Hybridization of Saccharomyces cerevisiae Sourdough Strains with Cryotolerant Saccharomyces bayanus NBRC1948 as a Strategy to Increase Diversity of Strains Available for Lager Beer Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Culture Conditions and Chemicals
2.2. Maltose and Glucose Consumpion Tests
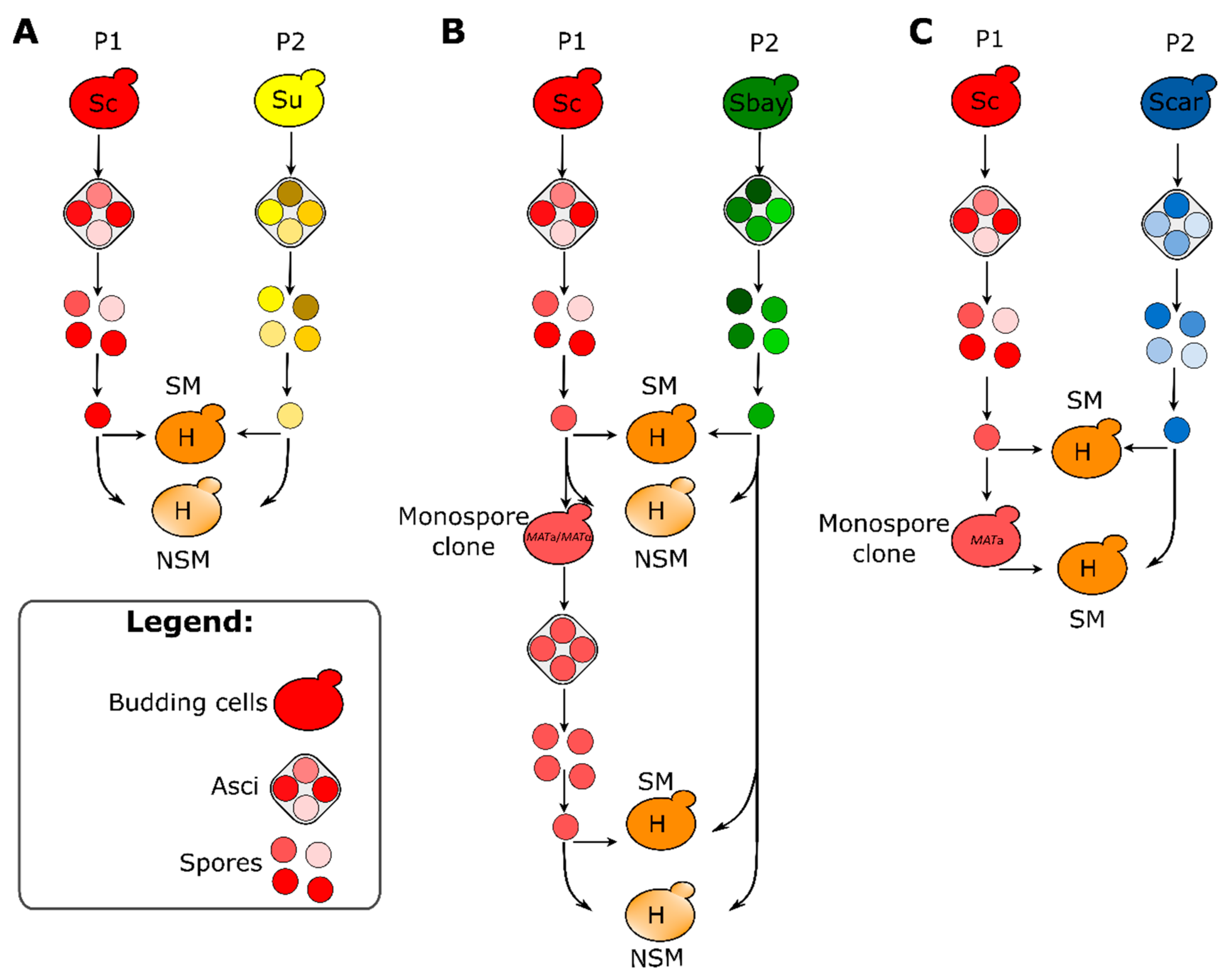
2.3. Sporulation Efficiency, Spore Viability and Generation of Spore Clones
2.4. Mating Competence Assay
2.5. Construction of Inter-Specific Hybrids
2.6. Molecular Methods
2.7. Wort Fermentations
2.8. Chemical Analysis
2.9. Statistical Analysis
3. Results
3.1. Maltose Fermentation Screening
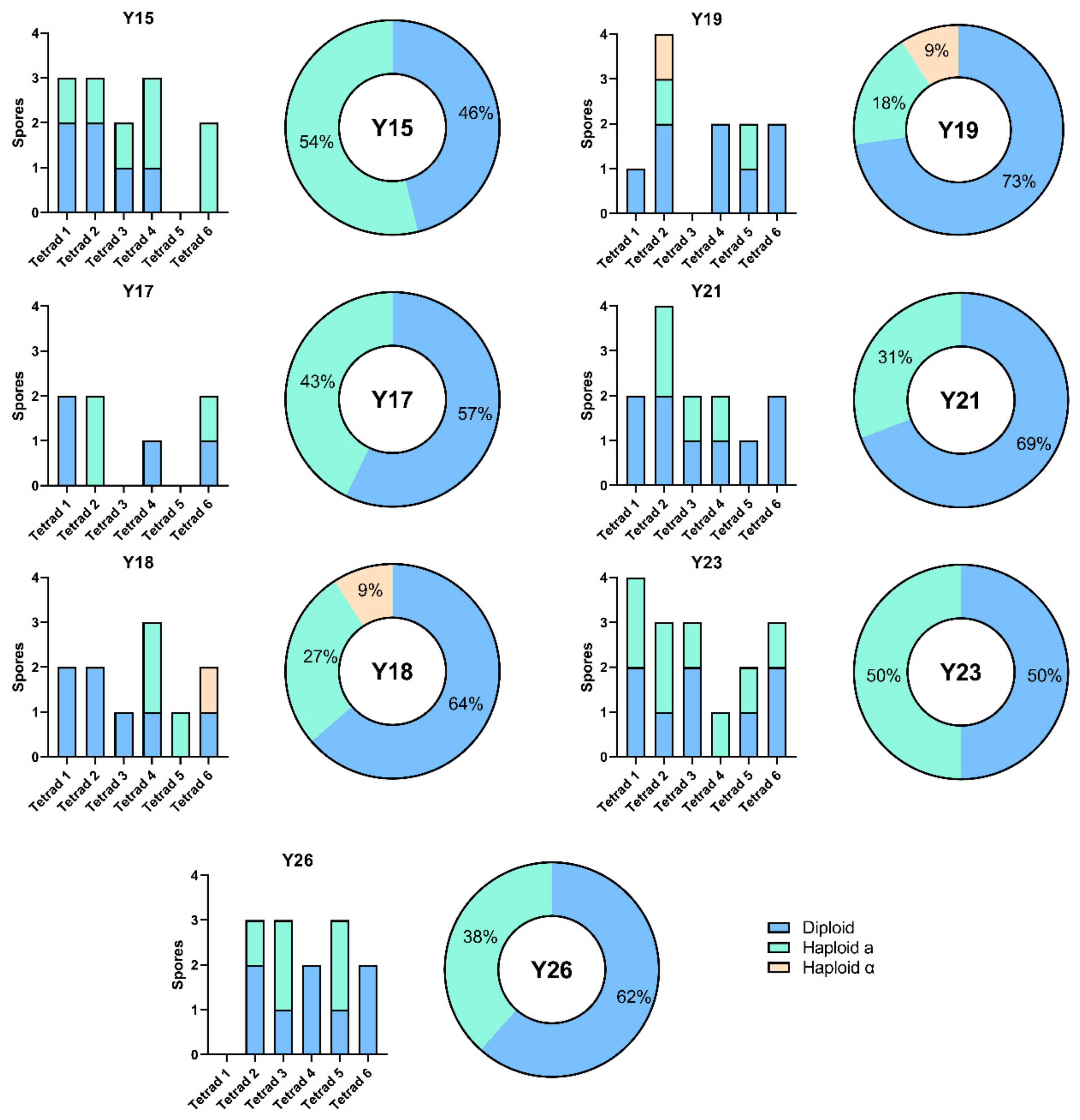
3.2. Sporulation Efficiency and Spore Viability
3.3. MAT Genotyping of Strains and Their Meiotic Segregants
3.4. Hybrid Construction
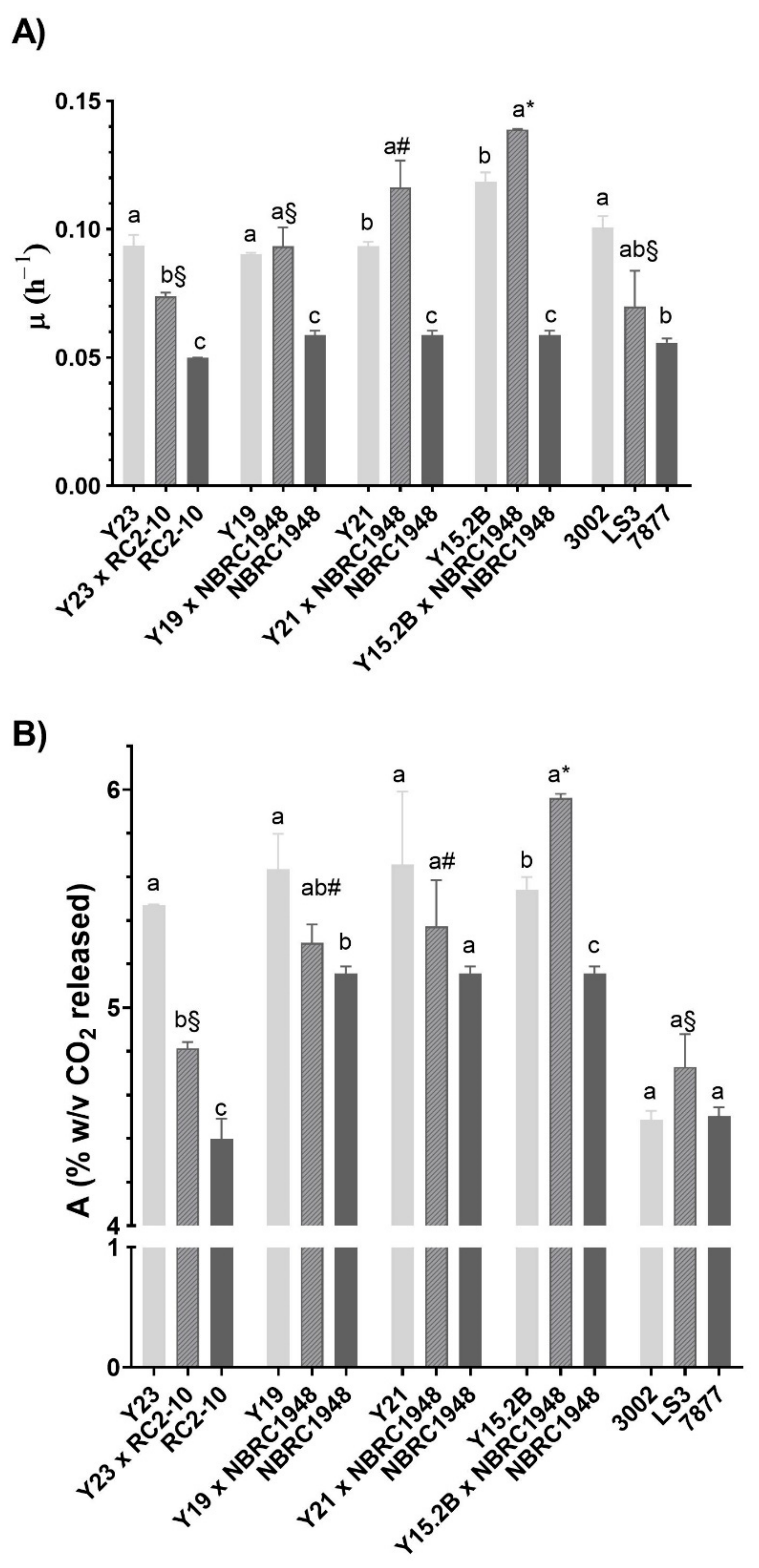
3.5. Laboratory-Scale Wort Fermentation
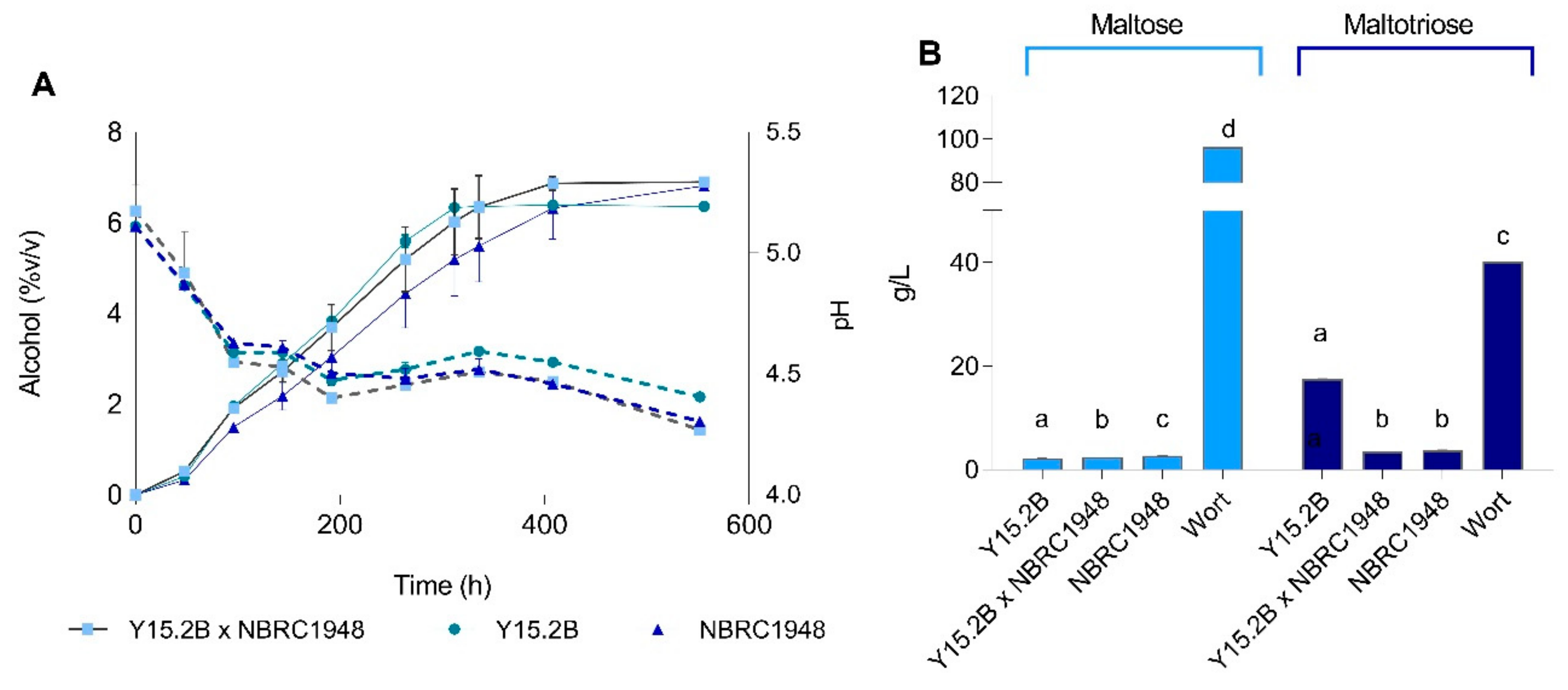
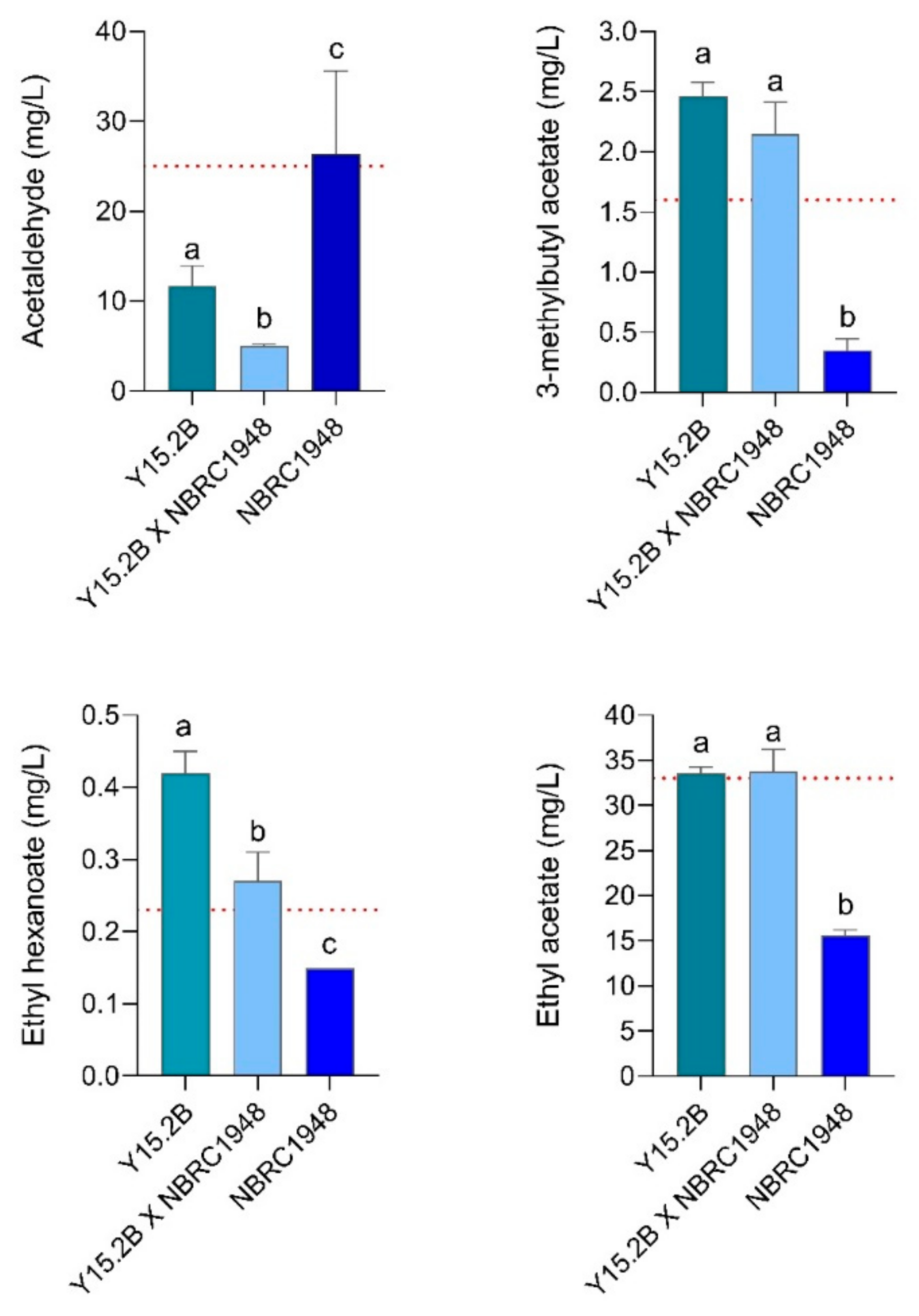
3.6. Two L-Scale Wort Fermentation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbott, R.; Albach, D.; Ansell, S.; Arntzen, J.W.; Baird, S.J.E.; Bierne, N.; Boughman, J.; Brelsford, A.; Buerkle, C.A.; Buggs, R.; et al. Hybridization and speciation. J. Evol. Biol. 2013, 26, 229–246. [Google Scholar] [CrossRef]
- Scannell, D.R.; Butler, G.; Wolfe, K.H. Yeast genome evolution—The origin of the species. Yeast 2007, 24, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Fridman, E. Consequences of hybridization and heterozygosity on plant vigor and phenotypic stability. Plant Sci. 2015, 232, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.I.; Ting, C.T. Genes and speciation. Nat. Rev. Genet. 2004, 5, 114–122. [Google Scholar] [CrossRef]
- Dagilis, A.J.; Kirkpatrick, M.; Bolnick, D.I. The evolution of hybrid fitness during speciation. PLoS Genet. 2019, 15, e1008125. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.A.; Travis, M.P. Hybridization, transgressive segregation, genetic covariation, and adaptive radiation. Trends Ecol. Evol. 2005, 20, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, L.M.; Sinha, H.; Richards, D.R.; Spiegelman, J.I.; Oefner, P.J.; McCusker, J.H.; Davis, R.W. Dissecting the architecture of a quantitative trait locus in yeast. Nature 2002, 416, 326–330. [Google Scholar] [CrossRef]
- Libkind, D.; Hittinger, C.T.; Valério, E.; Gonçalves, C.; Dover, J.; Johnston, M.; Gonçalves, P.; Sampaio, J.P. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. USA 2011, 108, 14539–14544. [Google Scholar] [CrossRef]
- Kellis, M.; Patterson, N.; Endrizzi, M.; Birren, B.; Lander, E.S. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 2003, 423, 241–254. [Google Scholar] [CrossRef]
- Hittinger, C.T. Saccharomyces diversity and evolution: A budding model genus. Trends Genet. 2013, 29, 309–317. [Google Scholar] [CrossRef]
- Sipiczki, M. Interspecies hybridization and recombination in Saccharomyces wine yeasts. FEMS Yeast Res. 2008, 8, 996–1007. [Google Scholar] [CrossRef]
- Albertin, W.; Marullo, P. Polyploidy in fungi: Evolution after whole-genome duplication. Proc. R. Soc. B Biol. Sci. 2012, 279, 2497–2509. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.; Dujon, B. Evolutionary role of interspecies hybridization and genetic exchanges in yeasts. Microbiol. Mol. Biol. Rev. 2012, 76, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Gallone, B.; Steensels, J.; Mertens, S.; Dzialo, M.C.; Gordon, J.L.; Wauters, R.; Theßeling, F.A.; Bellinazzo, F.; Saels, V.; Herrera-Malaver, B.; et al. Interspecific hybridization facilitates niche adaptation in beer yeast. Nat. Ecol. Evol. 2019, 3, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Langdon, Q.K.; Peris, D.; Baker, E.P.; Opulente, D.A.; Nguyen, H.V.; Bond, U.; Gonçalves, P.; Sampaio, J.P.; Libkind, D.; Hittinger, C.T. Fermentation innovation through complex hybridization of wild and domesticated yeasts. Nat. Ecol. Evol. 2019, 3, 1576–1586. [Google Scholar] [CrossRef]
- de Barros Lopes, M.; Bellon, J.R.; Shirley, N.J.; Ganter, P.F. Evidence for multiple interspecific hybridization in Saccharomyces sensu stricto species. FEMS Yeast Res. 2002, 1, 323–331. [Google Scholar] [CrossRef][Green Version]
- Dunn, B.; Sherlock, G. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 2008, 18, 1610–1623. [Google Scholar] [CrossRef]
- Liti, G.; Barton, D.B.H.; Louis, E.L. Sequence diversity, reproductive isolation and species concepts in Saccharomyces. Genetics 2006, 174, 839–850. [Google Scholar] [CrossRef]
- Nakao, Y.; Kanamori, T.; Itoh, T.; Kodama, Y.; Rainieri, S.; Nakamura, N.; Shimonaga, T.; Hattori, M.; Ashikari, T. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 2009, 16, 115–129. [Google Scholar] [CrossRef]
- Walther, A.; Hesselbart, A.; Wendland, J. Genome sequence of Saccharomyces carlsbergensis, the world’s first pure culture lager yeast. G3 (Bethesda) 2014, 4, 783–793. [Google Scholar] [CrossRef]
- Hebly, M.; Brickwedde, A.; Bolat, I.; Driessen, M.; de Hulster, E.; van den Broek, M.; Pronk, J.; Geertman, J.; Daran, J.; Daran-Lapujade, P. S. cerevisiae × S. eubayanus interspecific hybrid, best of both worlds and beyond. FEMS Yeast Res. 2015, 15, fov005. [Google Scholar] [CrossRef]
- Gibson, B.R.; Storgårds, E.; Krogerus, K.; Vidgren, V. Comparative physiology and fermentation performance of Saaz and Frohberg lager yeast strains and the parental species Saccharomyces eubayanus. Yeast 2013, 30, 255–266. [Google Scholar] [CrossRef]
- Brouwers, N.; Brickwedde, A.; de Vries, A.R.G.; van den Broek, M.; Weening, S.M.; van den Eijnden, L.; Diderich, J.A.; Bai, F.Y.; Pronk, J.T.; Daran, J.G. The genome sequences of Himalayan Saccharomyces eubayanus revealed genetic markers explaining heterotic maltotriose consumption by hybrid Saccharomyces pastorianus. Appl. Environ. Microbiol. 2019, 85, e01516–e01519. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, F.; Vidgren, V.; Ruohonen, L.; Gibson, B. Maltose and maltotriose utilisation by group I strains of the hybrid lager yeast Saccharomyces pastorianus. FEMS Yeast Res. 2016, 16. [Google Scholar] [CrossRef]
- Okuno, M.; Kajitani, R.; Ryusui, R.; Morimoto, H.; Kodama, Y.; Itoh, T. Next-generation sequencing analysis of lager brewing yeast strains reveals the evolutionary history of interspecies hybridization. DNA Res. 2016, 23, 67–80. [Google Scholar] [CrossRef]
- Salazar, A.N.; de Vries, A.R.G.; van den Broek, M.; Brouwers, N.; de la Torre Cortès, P.; Kuijpers, N.G.A.; Daran, J.-M.G.; Abeel, T. Chromosome level assembly and comparative genome analysis confirm lager-brewing yeasts originated from a single hybridization. BMC Genom. 2019, 20, 916. [Google Scholar] [CrossRef]
- Iattici, F.; Catallo, M.; Solieri, L. Designing new yeasts for craft brewing: When natural biodiversity meets biotechnology. Beverages 2020, 6, 3. [Google Scholar] [CrossRef]
- Krogerus, K.; Magalhães, F.; Vidgren, V.; Gibson, B. New lager yeast strains generated by interspecific hybridization. J. Ind. Microbiol. Biotechnol. 2015, 42, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Mertens, S.; Steensels, J.; Saels, V.; De Rouck, G.; Aerts, G.; Verstrepen, K.J. A large set of newly created interspecific yeast hybrids increases aromatic diversity in lager beers. Appl. Environ. Microbiol. 2015, 81, 8202–8214. [Google Scholar] [CrossRef]
- Peris, D.; Sylvester, K.; Libkind, D.; Gonçalves, P.; Sampaio, J.P.; Alexander, W.G.; Hittinger, C.T. Population structure and reticulate evolution of Saccharomyces eubayanus and its lager-brewing hybrids. Mol. Ecol. 2014, 23, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Bing, J.; Han, P.J.; Liu, W.Q.; Wang, Q.M.; Bai, F.Y. Evidence for a Far East Asian origin of lager beer yeast. Curr. Biol. 2014, 24, R380–R381. [Google Scholar] [CrossRef]
- Gayevskiy, V.; Goddard, M.R. Saccharomyces eubayanus and Saccharomyces arboricola reside in North Island native New Zealand forests. Environ. Microbiol. 2015, 18, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Nikulin, J.; Krogerus, K.; Gibson, B. Alternative Saccharomyces interspecies hybrid combinations and their potential for low-temperature wort fermentation. Yeast 2018, 35, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Gunge, N.; Nakatomi, Y. Genetic mechanisms of rare matings of the yeast Saccharomyces cerevisiae heterozygous for mating type. Genetics 1972, 70, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, K.; Seppänen-Laakso, T.; Castillo, S.; Gibson, B. Inheritance of brewing-relevant phenotypes in constructed Saccharomyces cerevisiae × Saccharomyces eubayanus hybrids. Microb. Cell Fact. 2017, 16, 1–22. [Google Scholar] [CrossRef]
- Lenz, S.D.; Riles, L.; Fay, J.C. Heterochronic meiotic misexpression in an interspecific yeast hybrid. Mol. Biol. Evol. 2014, 31, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, I.; Reikhav, S.; Levy, A.A.; Barkai, N. A yeast hybrid provides insight into the evolution of gene expression regulation. Science 2009, 324, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Alexander, W.G.; Peris, D.; Pfannenstiel, B.T.; Opulente, D.; Kuang, M.; Hittinger, C.T. Efficient engineering of marker-free synthetic allotetraploids of Saccharomyces. Fungal Genet. Biol. 2016, 89, 10–17. [Google Scholar] [CrossRef]
- Peris, D.; Alexander, W.G.; Fisher, K.J.; Moriarty, R.V.; Basuino, M.G.; Ubbelohde, E.J.; Wrobel, R.L.; Hittinger, C.T. Synthetic hybrids of six yeast species. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Marongiu, A.; Zara, G.; Legras, J.-L.; Del Caro, A.; Mascia, I.; Fadda, C.; Budroni, M. Novel starters for old processes: Use of Saccharomyces cerevisiae strains isolated from artisanal sourdough for craft beer production at a brewery scale. J. Ind. Microbiol. Biotechnol. 2015, 42, 85–92. [Google Scholar] [CrossRef]
- da Conceição, L.; Saraiva, M.; Diniz, R.; Oliveira, J.; Barbosa, G.; Alvarez, F.; da Mata Correa, L.; Mezadri, H.; Coutrim, M.; Afonso, R.; et al. Biotechnological potential of yeast isolates from cachaça: The Brazilian spirit. J. Ind. Microbiol. Biotechnol. 2015, 42, 237–246. [Google Scholar] [CrossRef]
- Araújo, T.M.; Souza, M.T.; Diniz, R.H.S.; Yamakawa, C.K.; Soares, L.B.; Lenczak, J.L.; de Castro Oliveira, J.V.; Goldman, G.H.; Barbosa, E.A.; Campos, A.C.S.; et al. Cachaça yeast strains: Alternative starters to produce beer and bioethanol. Antonie Van Leeuwenhoek 2018, 111, 1749–1766. [Google Scholar] [CrossRef]
- Rossi, S.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Evaluation of Saccharomyces cerevisiae strains isolated from non-brewing environments in beer production. J. Inst. Brew. 2018, 124, 381–388. [Google Scholar] [CrossRef]
- Cubillos, F.A.; Gibson, B.; Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J. Bioprospecting for brewers: Exploiting natural diversity for naturally diverse beers. Yeast 2019, 36, 383–398. [Google Scholar] [CrossRef]
- Catallo, M.; Nikulin, J.; Johansson, L.; Krogerus, K.; Laitinen, M.; Magalhães, F.; Piironen, M.; Mikkelson, A.; Randazzo, C.L.; Solieri, L.; et al. Sourdough derived strains of Saccharomyces cerevisiae and their potential for farmhouse ale brewing. J. Inst. Brew. 2020. [Google Scholar] [CrossRef]
- Nguyen, H.-V.; Legras, J.-L.; Neuvéglise, C.; Gaillardin, C. Deciphering the hybridization history leading to the lager lineage based on the mosaic genomes of Saccharomyces bayanus strains NBRC1948 and CBS380T. PLoS ONE 2011, 6, e25821. [Google Scholar] [CrossRef]
- Pérez-Través, L.; Lopes, C.A.; Querol, Q.; Barrio, B. On the complexity of the Saccharomyces bayanus taxon: Hybridization and potential hybrid speciation. PLoS ONE 2014, 9, e93729. [Google Scholar] [CrossRef]
- Giudici, P.; Restuccia, C.; Randazzo, C.; Melia, V.; Corte, V. Biodiversità fenotipica di lieviti isolati da mosti cotti e vini siciliani. Ind. Bevande 1990, 26, 252–259. [Google Scholar]
- Solieri, L.; Antúnez, O.; Pérez-Ortín, J.E.; Barrio, E.; Giudici, P. Mitochondrial inheritance and fermentative: Oxidative balance in hybrids between Saccharomyces cerevisiae and Saccharomyces uvarum. Yeast 2008, 25, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, R. Methods for Isolation, Phenotypic Characterization and Maintenance of Yeasts. In The Yeasts: A Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, W.F., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1, pp. 87–110. [Google Scholar]
- Murphy, H.A.; Zeyl, C.W. Prezygotic isolation between Saccharomyces cerevisiae and Saccharomyces paradoxus through differences in mating speed and germination timing. Evolution 2012, 66, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Lõoke, M.; Kristjuhan, K.; Kristjuhan, A. Extraction of genomic DNA from yeasts for PCR-based applications. BioTechniques 2011, 50, 325–328. [Google Scholar] [CrossRef]
- Huxley, C.; Green, E.D.; Dunham, I. Rapid assessment of S. cerevisiae mating type by PCR. Trends Genet. 1990, 6, 236. [Google Scholar]
- Solieri, L.; Verspohl, A.; Bonciani, T.; Caggia, C.; Giudici, P. Fast method for identifying inter- and intra-species Saccharomyces hybrids in extensive genetic improvement programs based on yeast breeding. J. Appl. Microbiol. 2015, 119, 149–161. [Google Scholar] [CrossRef]
- Pengelly, R.J.; Wheals, A.E. Rapid identification of Saccharomyces eubayanus and its hybrids. FEMS Yeast Res. 2013, 13, 156–161. [Google Scholar] [CrossRef]
- Dakal, T.C.; Solieri, L.; Giudici, P. Evaluation of fingerprinting techniques to assess genotype variation among Zygosaccharomyces strains. Food Microbiol. 2018, 72, 135–145. [Google Scholar] [CrossRef]
- Krogerus, K.; Holmström, S.; Gibson, B. Enhanced wort fermentation with de novo lager hybrids adapted to high-ethanol environments. Appl. Environ. Microbiol. 2019, 84, e02302–e02317. [Google Scholar] [CrossRef]
- Kahm, M.; Hasenbrink, G.; Lichtenberg-Frate, H.; Ludwig, J.; Kschischo, M. Grofit: Fitting biological growth curves. J. Stat. Softw. 2010, 33, 1–21. [Google Scholar] [CrossRef]
- Oura, E.; Suomalainen, H.; Viskari, R. Breadmaking. In Economic Microbiology; Rose, A.H., Ed.; Academic Press: London, UK, 1982; pp. 512–546. [Google Scholar]
- Zastrow, C.; Hollatz, C.; De Araujo, P.; Stambuk, B. Maltotriose fermentation by Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2001, 27, 34–38. [Google Scholar] [CrossRef]
- Mortimer, R.K. Evolution and variation of the yeast (Saccharomyces) genome. Genome Res. 2000, 10, 403–409. [Google Scholar] [CrossRef]
- Steensels, J.; Snoek, T.; Meersman, E.; Nicolino, M.P.; Voordeckers, K.; Verstrepen, K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef]
- Maclean, C.J.; Greig, D. Prezygotic reproductive isolation between Saccharomyces cerevisiae and Saccharomyces paradoxus. BMC Evol. Biol. 2008, 8, 1. [Google Scholar] [CrossRef]
- Verspohl, A.; Pignedoli, S.; Giudici, P. The inheritance of mitochondrial DNA in interspecific Saccharomyces hybrids and their properties in winemaking. Yeast 2018, 35, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, K.; Magalhães, F.; Vidgren, V.; Gibson, B. Novel brewing yeast hybrids: Creation and application. Appl. Microbiol. Biotechnol. 2017, 101, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M.C. Flavor chemistry of beer. II. Flavor and threshold of 239 aroma volatiles. Technol. Quart. Master. Brew. Assoc. Am. 1975, 12, 151–168. [Google Scholar]
- Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; Hilbert, F.; Lindqvist, R.; Nauta, M. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 9: Suitability of taxonomic units notified to EFSA until September 2018. Efsa J. 2019, 17, e05555. [Google Scholar]
- Capozzi, V.; Fragasso, M.; Russo, P. Microbiological safety and the management of microbial resources in artisanal foods and beverages: The need for a transdisciplinary assessment to conciliate actual trends and risks avoidance. Microorganisms 2020, 8, 306. [Google Scholar] [CrossRef]
- de Vries, A.R.G.; Koster, C.C.; Weening, S.M.; Luttik, M.A.H.; Kuijpers, N.G.A.; Geertman, J.-M.A.; Pronk, J.T.; Daran, J.-M.G. Phenotype-independent isolation of interspecies Saccharomyces hybrids by dual-dye fluorescent staining and fluorescence-activated cell sorting. Front. Microbiol. 2019, 10, 871. [Google Scholar] [CrossRef]
- Butler, G.; Kenny, C.; Fagan, A.; Kurischko, C.; Gaillardin, C.; Wolfe, K.H. Evolution of the MAT locus and its Ho endonuclease in yeast species. Proc. Natl. Acad. Sci. USA 2004, 101, 1632–1637. [Google Scholar] [CrossRef]
- Katz Ezov, T.; Chang, S.L.; Frenkel, Z.; Segrè, A.V.; Bahalul, M.; Murray, A.W.; Leu, J.Y.; Korol, A.; Kashi, Y. Heterothallism in Saccharomyces cerevisiae isolates from nature: Effect of HO locus on the mode of reproduction. Mol. Ecol. 2010, 19, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Liti, G.; Llorente, B. The budding yeast life cycle: More complex than anticipated? Yeast 2021, 38. [Google Scholar] [CrossRef]
- Figueiredo, B.I.C.; Saraiva, M.A.F.; de Souza Pimenta, P.P.; de Souza Testasicca, M.C.; Sampaio, G.M.S.; da Cunha, A.C.; Afonso, L.C.C.; de Queiroz, M.V.; de Miranda Castro, I.; Brandao, R.L. New lager brewery strains obtained by crossing techniques using cachaça (Brazilian spirit) yeasts. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Korhola, M.; Naumova, E.S.; Partti, E.; Aittamaa, M.; Turakainen, H.; Naumov, G.I. Exploiting heterozygosity in industrial yeasts to create new and improved baker’s yeasts. Yeast 2019, 36, 571–587. [Google Scholar] [CrossRef]
- Barbosa, R.; Almeida, P.; Safar, S.V.; Santos, R.O.; Morais, P.B.; Nielly-Thibault, L.; Leducq, J.-B.; Landry, C.R.; Gonçalves, P.; Rosa, C.A.; et al. Evidence of natural hybridization in Brazilian wild lineages of Saccharomyces cerevisiae. Genome Biol. Evol. 2016, 8, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Pontes, A.; Čadež, N.; Gonçalves, P.; Sampaio, J.P. A quasi-domesticate relic hybrid population of Saccharomyces cerevisiae × S. paradoxus adapted to olive brine. Front. Genet. 2019, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- D’Angiolo, M.; De Chiara, M.; Yue, J.-X.; Irizar, A.; Stenberg, S.; Persson, K.; Llored, A.; Barré, B.; Schacherer, J.; Marangoni, R.; et al. A yeast living ancestor reveals the origin of genomic introgressions. Nature 2020, 587, 420–425. [Google Scholar] [CrossRef]
- Bellon, J.R.; Schmid, F.; Capone, D.L.; Dunn, B.L.; Chambers, P.J. Introducing a new breed of wine yeast: Interspecific hybridization between a commercial Saccharomyces cerevisiae wine yeast and Saccharomyces mikatae. PLoS ONE 2013, 8, e62053. [Google Scholar] [CrossRef] [PubMed]
- Origone, A.C.; Rodríguez, M.E.; Oteiza, J.M.; Querol, A.; Lopes, C.A. Saccharomyces cerevisiae × Saccharomyces uvarum hybrids generated under different conditions share similar winemaking features. Yeast 2018, 35, 157–171. [Google Scholar] [CrossRef]
- Kanter, J.P.; Benito, S.; Brezina, S.; Beisert, B.; Fritsch, S.; Patz, C.D.; Rauhut, D. The impact of hybrid yeasts on the aroma profile of cool climate Riesling wines. Food Chem. X 2020, 5, 100072. [Google Scholar] [CrossRef]
- Krogerus, K.; Preiss, R.; Gibson, B. A unique Saccharomyces cerevisiae × Saccharomyces uvarum hybrid isolated from Norwegian farmhouse beer: Characterization and reconstruction. Front. Microbiol. 2018, 9, 2253. [Google Scholar] [CrossRef]
- Dietvorst, J.; Londesborough, J.; Steensma, H.Y. Maltotriose utilization in lager yeast strains: MTT1 encodes a maltotriose transporter. Yeast 2005, 22, 775–788. [Google Scholar] [CrossRef]
- Vidgren, V.; Multanen, J.-P.; Ruohonen, L.; Londesborough, J. The temperature dependence of maltose transport in ale and lager strains of brewer’s yeast. FEMS Yeast Res. 2010, 10, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Salema-Oom, M.; Pinto, V.V.; Goncalves, P.; Spencer-Martins, I. Maltotriose utilization by industrial Saccharomyces strains: Characterization of a new member of the α-glucoside transporter family. Appl. Environ. Microbiol. 2005, 71, 5044–5049. [Google Scholar] [CrossRef]

| Strains | Code | Origin/Characteristics |
|---|---|---|
| S. cerevisiae | Y15, Y17, Y18, Y19 | Sourdough of Maiorca flour (Maletto, CT) |
| Y21 | Sourdough of Maiorca flour (Castellammare, TP) | |
| Y23 | Sourdough of Maiorca flour (Catania, CT) | |
| Y26 | Sourdough of Maiorca flour (Balestrate, PA) | |
| BY4741 | Euroscarf/MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0 | |
| BY4742 | Euroscarf/MATα, his3∆1, leu2∆0, lys2∆0, ura3∆0 | |
| BY4743 | Euroscarf/ MATa/MATα, his3∆1/ his3∆1, leu2∆0/ leu2∆0, lys2∆0/ lys2∆0, ura3∆0/ ura3∆0 | |
| 3002 | Wine [48] | |
| S. pastorianus | Fermolager W | Lager Frohberg yeast (AEB spa, Brescia) |
| S. uvarum | RC2-10 | Grape fermenting yeast (Alsace); kindly provided by Philippe Marullo |
| 7877 | wine; DIROVAL 1 collection | |
| S. cerevisiae × S. uvarum hybrid | LS3 | [49] |
| S. bayanus | NBRC1948 | - |
| S. cariocanus | CBS8841 | Drosophila sp. (Rio de Janeiro, Brazil) |
| Species | Strain | Fermentation Assay | Optical Density (OD600 nm) | |
|---|---|---|---|---|
| Glucose (2% w/v) | Maltose (2% v/w) | |||
| S. cerevisiae | Y15 | s | 1.402 b,* ± 0.031 | 1.098 b,§ ± 0.02 |
| Y17 | s | 1.145 c,* ± 0.016 | 0.677 c,§ ± 0.06 | |
| Y18 | s | 1.438 b,* ± 0.042 | 1.029 b,§ ± 0.093 | |
| Y19 | + | 1.428 b,* ± 0.016 | 1.257 a,b,* ± 0.046 | |
| Y21 | s | 1.970 a,* ± 0.203 | 1.412 a,§ ± 0.05 | |
| Y23 | s | 1.551 b,* ± 0.228 | 1.495 a,* ± 0.119 | |
| Y26 | + | 1.687 a,* ± 0.12 | 1.252 a,b,§ ± 0.101 | |
| S. uvarum | RC2-10 | s | nd | nd |
| S. bayanus | NBRC1948 | s | nd | nd |
| S. cariocanus | CBS8841 | - | nd | nd |
| Species | Strain Code | Sporulation | Sporulation Efficiency (%) | Viability (%) | MAT Genotype |
|---|---|---|---|---|---|
| S. cerevisiae | Y15 | + | 32.9 ± 0.07 | 47.2 | MATa/MATα |
| Y17 | + | 35.9 ± 0.04 | 29.2 | MATa/MATα MATa/MATα | |
| Y18 | + | 19.0 ± 0.04 | 45.8 | MATa/MATα | |
| Y19 | + | 35.4 ± 0.00 | 45.8 | MATa/MATα | |
| Y21 | + | 34.5 ± 0.04 | 54.2 | MATa/MATα | |
| Y23 | + | 32.1 ± 0.04 | 66.7 | MATa/MATα | |
| Y26 | w | 17.2 ± 0.01 | 54.2 | MATa/MATα | |
| S. uvarum | RC2-10 | w | 20.4 ± 0.01 | nd | nd |
| S. bayanus | NBRC1948 | w | 30.9 ± 0.02 | nd | nd |
| S. cariocanus | CBS8841 | + | nd | nd | nd |
| Parental Strains | Type of Mating | Hybridization Frequency (%) | |
|---|---|---|---|
| SM | NSM | ||
| Y15 × RC2-10 | Sc × Su direct spore-to-spore | 0 | 23 |
| Y23 × RC2-10 | Sc × Su direct spore-to-spore | 27 | 31 |
| Y15.2B × NBRC1948 | Sc segregant spore-to-Sbay spore | 10 | 41 |
| Y19 × NBRC1948 | Sc × Sbay direct spore-to-spore | 31 | 42 |
| Y21 × NBRC1948 | Sc × Sbay direct spore-to-spore | 13 | 33 |
| Y19 × CBS8841 | Sc × Scar direct spore-to-spore | 22 | nd |
| Y19.5A × CBS8841 | Sc cell-to-Scar spore | 9 | nd |
| Parental Strains | Type of Mating | Hybridization Frequency (%) | |||
|---|---|---|---|---|---|
| 35 Days Age | 105 Days Age | ||||
| SM | NSM | SM | NSM | ||
| Y15 × RC2-10 | Sc × Su direct spore-to-spore | 0 | 23 | 10 | 28 |
| Y21 × NBRC1948 | Sc × Sbay direct spore-to-spore | 13 | 33 | 39 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catallo, M.; Iattici, F.; Randazzo, C.L.; Caggia, C.; Krogerus, K.; Magalhães, F.; Gibson, B.; Solieri, L. Hybridization of Saccharomyces cerevisiae Sourdough Strains with Cryotolerant Saccharomyces bayanus NBRC1948 as a Strategy to Increase Diversity of Strains Available for Lager Beer Fermentation. Microorganisms 2021, 9, 514. https://doi.org/10.3390/microorganisms9030514
Catallo M, Iattici F, Randazzo CL, Caggia C, Krogerus K, Magalhães F, Gibson B, Solieri L. Hybridization of Saccharomyces cerevisiae Sourdough Strains with Cryotolerant Saccharomyces bayanus NBRC1948 as a Strategy to Increase Diversity of Strains Available for Lager Beer Fermentation. Microorganisms. 2021; 9(3):514. https://doi.org/10.3390/microorganisms9030514
Chicago/Turabian StyleCatallo, Martina, Fabrizio Iattici, Cinzia L. Randazzo, Cinzia Caggia, Kristoffer Krogerus, Frederico Magalhães, Brian Gibson, and Lisa Solieri. 2021. "Hybridization of Saccharomyces cerevisiae Sourdough Strains with Cryotolerant Saccharomyces bayanus NBRC1948 as a Strategy to Increase Diversity of Strains Available for Lager Beer Fermentation" Microorganisms 9, no. 3: 514. https://doi.org/10.3390/microorganisms9030514
APA StyleCatallo, M., Iattici, F., Randazzo, C. L., Caggia, C., Krogerus, K., Magalhães, F., Gibson, B., & Solieri, L. (2021). Hybridization of Saccharomyces cerevisiae Sourdough Strains with Cryotolerant Saccharomyces bayanus NBRC1948 as a Strategy to Increase Diversity of Strains Available for Lager Beer Fermentation. Microorganisms, 9(3), 514. https://doi.org/10.3390/microorganisms9030514









