Effect of Colonization of Trichoderma harzianum on Growth Development and CBD Content of Hemp (Cannabis sativa L.)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Agronomic Characteristics
3.2. Yield Characteristics and CBD Content
4. Discussion
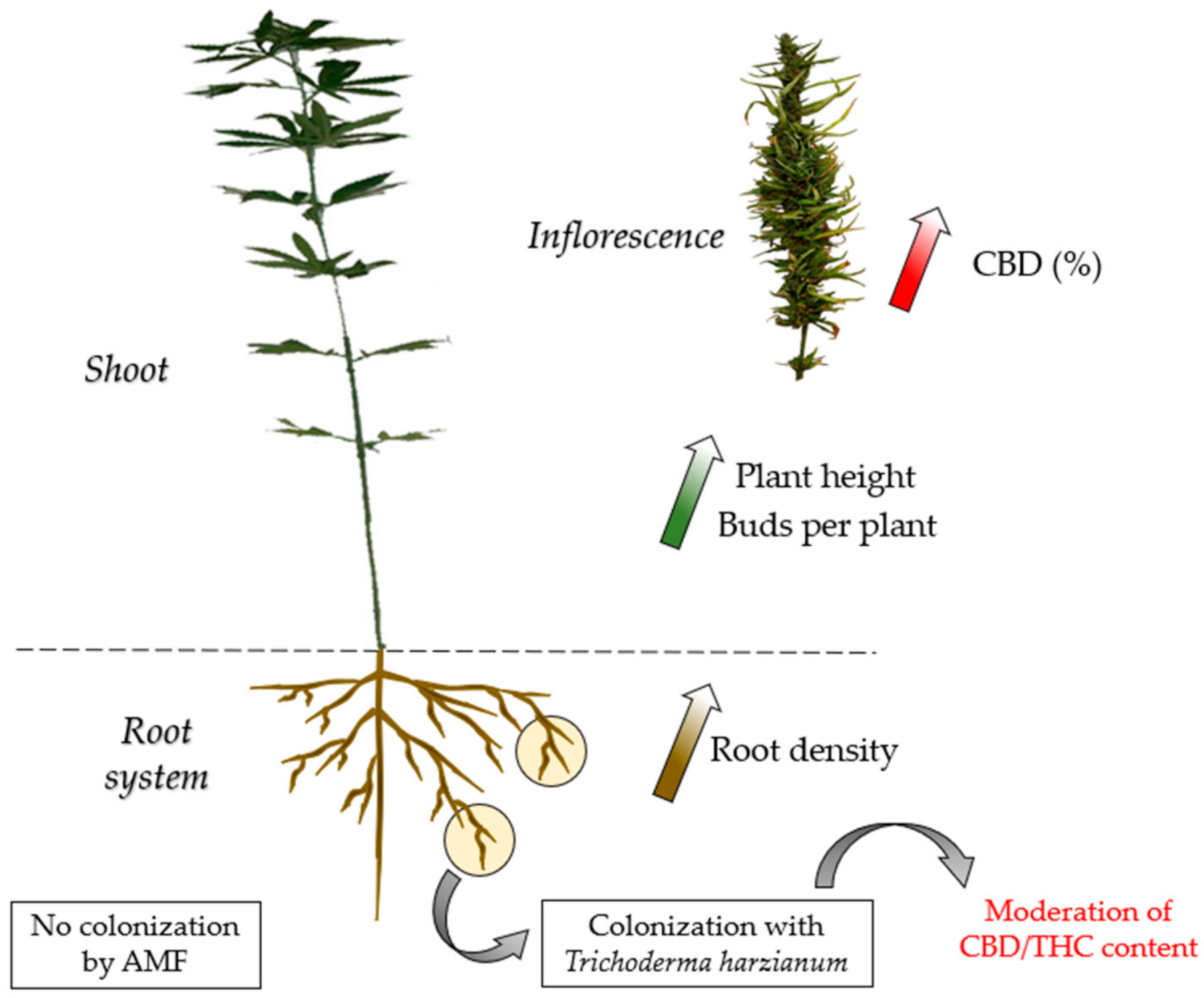
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Schultes, R.E.; Klein, W.M.; Plowman, T.; Lockwood, T.E. Cannabis: An example of taxonomic neglect. Bot. Mus. Leafl. Harv. Univ. 1974, 23, 337–367. [Google Scholar]
- Papastylianou, P.; Kakabouki, I.; Travlos, I. Effect of nitrogen fertilization on growth and yield of industrial hemp (Cannabis sativa L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 197–201. [Google Scholar] [CrossRef]
- Groom, Q.; Clarke, R.C.; Merlin, M.D. Cannabis: Evolution and Ethnobotany. Plant Ecol. Evoution 2014, 147, 149. [Google Scholar] [CrossRef]
- Allegret, S. The history of hemp. In Hemp: Industrial Production and Uses; Bouloc, P., Allegret, S., Arnaud, L., Eds.; CAB International: Bar sur Aube, France, 2013; pp. 4–25. ISBN 9781845937928. [Google Scholar]
- Kousta, A.; Papastylianou, P.; Cheimona, N.; Travlos, I.; Kakabouki, I.; Bilalis, D. Effect of Fertilization and Weed Man-agement on Weed Flora of Hemp Crop. Bull. UASVM Hortic. 2020, 77, 2. [Google Scholar]
- Amaducci, S.; Scordia, D.; Liu, F.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S. Key cultivation techniques for hemp in Europe and China. Ind. Crop. Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- European Commission. Common Catalogue of Varieties of Agricultural Plant Species. Available online: https://op.europa.eu/ (accessed on 16 June 2020).
- European Commission. Directive 2012/27/Eu of the European Parliament and of the Council of 25 October 2012 on the Energy Efficiency, Amending Directive 2009/125/EC and 2010/30/EU and Repealing Directives 2004/8/EC and 2006/32/EC (Text with EEA Relevance). Available online: http://eur-lex.europa.eu (accessed on 15 July 2019).
- Mark, T.; Shepherd, J.; Olson, D.; Snell, W.; Proper, S.; Thornsbury, S. February Economic Viability of Industrial Hemp in the United States: A Review of State Pilot Programs EIB-217; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2020.
- Salentijn, E.M.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New developments in fiber hemp (Cannabis sativa L.) breeding. Ind. Crop. Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Tsaliki, E.; Kalivas, A.; Jankauskiene, Z.; Irakli, M.; Cook, C.; Grigoriadis, I.; Panoras, I.; Vasilakoglou, I.; Dhima, K. Fibre and Seed Productivity of Industrial Hemp (Cannabis sativa L.) Varieties under Mediterranean Conditions. Agron. 2021, 11, 171. [Google Scholar] [CrossRef]
- Alexander, S.P. Therapeutic potential of cannabis-related drugs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, J.; Bernstein, N. Chemical and Physical Elicitation for Enhanced Cannabinoid Production in Cannabis. In Cannabis sativa L.—Botany and Biotechnology; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 439–456. [Google Scholar]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and humic acid supplementation on the chemical pro-file of medical cannabis (Cannabis sativa L). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef]
- Johnson, R. Hemp as an Agricultural Commodity. Available online: https://fas.org/sgp/crs/misc/RL32725.pdf (accessed on 23 December 2019).
- Backer, R.; Schwinghamer, T.; Rosenbaum, P.; Mccarty, V.; Bilodeau, S.E.; Lyu, D.; Ahmed, B.; Robinson, G.; Lefsrud, M.; Wilkins, O.; et al. Closing the Yield Gap for Cannabis: A Meta-Analysis of Factors Determining Cannabis Yield. Front. Plant Sci. 2019, 10, 495. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Amaducci, S. Comparing hemp (Cannabis sativa L.) cultivars for dual-purpose production under contrasting environments. Ind. Crop. Prod. 2016, 87, 33–44. [Google Scholar] [CrossRef]
- Folina, A.; Roussis, I.; Kouneli, V.; Kakabouki, I.; Karidogianni, S.; Bilalis, D.; Kadoglou, N. Evaluation of Woven Agro-textiles in the Development of Hemp (Cannabis sativa L.) in Greenhouse. Bull. UASVM Hortic. 2020, 77, 53–62. [Google Scholar]
- Carus, M.; Sarmento, L. The European Hemp Industry: Cultivation, Processing and Applications for Fibres, Shivs, Seeds and Flowers; European Industrial Hemp Association: Brussels, Belgium, 2016; pp. 1–9. [Google Scholar]
- Calzolari, D.; Magagnini, G.; Lucini, L.; Grassi, G.; Appendino, G.B.; Amaducci, S. High added-valuecompounds from Cannabis threshing residues. Ind. Crop. Prod. 2017, 108, 558–563. [Google Scholar] [CrossRef]
- Welling, M.T.; Liu, L.; Shapter, T.; Raymond, C.A.; King, G.J. Characterisation of cannabinoid composition in a diverse Cannabis sativa L. germplasm collection. Euphytica 2016, 208, 463–475. [Google Scholar] [CrossRef]
- Toth, J.A.; Stack, G.M.; Cala, A.R.; Carlson, C.H.; Wilk, R.L.; Crawford, J.L.; Viands, D.R.; Philippe, G.; Smart, C.D.; Rose, J.K.C.; et al. Development and validation of genetic markers for sex and cannabinoid chemotype in Cannabis sativa L. GCB Bioenergy 2020, 12, 213–222. [Google Scholar] [CrossRef]
- Ogden, M.; Hoefgen, R.; Roessner, U.; Persson, S.; Khan, G.A. Feeding the walls: How does nutrient availability regulate cell wall composition? Int. J. Mol. Sci. 2018, 19, 2691. [Google Scholar] [CrossRef] [PubMed]
- Kakabouki, I.; Kousta, A.; Folina, A.; Karydogianni, S.; Zisi, C.; Varvara, K.; Papastylianou, P. Effect of Fertilization with Urea and Inhibitors on Growth, Yield and CBD Concentration of Hemp (Cannabis sativa L.). Sustainability 2021, 13, 2157. [Google Scholar] [CrossRef]
- Tedeschi, A.; Volpe, M.G.; Polimeno, F.; Siano, F.; Maglione, G.; Di Tommasi, P.; Vasca, E.; Magliulo, V.; Vitale, L. Soil Fertilization with Urea Has Little Effect on Seed Quality but Reduces Soil N2O Emissions from a Hemp Cultivation. Agriculture 2020, 10, 240. [Google Scholar] [CrossRef]
- Cai, F.; Chen, W.; Wei, Z.; Pang, G.; Li, R.; Ran, W.; Shen, Q. Colonization of Trichoderma harzianum strain SQR-T037 on tomato roots and its relationship to plant growth, nutrient availability and soil microflora. Plant Soil 2014, 388, 337–350. [Google Scholar] [CrossRef]
- Altomare, C.; Tringovska, I. Beneficial Soil Microorganisms, an Ecological Alternative for Soil Fertility Management. Sustain. Agric. Rev. 2011, 161–214. [Google Scholar] [CrossRef]
- Shaharoona, B.; Naveed, M.; Arshad, M.; Zahir, Z.A. Fertilizer-dependent efficiency of Pseudomonads for improving growth, yield, and nutrient use efficiency of wheat (Triticum aestivum L.). Appl. Microbiol. Biotechnol. 2008, 79, 147–155. [Google Scholar] [CrossRef]
- Adesemoye, A.; Torbert, H.; Kloepper, J. Increased plant uptake of nitrogen from 15N-depleted fertilizer using plant growth-promoting rhizobacteria. Appl. Soil Ecol. 2010, 46, 54–58. [Google Scholar] [CrossRef]
- Kaewchai, S. Mycofungicides and fungal biofertilizers. Fungal Divers. 2009, 38, 25–50. [Google Scholar]
- Debbi, A.; Boureghda, H.; Monte, E.; Hermosa, R. Distribution and Genetic Variability of Fusarium oxysporum Associated with Tomato Diseases in Algeria and a Biocontrol Strategy with Indigenous Trichoderma spp. Front. Microbiol. 2018, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Vicente, I.; Baroncelli, R.; Morán-Diez, M.E.; Bernardi, R.; Puntoni, G.; Hermosa, R.; Monte, E.; Vannacci, G.; Sarrocco, S. Combined Comparative Genomics and Gene Expression Analyses Provide Insights into the Terpene Synthases Inventory in Trichoderma. Microorganisms 2020, 8, 1603. [Google Scholar] [CrossRef]
- Gachomo, E.W.; Kotchoni, S.O. The Use of Trichoderma harzianum and T. viride as Potential Biocontrol Agents against Peanut Microflora and Their Effectiveness in Reducing Aflatoxin Contamination of Infected Kernels. Biotechnology 2008, 7, 439–447. [Google Scholar] [CrossRef]
- Coppola, M.; Diretto, G.; Digilio, M.C.; Woo, S.L.; Giuliano, G.; Molisso, D.; Pennacchio, F.; Lorito, M.; Rao, R. Transcriptome and Metabolome Reprogramming in Tomato Plants by Trichoderma harzianum strain T22 Primes and Enhances Defense Responses against Aphids. Front. Physiol. 2019, 10, 745. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhuang, L.; Yu, Y.; Liu, J.; Zhang, L.; Gao, Z.; Wu, Y.; Gao, W.; Ding, G. A rhizo-sphere-derived consortium of Bacillus subtilis and Trichoderma harzianum suppresses common scab of potato and increases yield. Comput. Struct. Biotechnol. J. 2019, 17, 645–653. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Kucuk, C. Enhanced Root and Shoot Growth of Wheat (Triticum aestivum L.) by Trichoderma harzianum from Turkey. Pak. J. Biol. Sci. 2013, 17, 122–125. [Google Scholar] [CrossRef]
- Bal, U.; Altintas, S. Application of the antagonistic fungus Trichoderma harzianum (TrichoFlow WP™) to root zone in-creases yield of bell peppers grown in soil. Biol. Agric. Hortic. 2006, 24, 149–163. [Google Scholar] [CrossRef]
- Hoyos-Carvajal, L.; Orduz, S.; Bissett, J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Control. 2009, 51, 409–416. [Google Scholar] [CrossRef]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- Buysens, C.; César, V.; Ferrais, F.; de Boulois, H.D.; Declerck, S. Inoculation of Medicago sativa cover crop with Rhizoph-agusirregularis and Trichoderma harzianum increases the yield of subsequently-grown potato under low nutrient condi-tions. Appl. Soil Ecol. 2016, 105, 137–143. [Google Scholar] [CrossRef]
- Bal, U.; Altintas, S. Effects of Trichoderma harzianum on lettuce in protected cultivation. J. Cent. Eur. Agric. 2008, 9, 63–70. [Google Scholar]
- Goicoechea, N.; Antolín, M.C. Increased nutritional value in food crops. Microb. Biotechnol. 2017, 10, 1004–1007. [Google Scholar] [CrossRef]
- Kakabouki, I.; Tsirogiannis, D.; Karydogianni, S.; Folina, A.; Zisi, C.; Platanopoulos, E.; Papadopoulos, G.; Grammenos, G.; Bilalis, D. Interaction of arbuscular mycorrhizal fungi and trichoderma on growth of root system and on yield of in-dustrial hemp (Cannabis sativa var.’Uso’). Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Hortic. 2020, 77, 25–29. [Google Scholar]
- Kokko, E.; Volkmar, K.; Gowen, B.; Entz, T. Determination of total root surface area in soil core samples by image analysis. Soil Tillage Res. 1993, 26, 33–43. [Google Scholar] [CrossRef]
- Bilalis, D.; Katsenios, N.; Efthimiadou, A.; Efthimiadis, P.; Karkanis, A. Pulsed electromagnetic fields effect in oregano rooting and vegetative propagation: A potential new organic method. Acta Agric. Scand. Sect. B Plant Soil Sci. 2012, 62, 94–99. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Isermeyer, H. Eine einfache Methode zur Bestimmung der Bodenatmung und der Karbonate im Boden. J. Plant Nutr. Soil Sci. 1952, 56, 26–38. [Google Scholar] [CrossRef]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Bjorkman, T.; Harman, G.E. Solubilization of Phosphates and Micronutrients by the Plant-Growth-Promoting and Biocontrol Fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Rabeendran, N.; Moot, D.J.; Jones, E.E.; Stewart, A. Inconsistent growth promotion of cabbage and lettuce from Trichoderma isolates. N. Z. Plant Prot. 2000, 53, 143–146. [Google Scholar] [CrossRef]
- Kleifeld, O.; Chet, I. Trichoderma harzianum—Interaction with plants and effect on growth response. Plant Soil 1992, 144, 267–272. [Google Scholar] [CrossRef]
- Powel, C.L.; Bagyaraj, D.J. VA Mycorrhiza; CRC Press: Boca Raton, FL, USA, 1984; pp. 1–60. [Google Scholar] [CrossRef]
- Windham, M.T.; Elad, Y.; Baker, R. A mechanism for increased plant growth induced by Trichoderma spp. Phytopathology 1986, 76, 518–521. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chang, Y.C.; Baker, R.; Kleifeld, O.; Chet, I. Increased growth of plants in the presence of the biological control agent Trichoderma harzianum. Plant Dis. 1986, 70, 145–148. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: San Diego, CA, USA, 2010. [Google Scholar]
- Gorelick, J.; Bernstein, N. Elicitation: An underutilized tool in the development of medicinal plants as a source of therapeutic secondary metabolites. Adv. Agron. 2014, 124, 201–230. [Google Scholar]
- Coffman, C.B.; Gentner, W.A. Responses of Greenhouse-grown Cannabis sativa L. to Nitrogen, Phosphorus, and Potassium. Agron. J. 1977, 69, 832–836. [Google Scholar] [CrossRef]
- Harman, G.E. Trichoderma—Not just for biocontrol anymore. Phytoparasitica 2011, 39, 103–108. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Porras-Troncoso, M.D.; Olmedo-Monfil, V.; Herrera-Estrella, A. Trichoderma Species: Versatile Plant Symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef]
- Vinale, F.; Manganiello, G.; Nigro, M.; Mazzei, P.; Piccolo, A.; Pascale, A.; Ruocco, M.; Marra, R.; Lombardi, N.; Lan-zuise, S.; et al. A novel fungal metabolite with beneficial properties for agricultural applications. Molecules 2014, 19, 9760–9772. [Google Scholar] [CrossRef] [PubMed]
- Şesan, T.E.; Oancea, A.O.; Ştefan, L.M.; Mănoiu, V.S.; Ghiurea, M.; Răut, I.; Constantinescu-Aruxandei, D.; Toma, A.; Savin, S.; Bira, A.F.; et al. Effects of Foliar Treatment with a Trichoderma Plant Biostimulant Consortium on Passiflora caerulea L. Yield and Quality. Microorganisms 2020, 8, 123. [Google Scholar] [CrossRef]
- Zúñiga-Silgado, D.; Rivera-Leyva, J.C.; Coleman, J.J.; Sánchez-Reyez, A.; Valencia-Díaz, S.; Serrano, M.; De-Bashan, L.E.; Folch-Mallol, J.L. Soil Type Affects Organic Acid Production and Phosphorus Solubilization Efficiency Mediated by Several Native Fungal Strains from Mexico. Microorganisms 2020, 8, 1337. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, T.; Raza, W.; Liu, D.; Shen, Q. The Growth Promotion of Peppers (Capsicum annuum L.) by Trichoderma guizhouense NJAU4742-Based Biological Organic Fertilizer: Possible Role of Increasing Nutrient Availabilities. Microorganisms 2020, 8, 1296. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Mulè, G.; Favilla, M.; Altomare, C. Isolation and characterisation of a trichodiene synthase homologous gene in Trichoderma harzianum. Physiol. Mol. Plant Pathol. 2004, 65, 11–20. [Google Scholar] [CrossRef]
- Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Wang, X.; Liu, B. Trichoderma Enhances Net Photosynthesis, Water Use Efficiency, and Growth of Wheat (Triticum aestivum L.) under Salt Stress. Microorganisms 2020, 8, 1565. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Almeida, N.O.; Da Rocha, M.R.; Rezende, M.H.; Carneiro, R.G.D.S.; Ulhoa, C.J. Anatomical changes induced by isolates of Trichoderma spp. in soybean plants. PLoS ONE 2020, 15, e0242480. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Prieto, S.; Porteous-Álvarez, A.; Mezquita-García, S.; Rodríguez-González, Á.; Carro-Huerga, G.; del Ser-Herrero, S.; Gutiérrez, S.; Casquero, P. Influence of Physicochemical Characteristics of Bean Crop Soil in Trichoderma spp. Development. Agronomy 2021, 11, 274. [Google Scholar] [CrossRef]
- Stummer, B.E.; Zhang, Q.; Zhang, X.; Warren, R.; Harvey, P.R. Quantification of Trichoderma afroharzianum, Trichoderma harzianum and Trichoderma gamsii inoculants in soil, the wheat rhizosphere and in planta suppression of the crown rot pathogen Fusarium pseudograminearum. J. Appl. Microbiol. 2020, 129, 971–990. [Google Scholar] [CrossRef]
- Ortuño, N.; Castillo, J.A.; Miranda, C.; Claros, M.; Soto, X. The use of secondary metabolites extracted from Trichoderma for plant growth promotion in the Andean highlands. Renew. Agric. Food Syst. 2016, 32, 366–375. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Alhaithloul, H.A.S.; Qari, S.H.; Soliman, M.H.; Hasanuzzaman, M. Pretreatment with Trichoderma harzianum alleviates waterlogging-induced growth alterations in tomato seedlings by modulating physiological, biochemical, and molecular mechanisms. Environ. Exp. Bot. 2020, 171, 103946. [Google Scholar] [CrossRef]
- Aleksandr, L.; Bitiutskikh, K. Investigation on the use of hemp flour in cookie production. Bulg. J. Agric. Sci. 2017, 23, 664–667. [Google Scholar]
- Caplan, D.; Dixon, M.; Zheng, Y. Optimal Rate of Organic Fertilizer during the Flowering Stage for Cannabis Grown in Two Coir-based Substrates. HortScience 2017, 52, 1796–1803. [Google Scholar] [CrossRef]
- Lowe, H.; Steele, B.; Bryant, J.; Toyang, N.; Ngwa, W. Non-Cannabinoid Metabolites of Cannabis sativa L. with Therapeutic Potential. Plants 2021, 10, 400. [Google Scholar] [CrossRef]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Q.; Gao, S.-S.; Young, A.E.; Jacobsen, S.E.; Tang, Y. Genome mining and biosynthesis of a polyketide from a biofertilizer fungus that can facilitate reductive iron assimilation in plant. Proc. Natl. Acad. Sci. USA 2019, 116, 5499–5504. [Google Scholar] [CrossRef]
- Cockson, P.; Schroeder-Moreno, M.; Veazie, P.; Barajas, G.; Logan, D.; Davis, M.; Whipker, B.E. Impact of Phosphorus on Cannabis sativa Reproduction, Cannabinoids, and Terpenes. Appl. Sci. 2020, 10, 7875. [Google Scholar] [CrossRef]
- Hewedy, O.A.; Lateif, K.S.A.; Seleiman, M.F.; Shami, A.; Albarakaty, F.M.; El-Meihy, R.M. Phylogenetic Diversity of Trichoderma Strains and Their Antagonistic Potential against Soil-Borne Pathogens under Stress Conditions. Biology 2020, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A Review on the Current State of Knowledge of Growing Conditions, Agronomic Soil Health Practices and Utilities of Hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-Pentyl-α-pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop. Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Li, J.; Philp, J.; Li, J.; Wei, Y.; Li, H.; Yang, K.; Ryder, M.; Toh, R.; Zhou, Y.; Denton, M.D.; et al. Trichoderma harzianum Inoculation Reduces the Incidence of Clubroot Disease in Chinese Cabbage by Regulating the Rhizosphere Microbial Community. Microorganisms 2020, 8, 1325. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode. Microorganisms 2020, 8, 401. [Google Scholar] [CrossRef]
- Illescas, M.; Rubio, M.B.; Hernández-Ruiz, V.; Morán-Diez, M.E.; De Alba, A.E.M.; Nicolás, C.; Monte, E.; Hermosa, R. Effect of Inorganic N Top Dressing and Trichoderma harzianum Seed-Inoculation on Crop Yield and the Shaping of Root Microbial Communities of Wheat Plants Cultivated Under High Basal N Fertilization. Front. Plant Sci. 2020, 11, 575861. [Google Scholar] [CrossRef]
- Poveda, J.; Hermosa, R.; Monte, E.; Nicolás, C. Trichoderma harzianum favours the access of arbuscular mycorrhizal fungi to non-host Brassicaceae roots and increases plant productivity. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
| pH | Olsen-P (mg kg−1 Soil) | Available Potassium (K) (mg kg−1 Soil) | Organic Matter (%) | |
|---|---|---|---|---|
| Soil | 7.36 | 15 | 215 | 1.36 |
| Compost | 7.6 | 410 | 620 | 42.41 |
| Treatment | Trichoderma harzianum Amount Added per Pot (g) | Total Applied Fungus Spores CFU kg−1 |
|---|---|---|
| Control | − | − |
| T1 | 2 | 2 × 1012 |
| T2 | 4 | 4 × 1012 |
| Root Density (mm cm−3) | AMF (%) | CO2 mg/100 g/24 h/25 °C | Plant Height (cm) | DW/Plant (g) | Number of Buds per Plant | Bud Weight Fresh (g) | Bud Moisture (%) | Bud Weight Dry (g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fedora 17 | Felina | Fedora 17 | Felina | Fedora 17 | Felina | Fedora 17 | Felina | Fedora 17 | Felina | Fedora 17 | Felina | Fedora 17 | Felina | Fedora 17 | Felina | Fedora 17 | Felina | |
| Control | 1.99 a | 2.02 a | 19.75 a | 19.75 a | 51.50 a | 50.25 a | 126.75 a | 127.50 a | 199.75 a | 201.75 a | 1.12 a | 1.16 a | 113.75 a | 114.75 a | 56.25 a | 54.50 a | 49.79 ns | 52.28 ns |
| T1 | 2.25 b | 2.25 b | 22.75 b | 22.75 b | 58.50 ab | 60.25 b | 136.25 b | 136 b | 221.25 b | 221.25 b | 1.19 ab | 1.21 ab | 117.75 a | 118 a | 60.50 b | 61 b | 46.59 ns | 46.09 ns |
| T2 | 2.32 b | 2.30 b | 25.50 c | 26.50 c | 64.50 b | 67.75 b | 142 b | 143.50 b | 231.25 b | 232 b | 1.26 b | 1.27 b | 124.50 b | 131 b | 65 c | 63 b | 43.64 ns | 48.44 ns |
| FDoses | 23.39 *** | 34.32 *** | 25.23 *** | 22.62 *** | 38.75 *** | 8.78 ** | 7.58 ** | 34.70 *** | ns | |||||||||
| FVariety | ns | ns | ns | ns | ns | ns | ns | ns | ns | |||||||||
| FDoses × Variety | ns | ns | ns | ns | ns | ns | ns | ns | ns | |||||||||
| Bud Dry Yield(g plant−1) | Bud Length(cm) | Bud Compact Index(fw/cm gr/cm) | CBD(%) | CBD Yield(g plant−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fedora 17 | Felina | Fedora 17 | Felina | Fedora 17 | Felina | Fedora 17 | Felina | Fedora 17 | Felina | |
| Control | 56.14 ns | 60.65 ns | 32.25 ns | 34.25 ns | 3.54 a | 3.35 a | 1.14 a | 1.18 a | 0.65 ns | 0.72 ns |
| T1 | 55.66 ns | 55.91 ns | 33.75 ns | 32.75 ns | 3.49 a | 3.63 b | 1.23 a | 1.24 b | 0.69 ns | 0.70 ns |
| T2 | 55.19 ns | 61.70 ns | 33 ns | 33.50 ns | 3.78 b | 3.91 c | 1.32 b | 1.29 b | 0.73 ns | 0.80 ns |
| FDoses | ns | ns | 3.90 * | 7.27 ** | ns | |||||
| FVariety | ns | ns | ns | ns | ns | |||||
| FDoses × Variety | ns | ns | ns | ns | ns | |||||
| Root Density (mm cm−3) | AMF(%) | CO2 mg/100 g/24 h/25 °C | Plant Height (cm) | DW/Plant (g) | Number of Buds per Plant | Bud Weight Fresh (g) | Bud Moisture (%) | Bud Weight Dry (g) | Bud Dry Yield(g plant−1) | CBD(%) | CBD Yield(g plant−1) | Bud Length (cm) | Bud Compact Index (fw/cm gr/cm) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root density (mm cm−3) | 1.00 | 0.67 *** | 0.60 ** | 0.76 *** | 0.94 *** | 0.81 *** | 0.68 *** | 0.63 *** | −0.14 ns | 0.28 ns | 0.84 *** | 0.56 ** | 0.18 ns | 0.46 * |
| AMF (%) | 1.00 | 0.70 *** | 0.84 *** | 0.81 *** | 0.56 ** | 0.64 *** | 0.81 *** | −0.37 ns | −0.02 ns | 0.50 * | 0.20 ns | −0.07 ns | 0.57 ** | |
| CO2 mg/100 g/24 h/25 °C | 1.00 | 0.59 ** | 0.72 *** | 0.52 ** | 0.38 ns | 0.77 *** | −0.51 * | −0.15 ns | 0.41 * | 0.06 ns | −0.21 ns | 0.44 * | ||
| Plant height (cm) | 1.00 | 0.81 *** | 0.71 *** | 0.71 *** | 0.71 *** | −0.22 ns | 0.17 ns | 0.61 ** | 0.38 ns | 0.09 ns | 0.53 ** | |||
| DW/plant (g) | 1.00 | 0.73 *** | 0.65 *** | 0.73 *** | −0.27 ns | 0.14 ns | 0.78 *** | 0.43 * | 0.12 ns | 0.47 * | ||||
| No buds per plant | 1.00 | 0.82 *** | 0.38 ns | 0.22 ns | 0.65 *** | 0.84 *** | 0.83 *** | −0.02 ns | 0.70 *** | |||||
| Bud weight fresh (g) | 1.00 | 0.37 ns | 0.35 ns | 0.66 *** | 0.69 *** | 0.77 *** | 0.06 ns | 0.78 *** | ||||||
| Bud moisture (%) | 1.00 | −0.74 *** | −0.40 ns | 0.44 * | −0.12 ns | −0.12 ns | 0.36 ns | |||||||
| Bud weight Dry (g) | 1.00 | 0.88 *** | 0.06 ns | 0.68 *** | 0.16 ns | 0.20 ns | ||||||||
| Bud Dry yield(g plant−1) | 1.00 | 0.45 * | 0.93 *** | 0.11 ns | 0.49 * | |||||||||
| CBD (%) | 1.00 | 0.74 *** | 0.14 ns | 0.48 * | ||||||||||
| CBD yield (g plant−1) | 1.00 | 0.15 ns | 0.56 ** | |||||||||||
| Bud length (cm) | 1.00 | −0.57 ** | ||||||||||||
| Bud Compact index(fw/cm gr/cm) | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Karydogianni, S.; Zisi, C.; Kouneli, V.; Konstantinou, A.; Folina, A.; Konstantas, A.; et al. Effect of Colonization of Trichoderma harzianum on Growth Development and CBD Content of Hemp (Cannabis sativa L.). Microorganisms 2021, 9, 518. https://doi.org/10.3390/microorganisms9030518
Kakabouki I, Tataridas A, Mavroeidis A, Kousta A, Karydogianni S, Zisi C, Kouneli V, Konstantinou A, Folina A, Konstantas A, et al. Effect of Colonization of Trichoderma harzianum on Growth Development and CBD Content of Hemp (Cannabis sativa L.). Microorganisms. 2021; 9(3):518. https://doi.org/10.3390/microorganisms9030518
Chicago/Turabian StyleKakabouki, Ioanna, Alexandros Tataridas, Antonios Mavroeidis, Angeliki Kousta, Stella Karydogianni, Charikleia Zisi, Varvara Kouneli, Artemis Konstantinou, Antigolena Folina, Aristidis Konstantas, and et al. 2021. "Effect of Colonization of Trichoderma harzianum on Growth Development and CBD Content of Hemp (Cannabis sativa L.)" Microorganisms 9, no. 3: 518. https://doi.org/10.3390/microorganisms9030518
APA StyleKakabouki, I., Tataridas, A., Mavroeidis, A., Kousta, A., Karydogianni, S., Zisi, C., Kouneli, V., Konstantinou, A., Folina, A., Konstantas, A., & Papastylianou, P. (2021). Effect of Colonization of Trichoderma harzianum on Growth Development and CBD Content of Hemp (Cannabis sativa L.). Microorganisms, 9(3), 518. https://doi.org/10.3390/microorganisms9030518








