Scholar] [CrossRef]
- Swartley, J.S.; Marfin, A.A.; Edupuganti, S.; Liu, L.J.; Cieslak, P.; Perkins, B.; Wenger, J.D.; Stephens, D.S. Capsule switching of Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 1997, 94, 271–276. [Google Scholar] [CrossRef]
- Tsang, R.S.; Law, D.K.; Tyler, S.D.; Stephens, G.S.; Bigham, M.; Zollinger, W.D. Potential capsule switching from serogroup Y to B: The characterization of three such Neisseria meningitidis isolates causing invasive meningococcal disease in Canada. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, M.; Guiyoule, A.; Ruckly, C.; Hong, E.; Alonso, J.M.; Taha, M.K. Conserved virulence of C to B capsule switched Neisseria meningitidis clinical isolates belonging to ET-37/ST-11 clonal complex. Microbes Infect. 2006, 8, 191–196. [Google Scholar] [CrossRef]
- Beddek, A.J.; Li, M.S.; Kroll, J.S.; Jordan, T.W.; Martin, D.R. Evidence for capsule switching between carried and disease-causing Neisseria meningitidis strains. Infect. Immun. 2009, 77, 2989–2994. [Google Scholar] [CrossRef]
- Harrison, L.H.; Shutt, K.A.; Schmink, S.E.; Marsh, J.W.; Harcourt, B.H.; Wang, X.; Whitney, A.M.; Stephens, D.S.; Cohn, A.A.; Messonnier, N.E.; et al. Population structure and capsular switching of invasive Neisseria meningitidis isolates in the pre-meningococcal conjugate vaccine era—United States, 2000–2005. J. Infect. Dis. 2010, 201, 1208–1224. [Google Scholar] [CrossRef]
- Harris, S.L.; Tan, C.; Andrew, L.; Hao, L.; Liberator, P.A.; Absalon, J.; Anderson, A.S.; Jones, T.R. The bivalent factor H binding protein meningococcal serogroup B vaccine elicits bactericidal antibodies against representative non-serogroup B meningococci. Vaccine 2018, 36, 6867–6874. [Google Scholar] [CrossRef] [PubMed]
- Muzzi, A.; Brozzi, A.; Serino, L.; Bodini, M.; Abad, R.; Caugant, D.; Comanducci, M.; Lemos, A.P.; Gorla, M.C.; Krizova, P.; et al. Genetic Meningococcal Antigen Typing System (gMATS): A genotyping tool that predicts 4CMenB strain coverage worldwide. Vaccine 2019, 37, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Biolchi, A.; De Angelis, G.; Moschioni, M.; Tomei, S.; Brunelli, B.; Giuliani, M.; Bambini, S.; Borrow, R.; Claus, H.; Gorla, M.C.O.; et al. Multicomponent meningococcal serogroup B vaccination elicits cross-reactive immunity in infants against genetically diverse serogroup C, W and Y invasive disease isolates. Vaccine 2020, 38, 7542–7550. [Google Scholar] [CrossRef] [PubMed]
- Linz, B.; Schenker, M.; Zhu, P.; Achtman, M. Frequent interspecific genetic exchange between commensal Neisseriae and Neisseria meningitidis. Mol. Microbiol. 2000, 36, 1049–1058. [Google Scholar] [CrossRef]

| Vaccine Product | Trade Name | Age Group | Year Licensed |
|---|---|---|---|
| Polysaccharide Conjugate (Groups A, C, W, and Y) | |||
| MenACWY-D | Menactra | 9 months–55 years | 2005 |
| MenACWY-CRM | Menveo | ≥2 months | 2010 |
| MenACWY-TT | MenQuadfi #/Nimenrix | ≥1 year/≥6 weeks | 2020/2012 |
| MenA-TT | MenAfriVac | 3 months–29 years | 2010 |
| Protein based (directed at group B) | |||
| MenB-FHbp | Trumenba | 10–25 years | 2014 |
| MenB-4C # | Bexsero | ≥2 months | 2015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzeng, Y.-L.; Stephens, D.S. A Narrative Review of the W, X, Y, E, and NG of Meningococcal Disease: Emerging Capsular Groups, Pathotypes, and Global Control. Microorganisms 2021, 9, 519. https://doi.org/10.3390/microorganisms9030519
Tzeng Y-L, Stephens DS. A Narrative Review of the W, X, Y, E, and NG of Meningococcal Disease: Emerging Capsular Groups, Pathotypes, and Global Control. Microorganisms. 2021; 9(3):519. https://doi.org/10.3390/microorganisms9030519
Chicago/Turabian StyleTzeng, Yih-Ling, and David S. Stephens. 2021. "A Narrative Review of the W, X, Y, E, and NG of Meningococcal Disease: Emerging Capsular Groups, Pathotypes, and Global Control" Microorganisms 9, no. 3: 519. https://doi.org/10.3390/microorganisms9030519
APA StyleTzeng, Y.-L., & Stephens, D. S. (2021). A Narrative Review of the W, X, Y, E, and NG of Meningococcal Disease: Emerging Capsular Groups, Pathotypes, and Global Control. Microorganisms, 9(3), 519. https://doi.org/10.3390/microorganisms9030519






