Plant-Growth Endophytic Bacteria Improve Nutrient Use Efficiency and Modulate Foliar N-Metabolites in Sugarcane Seedling
Abstract
1. Introduction
2. Material and Methods
2.1. Bacterial Strain Inocula Preparation
2.2. Evaluating the Sugarcane Seedlings Growth under Greenhouse Conditions
2.3. Evaluation of Plant Growth-Promotion and Plant Nutritional Status
2.4. Enzyme Extraction and Enzyme Activities Determination
2.5. Activity of Nitrate Reductase Enzyme (NR)
2.6. Activity of Nitrite Reductase Enzyme (NiR)
2.7. Activity of Glutamine Synthetase Enzyme (GS)
2.8. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR) Analysis
2.9. Profile of Amino Acids and Polyamines
2.10. Statistical Analysis
3. Results
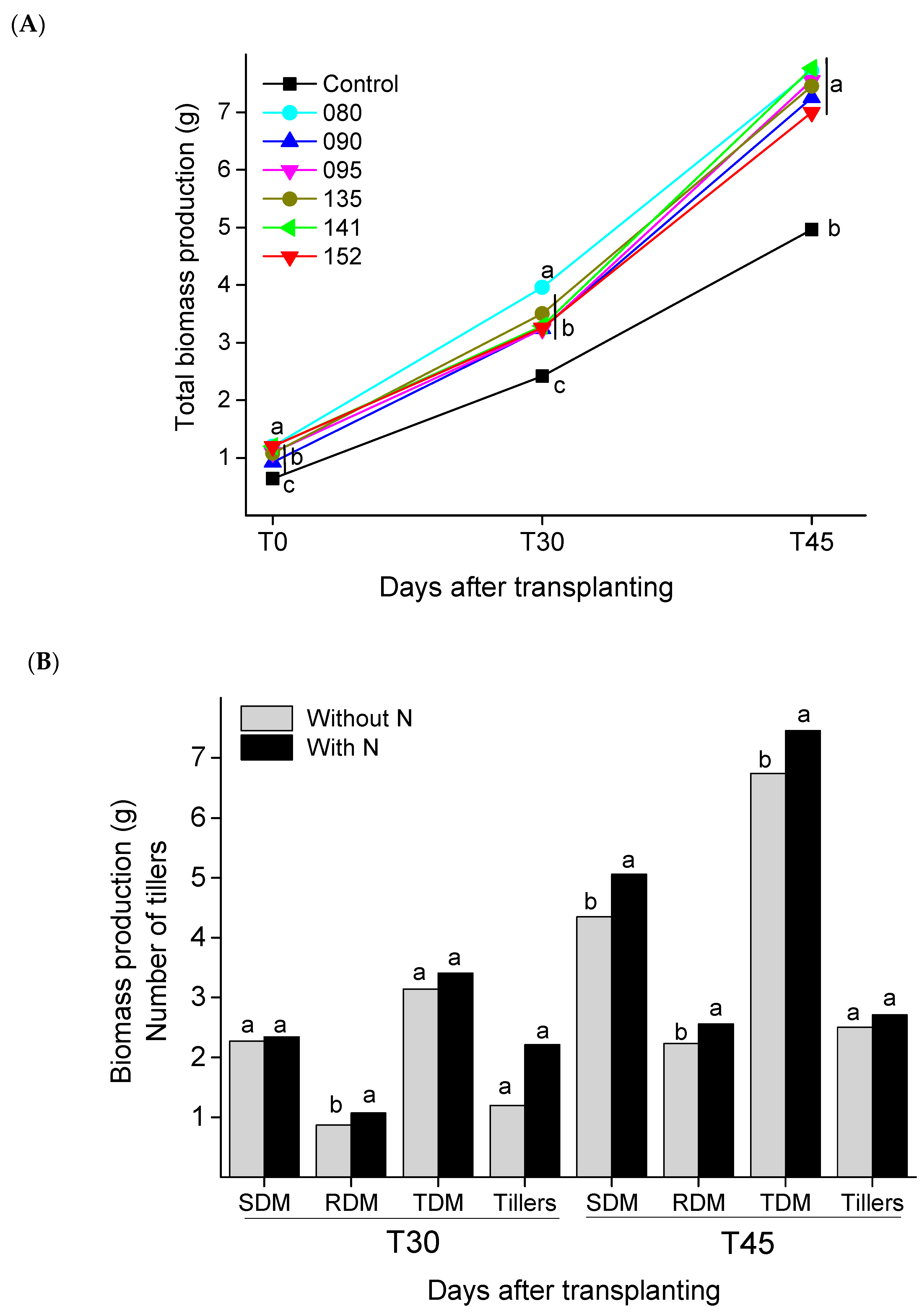
3.1. Plant Growth-Promotion and Nutritional Status of Plant
3.2. Enzymatic Analysis—Nitrogen Metabolism Enzymes (NR, NiR and GS)
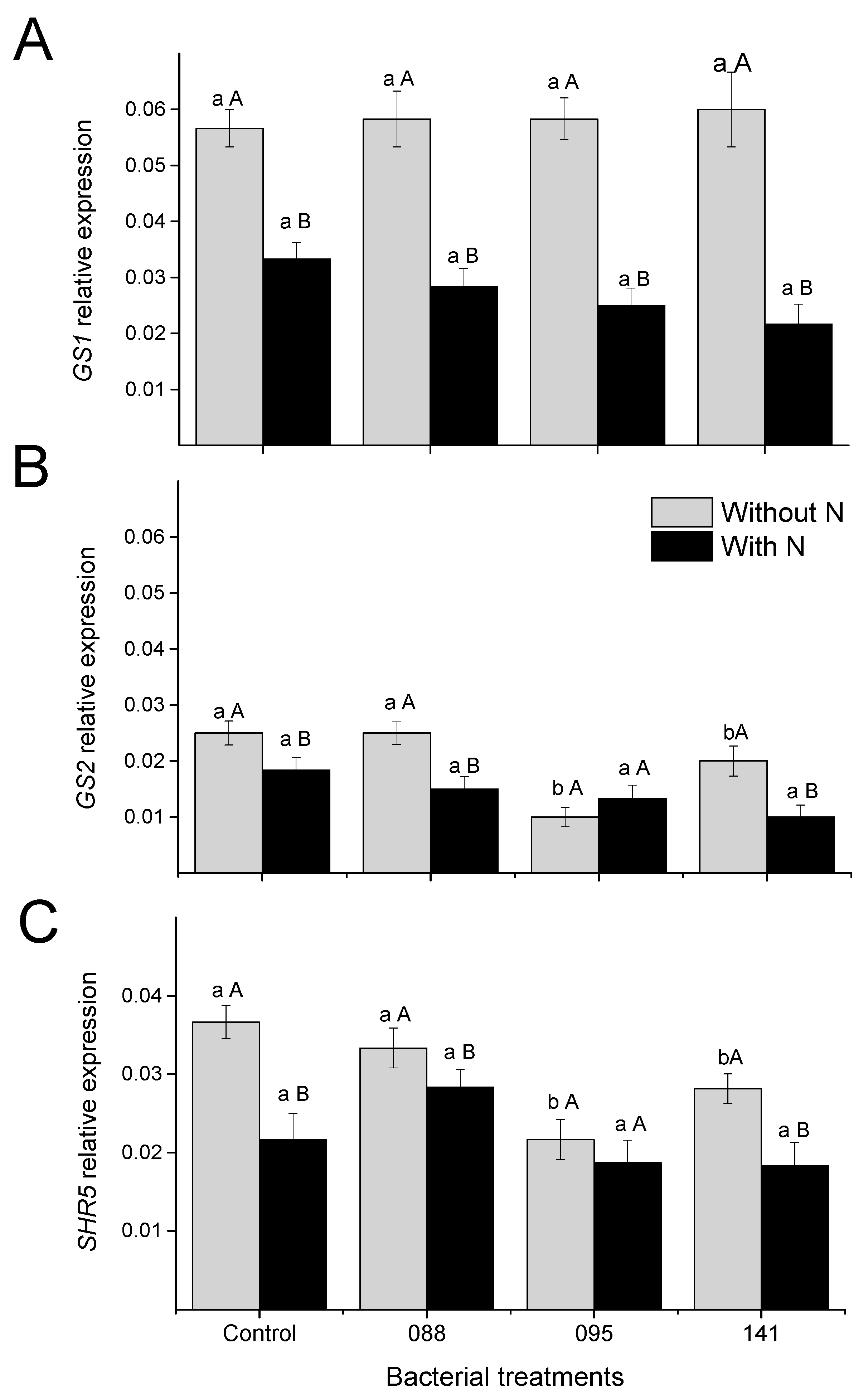
3.3. Relative Expression of the Genes GS1, GS2, and SHR5
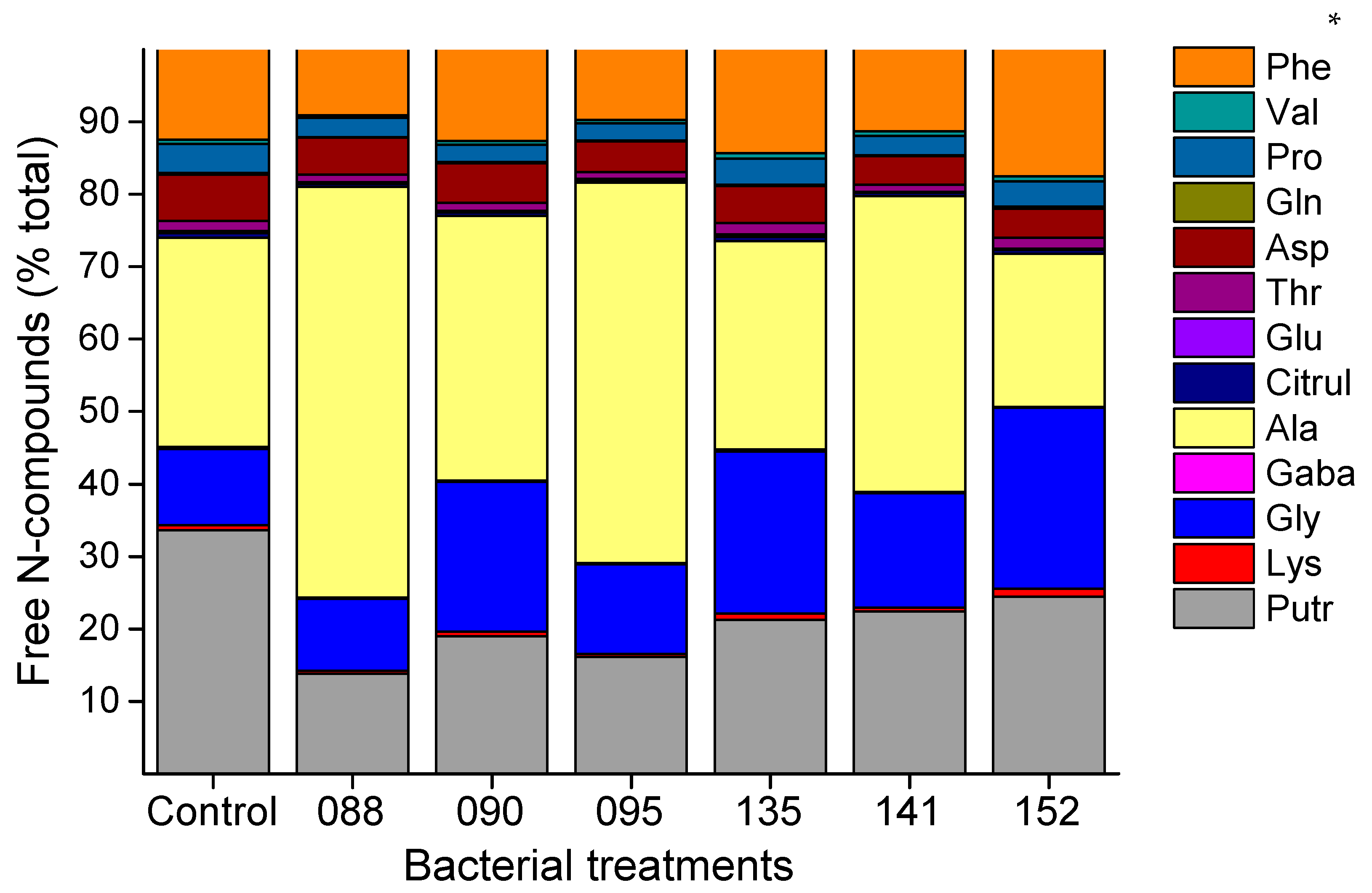
3.4. Free Amino Acid and Polyamines Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Otieno, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The interactions of rhizosphere with plant growth-promoting rhizobacteria in the rhizosphere: A review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef]
- Da Silveira, A.P.D.; Sala, V.M.R.; Cardoso, E.J.B.N.; Labanca, E.G.; Cipriano, M.A.P. Nitrogen metabolism and growth of wheat plant under diazotrophic endophytic bacteria inoculation. Appl. Soil Ecol. 2016, 107, 313–319. [Google Scholar] [CrossRef]
- Da Silveira, A.P.D.; Iório, R.D.P.F.; Marcos, F.C.C.; Fernandes, A.O.; De Souza, S.A.C.D.; Kuramae, E.E.; Cipriano, M.A.P. Exploitation of new endophytic bacteria and their ability to promote sugarcane growth and nitrogen nutrition. Antonie Leeuwenhoek 2019, 112, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.G.; da Silva Ribeiro, F.; Alves, G.C.; Santos, L.A.; Reis, V.M. Inoculation with five diazotrophs alters nitrogen metabolism during the initial growth of sugarcane varieties with contrasting responses to added nitrogen. Plant Soil 2020, 451, 25–44. [Google Scholar] [CrossRef]
- Vinagre, F.; Vargas, C.; Schwarcz, K.; Cavalcante, J.; Nogueira, E.M.; Baldani, J.I.; Ferreira, P.C.G.; Hemerly, A.S. SHR5: A novel plant receptor kinase involved in plant–N2-fixing endophytic bacteria association. J. Exp. Bot. 2006, 57, 559–569. [Google Scholar] [CrossRef]
- Carvalho, T.L.G.; Ballesteros, H.G.F.; Thiébaut, F.; Ferreira, P.C.G.; Hemerly, A.S. Nice to meet you: Genetic, epigenetic and metabolic controls of plant perception of beneficial associative and endophytic diazotrophic bacteria in non-leguminous plants. Plant Mol. Biol. 2016, 90, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Terra, L.A.; De Soares, C.P.; Meneses, C.H.S.G.; Sfeir, M.Z.T.; De Souza, E.M.; Silveira, V.; Vidal, M.S.; Baldani, J.I.; Schwab, S. Transcriptome and proteome profiles of the diazotroph Nitrospirillum amazonense strain CBAmC in response to the sugarcane apoplast fluid. Plant Soil 2019, 451, 145–168. [Google Scholar] [CrossRef]
- Wang, W.; Paschalidis, K.; Feng, J.-C.; Song, J.; Liu, J.-H. Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef]
- Rampazzo, P.; Marcos, F.; Cipriano, M.; Marchiori, P.; Freitas, S.; Machado, E.; Nascimento, L.; Brocchi, M.; Ribeiro, R. Rhizobacteria improve sugarcane growth and photosynthesis under well-watered conditions. Ann. Appl. Biol. 2018, 172, 309–320. [Google Scholar] [CrossRef]
- Labanca, E.; Andrade, S.; Kuramae, E.; Silveira, A. The modulation of sugarcane growth and nutritional profile under aluminum stress is dependent on beneficial endophytic bacteria and plantlet origin. Appl. Soil Ecol. 2020, 156, 103715. [Google Scholar] [CrossRef]
- De Carvalho, T.L.G.; Ferreira, P.C.G.; Hemerly, A.S. Sugarcane genetic controls involved in the association with beneficial endophytic nitrogen fixing bacteria. Trop. Plant Biol. 2011, 4, 31–41. [Google Scholar] [CrossRef]
- Prima-A-Plant Group; Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; et al. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.S.C.; Okura, V.K.; Armanhi, J.S.L.; Jorrín, B.; Lozano, N.; Da Silva, M.J.; González-Guerrero, M.; De Araújo, L.M.; Verza, N.C.; Bagheri, H.C.; et al. Unlocking the bacterial and fungal communities assemblages of sugarcane microbiome. Sci. Rep. 2016, 6, 28774. [Google Scholar] [CrossRef] [PubMed]
- Marcos, F.C.C.; Iório, R.D.P.F.; Da Silveira, A.P.D.; Ribeiro, R.V.; Machado, E.C.; Lagôa, A.M.M.D.A. Endophytic bacteria affect sugarcane physiology without changing plant growth. Bragantia 2015, 75, 1–9. [Google Scholar] [CrossRef]
- Schlemper, T.R.; Dimitrov, M.R.; Gutierrez, F.A.S.; Van Veen, J.A.; Silveira, A.P.; Kuramae, E.E. Effect of Burkholderia tropica and Herbaspirillum frisingense strains on sorghum growth is plant genotype dependent. PeerJ 2018, 6, e5346. [Google Scholar] [CrossRef]
- Kuramae, E.E.; Derksen, S.; Schlemper, T.R.; Dimitrov, M.R.; Costa, O.Y.A.; Da Silveira, A.P.D. Sorghum growth promotion by Paraburkholderia tropica and Herbaspirillum frisingense: Putative mechanisms revealed by genomics and metagenomics. Microorganisms 2020, 8, 725. [Google Scholar] [CrossRef]
- Van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química para Avaliação da Fertilidade de Solos Tropicais; Intituto Agronômico: Campinas, Brazil, 2001; pp. 1–285. [Google Scholar]
- Bataglia, O.C.; Furlani, A.M.C.; Teixeira, J.P.F.; Furlani, P.R.; Gallo, J.R. Métodos de Análise Química de Plantas; Instituto Agronômico, Boletim Técnico: Campinas, Brazil, 1983; Volume 78, p. 48. [Google Scholar]
- Siddiqi, M.Y.; Glass, A.D. Utilization index: A modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J. Plant Nutr. 1981, 4, 289–302. [Google Scholar] [CrossRef]
- Casagrande, A.A. Tópicos de Morfologia e Fisiologia de Cana-de-Açúcar; Jaboticabal: FUNEP, Brazil, 1991; p. 157. [Google Scholar]
- Silveira, J.A.G.; Martins, M.O.; Lima, A.B.; Ferreira-Silva, S. Redução de nitrato e assimilação da amônia em sistemas vegetais: Mensuração de atividade enzimática e metabólitos. In Figueiredo MVB. Biotecnologia Aplicada à Agricultura: Textos de Apoio e Protocolos Experimentais; EMBRAPA: Brasília, Brazil, 2010; pp. 93–124. [Google Scholar]
- Iskandar, H.M.; Simpson, R.S.; Casu, R.E.; Bonnett, G.D.; MacLean, D.J.; Manners, J.M. Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression in sugarcane. Plant Mol. Biol. Rep. 2004, 22, 325–337. [Google Scholar] [CrossRef]
- Nogueira, E.D.M.; Olivares, F.L.; Japiassu, J.C.; Vilar, C.; Vinagre, F.; Baldani, J.I.; Hemerly, A.S. Characterization of glutamine synthetase genes in sugarcane genotypes with different rates of biological nitrogen fixation. Plant Sci. 2005, 169, 819–832. [Google Scholar] [CrossRef]
- Integrated DNA Technologies. OligoAnalyzerTM Tool. Available online: www.idtdna.com/calc/analyzer (accessed on 15 May 2020).
- National Center Biotechnology Information. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 14 May 2020).
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Tezotto, T.; Souza, S.C.R.; Mihail, J.; Favarin, J.L.; Mazzafera, P.; Bilyeu, K.; Polacco, J.C. Deletion of the single UreG urease activation gene in soybean NIL lines: Characterization and pleiotropic effects. Theor. Exp. Plant Physiol. 2016, 28, 307–320. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Oliveira, A.L.M.; Canuto, E.L.; Urquiaga, S.; Reis, V.M.; Baldanim, J.I. Yield of micropropagated sugarcane varieties in different soil types following inoculation with diazotrophic bacteria. Plant Soil. 2006, 284, 23–32. [Google Scholar] [CrossRef]
- Banik, A.; Mukkopadhaya, S.K.; Dangar, T.K. Characterization of N2-fixing plant growth promoting endo-phytic and epiphytic bacterial community of Indian cultivated and wild ric (Oryza spp.) genotpyes. Planta 2016, 243, 799–812. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; James, E.K.; Mathan, N.; Reddy, P.M.; Reinhold-Hurek, B.; Ladha, J.K. Endophytic colonization of rice by a diazotrophic strain of Serratia marcescens. J. Bacteriol. 2001, 183, 2634–2645. [Google Scholar] [CrossRef]
- Chauhan, H.; Bagyaraj, D.J.; Sharma, A. Plant growth-promoting bacterial endophytes from sugarcane and their potential in promoting growth of the host under field conditions. Exp. Agric. 2012, 49, 43–52. [Google Scholar] [CrossRef]
- Govindarajan, M.; Balandreau, J.; Muthukumarasamy, R.; Revathi, G.; Lakshminarasimhan, C. Improved yield of micropropagated sugarcane following inoculation by endophytic Burkholderia vietnamiensis. Plant Soil 2006, 280, 239–252. [Google Scholar] [CrossRef]
- Reis, F.B., Jr.; Machado, C.T.T.; Machado, A.T.; Sodek, L. Inoculação de Azozpirillum amazonense em dois genótipos de milho sob diferentes regimes de nitrogênio. Rev. Bras. Ciênc. Solo 2008, 32, 1139–1146. [Google Scholar] [CrossRef]
- Gírio, L.A.D.S.; Dias, F.L.F.; Reis, V.M.; Urquiaga, S.; Schultz, N.; Bolonhezi, D.; Mutton, M.A. Bactérias promotoras de crescimento e adubação nitrogenada no crescimento inicial de cana-de-açúcar proveniente de mudas pré-brotadas. Pesqui. Agropec. Bras. 2015, 50, 33–43. [Google Scholar] [CrossRef]
- Prieto, K.R.; Echaide-Aquino, F.; Huerta-Robles, A.; Valério, H.P.; Macedo-Raygoza, G.; Prado, F.M.; Medeiros, M.H.; Brito, H.F.; Da Silva, I.G.; Felinto, M.C.C.; et al. Endophytic bacteria and rare earth elements; promising candidates for nutrient use efficiency in plants. In Plant Macronutrient Use Efficiency; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 285–306. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Suman, A.; Gaur, A.; Shrivastava, A.K.; Yadav, R. Improving sugarcane growth and nutrient uptake by inoculating Glucanacetobacter diazotrophicus. Plant Growth Regul. 2005, 47, 155–162. [Google Scholar] [CrossRef]
- Barretti, P.B.; Souza, R.M.; Pozza, A.A.A.; Pozza, E.A.; Carvalho, J.G.; Souza, J.T. Aumento da eficiência nutricional de tomateiros inoculados com bactérias endofíticas promotoras de crescimento. Rev. Bras Ciênc. Solo 2008, 32, 1542–1548. [Google Scholar] [CrossRef][Green Version]
- Vitousek, P. Nutrient cycling and nutrient use efficiency. Am. Nat. 1982, 119, 553–572. [Google Scholar] [CrossRef]
- Kuss, A.V.; Kuss, V.V.; Lovato, T.; Flôres, M.L. Fixação de nitrogênio e produção de ácido indol acético in vitro por bactérias diazotróficas endofíticas. Pesqui. Agropec. Bras. 2007, 42, 1459–1465. [Google Scholar] [CrossRef]
- De Hita, D.; Fuentes, M.; Zamarreño, A.M.; Ruiz, Y.; Garcia-Mina, J.M. Culturable bacterial endophytes from sedimentary humic acid-treated plants. Front. Plant Sci. 2020, 11, 837. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.; Zinovkina, N.; Safronova, V.; Belimov, A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Mehnaz, S. Microbes—Friends and foes of sugarcane. J. Basic Microbiol. 2013, 53, 954–971. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, V.; Madhaiyan, M.; Thangaraju, M. Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere 2007, 66, 1794–1798. [Google Scholar] [CrossRef]
- Mantelin, S.; Touraine, B. Plant growth-promoting bacteria and nitrate availability: Impacts on roots development and nitrate uptake. J. Exp. Bot. 2004, 55, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.S.; Mariano, D.C.; Dallacort, R.; Albuquerque, A.N.; Lobato, A.K.S.; Guedes, E.M.S.; Oliveira Neto, C.F.; Conceição, H.E.O.; Alves, G.A.R. Azospirillum: A new and efficient alternative to biological nitrogen fixation in grasses. J. Food Agric. Environ. 2013, 11, 1142–1146. [Google Scholar]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef]
- Kuklinsky-Sobral, J.; Araujo, W.L.; Mendes, R.; Geraldi, I.O.; Pizzirani-Kleiner, A.A.; Azevedo, J.L. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ. Microbiol. 2004, 6, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.; Nijhuis, E.; Someus, E. Selection of phosphorus solubilizing bacteria with biocontrol potential for growth in phosphorus rich animal bone charcoal. Appl. Soil Ecol. 2010, 46, 464–469. [Google Scholar] [CrossRef]
- Donato, V.M.T.S.; Andrade, A.G.; Souza, E.S.; França, J.G.; Maciel, G.A. Atividade enzimática em variedades de cana-de-açúcar cultivadas in vitro sob diferentes níveis de nitrogênio. Pesqui. Agropec. Bras. 2004, 39, 1087–1093. [Google Scholar] [CrossRef][Green Version]
- Thaweenut, N.; Hachisuka, Y.; Ando, S.; Yanagisawa, S.; Yoneyama, T. Two seasons study on nifH gene expression and nitrogen fixation by diazotrophic endophytes in sugarcane (Saccharum app. Hybrids): Expression of nifH genes similar to those rhizobia. Plant Soil 2011, 38, 435–449. [Google Scholar] [CrossRef]
- Sevilla, M.; Burris, R.H.; Gunapala, N.; Kennedy, C. Comparison of benefit to sugarcane plant growth and 15N2 incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and Nif− mutant strains. Mol. Plant Microbe Interact. 2001, 14, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Mehnaz, S.; Kowalik, T.; Reynolds, B.; Lazarovits, G. Growth promoting effects of corn (Zea mays) bacterial isolates under greenhouse and field conditions. Soil Biol. Biochem. 2010, 42, 1848–1856. [Google Scholar] [CrossRef]
- Miflin, B.J.; Habash, D.Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Müller, C.; Matt, P.; Gibon, Y.; Carillo, P.; Morcuende, R.; Scheible, W.; Krapp, A. Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot. 2002, 53, 959–970. [Google Scholar] [CrossRef]
- Tobin, A.K.; Yamaya, T. Cellular compartmentation of ammonium assimilation in rice and barley. J. Exp. Bot. 2001, 53, 591–604. [Google Scholar] [CrossRef]
- Lemaitre, T.; Gaufichon, L.; Boutet-Mercey, S.; Christ, A.; Masclaux-Doubresse, C. Enzimatic and metabolic of nitrogen deficiency in Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Lea, P.J.; Raven, J.A.; Lindsey, K. Can genetic manipulation of plant nitrogen assimilation enzymes result in increased crop yield and greater N-use efficiency? An assessment. Ann. Appl. Biol. 2004, 145, 25–40. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Ainsworth, C. Leaf senescence in Brassica napus: Cloning of senescence related genes by substractive hybridization. Plant Mol. Biol. 1997, 33, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Caputo, C.; Criado, M.V.; Roberts, I.N.; Gelso, M.A.; Barneix, A.J. Regulation of glutamine synthetase 1 and amino acids transport in the phloem of young wheat plants. Plant Physiol. Biochem. 2009, 47, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cren, M.; Hirel, B. Glutamine synthetase in higher plants regulation of gene and protein expression from the organ to the cell. Plant Cell Physiol. 1999, 40, 1187–1193. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, B. Arbuscular mycorrhiza induced putrescine degradation into γ-aminobutyric acid, malic acid accumulation, and improvement of nitrogen assimilation in roots of water-stressed maize plants. Mycorrhiza 2020, 30, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.R.; Papini-Terzi, F.S.; Nishiyama, M.Y., Jr.; Vêncio, R.Z.N.; Vicentini, R.; Duarte, R.D.C.; De Rosa, V.E., Jr.; Vinagre, F.; Barsalobres, C.; Medeiros, A.H.; et al. Signal transduction-related responses to phytohormones and environmental challenges in sugarcane. BMC Genom. 2007, 8, 71. [Google Scholar] [CrossRef]
- Afzal, A.J.; Wood, A.J.; Lightfoot, D.A. Plant receptor-like serine threonine kinases: Roles in signaling and plant defense. Mol. Plant Microbe Interact. 2008, 21, 507–517. [Google Scholar] [CrossRef]
- Tör, M.; Lotze, M.T.; Holton, N. Receptor-mediated signalling in plants: Molecular patterns and programmes. J. Exp. Bot. 2009, 60, 3645–3654. [Google Scholar] [CrossRef]
- Weston, D.J.; Pelletier, D.A.; Morrell-Falvey, J.L.; Tschaplinski, T.J.; Jawdy, S.S.; Lu, T.-Y.; Allen, S.M.; Melton, S.J.; Martin, M.Z.; Schadt, C.W.; et al. Pseudomonas fluorescens induces strain-dependent and strain-independent host plant responses in defense networks, primary metabolism, photosynthesis, and fitness. Mol. Plant Microbe Interact. 2012, 25, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Tejera, N.; Ortega, E.; Rodes, R.; Lluch, C. Nitrogen compounds in the apoplastic sap of sugarcane stem: Some implications in the association with endophytes. J. Plant Physiol. 2006, 163, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Elshintinawy, F.; Elshourbagy, M.N. Alleviation of changes in protein metabolism in NaCl-stressed wheat seedlings by thiamine. Biol. Plant. 2001, 44, 541–545. [Google Scholar] [CrossRef]
- Viégas, R.A.; Silveira, J.A.G. Ammonia assimilation and proline accumulation in young cashew plants during long term exposure to NaCl-salinity. Rev. Bras. Fisiol. Veg. 1999, 11, 153–159. [Google Scholar]
- Alcázar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, U.; Weber, A.P. Regulation and evolution of C4 photosynthesis. Annu. Rev. Plant. Biol. 2020, 71, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Pottosin, I.; Lamade, E.; Tcherkez, G. What is the role of putrescine accumulated under potassium deficiency? Plant Cell Environ. 2020, 43, 1331–1347. [Google Scholar] [CrossRef] [PubMed]
| Strain Code | Species Identity | nifH | IAA | PS | References |
|---|---|---|---|---|---|
| IAC/BECa-088 | Paraburkholderia caribensis | + | − | − | [13,17] |
| IAC/BECa-090 | Kosakonia radicincitans | + | − | − | [17] |
| IAC/BECa-095 | Kosakonia radicincitans | + | + | − | [13] |
| IAC/BECa-135 | Paraburkholderia tropica | − | − | − | [13,18] |
| IAC/BECa-141 | Pseudomonas fluorescens | − | + | − | [1,13,18] |
| IAC/BECa-152 | Herbaspirillum frisingense | − | + | − | [6,13,18,19] |
| Time | T0 | T30 | T45 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | SDM | RDM | TDM | Tiller | SDM | RDM | TDM | Tiller | SDM | RDM | TDM | Tiller | |||||||||||
| g | g | g | |||||||||||||||||||||
| Control | 0.27 | c * | 0.37 | a | 0.64 | c | - | 1.58 | b | 0.84 | b | 2.42 | c | 2.17 | a | 3.45 | b | 1.51 | b | 4.96 | b | 2.25 | a |
| IAC/BECa-088 | 0.78 | a | 0.42 | a | 1.2 | a | - | 2.74 | a | 1.23 | a | 3.96 | a | 2.17 | a | 5.23 | a | 2.49 | a | 7.72 | a | 2.25 | a |
| IAC/BECa-090 | 0.51 | b | 0.41 | a | 0.92 | b | - | 2.32 | a | 0.92 | b | 3.24 | b | 2 | a | 4.89 | a | 2.37 | a | 7.25 | a | 2.83 | a |
| IAC/BECa-095 | 0.64 | a | 0.45 | a | 1.09 | a | - | 2.38 | a | 0.86 | b | 3.23 | b | 1.75 | a | 5.00 | a | 2.55 | a | 7.55 | a | 3.08 | a |
| IAC/BECa-135 | 0.7 | a | 0.38 | a | 1.08 | a | - | 2.54 | a | 0.96 | b | 3.5 | b | 2.33 | a | 5.11 | a | 2.33 | a | 7.45 | a | 2.5 | a |
| IAC/BECa-141 | 0.75 | a | 0.45 | a | 1.2 | a | - | 2.31 | a | 0.98 | b | 3.29 | b | 2.33 | a | 4.78 | a | 2.98 | a | 7.76 | a | 2.42 | a |
| IAC/BECa-152 | 0.75 | a | 0.45 | a | 1.2 | a | - | 2.26 | a | 1.00 | b | 3.26 | b | 1.83 | a | 4.45 | a | 2.55 | a | 7 | a | 2.92 | a |
| CV (%) | 19.5 | 9.97 | 12.68 | - | 17.03 | 18.19 | 14.52 | 30.98 | 11.73 | 16.48 | 11.89 | 31.26 | |||||||||||
| Nutrient | N | P | K | Ca | Mg | S | Fe | Mn | Cu | Zn | B | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrient Concentration | Treatments | g kg−1 | mg kg−1 | ||||||||||||||||||||
| control | 18.52 | a * | 1.83 | b | 27.38 | b | 4.73 | a | 2.72 | a | 2.85 | a | 68.80 | c | 57.80 | a | 8.50 | c | 32.08 | b | 1.58 | b | |
| IAC-BECa-088 | 17.88 | b | 2.06 | a | 30.53 | a | 5.02 | a | 2.82 | a | 3.20 | a | 69.08 | c | 65.93 | a | 11.18 | a | 37.03 | a | 2.74 | a | |
| IAC-BECa-090 | 19.28 | a | 1.68 | b | 27.03 | b | 5.03 | a | 2.90 | a | 2.92 | a | 80.43 | b | 66.70 | a | 9.15 | b | 33.58 | a | 2.33 | a | |
| IAC-BECa-095 | 18.47 | a | 1.71 | b | 24.85 | c | 4.83 | a | 2.87 | a | 2.95 | a | 139.62 | a | 67.35 | a | 9.60 | b | 35.00 | a | 2.38 | a | |
| IAC-BECa-135 | 17.62 | b | 1.78 | b | 25.03 | c | 4.78 | a | 2.80 | a | 2.97 | a | 79.24 | b | 59.51 | a | 9.00 | b | 28.99 | b | 2.54 | a | |
| IAC-BECa-141 | 17.85 | b | 1.75 | b | 25.07 | c | 4.62 | a | 2.90 | a | 2.88 | a | 71.39 | c | 63.84 | a | 8.27 | c | 32.40 | b | 2.31 | a | |
| IAC-BECa-152 | 18.19 | b | 1.43 | c | 21.80 | d | 4.12 | b | 2.65 | a | 2.55 | a | 69.78 | c | 56.72 | a | 7.90 | c | 30.18 | b | 2.26 | a | |
| CV (%) | 3.88 | 4.74 | 8.55 | 8.94 | 6.32 | 8.64 | 10.43 | 11.50 | 9.20 | 9.63 | 17.03 | ||||||||||||
| Nutrient Accumulation | Treatments | g plant−1 | mg plant−1 | ||||||||||||||||||||
| control | 0.029 | b | 0.0029 | d | 0.0436 | c | 0.0075 | b | 0.0043 | b | 0.0045 | b | 0.109 | c | 0.0894 | c | 0.0135 | c | 0.0508 | c | 0.0193 | a | |
| IAC-BECa-088 | 0.049 | a | 0.0056 | a | 0.0836 | a | 0.0137 | a | 0.0077 | a | 0.0088 | a | 0.189 | b | 0.1803 | a | 0.0308 | a | 0.1012 | a | 0.0173 | a | |
| IAC-BECa-090 | 0.045 | a | 0.0039 | c | 0.0626 | b | 0.0116 | a | 0.0067 | a | 0.0068 | a | 0.186 | b | 0.1548 | a | 0.0212 | b | 0.0779 | b | 0.0190 | a | |
| IAC-BECa-095 | 0.044 | a | 0.0041 | c | 0.0591 | b | 0.0115 | a | 0.0068 | a | 0.0070 | a | 0.328 | a | 0.1589 | a | 0.0228 | b | 0.0833 | b | 0.0202 | a | |
| IAC-BECa-135 | 0.045 | a | 0.0046 | b | 0.0645 | b | 0.0122 | a | 0.0071 | a | 0.0076 | a | 0.205 | b | 0.1522 | a | 0.0230 | b | 0.0745 | b | 0.0200 | a | |
| IAC-BECa-141 | 0.041 | a | 0.0040 | b | 0.0582 | b | 0.0107 | a | 0.0067 | a | 0.0067 | a | 0.164 | b | 0.1480 | a | 0.0191 | b | 0.0755 | b | 0.0191 | a | |
| IAC-BECa-152 | 0.041 | a | 0.0032 | d | 0.0494 | c | 0.0093 | b | 0.0059 | a | 0.0058 | b | 0.156 | b | 0.1266 | b | 0.0177 | c | 0.0682 | c | 0.0218 | a | |
| CV (%) | 15.67 | 17.12 | 20.96 | 18.30 | 16.07 | 20.43 | 15.79 | 18.21 | 20.34 | 20.99 | 19.24 | ||||||||||||
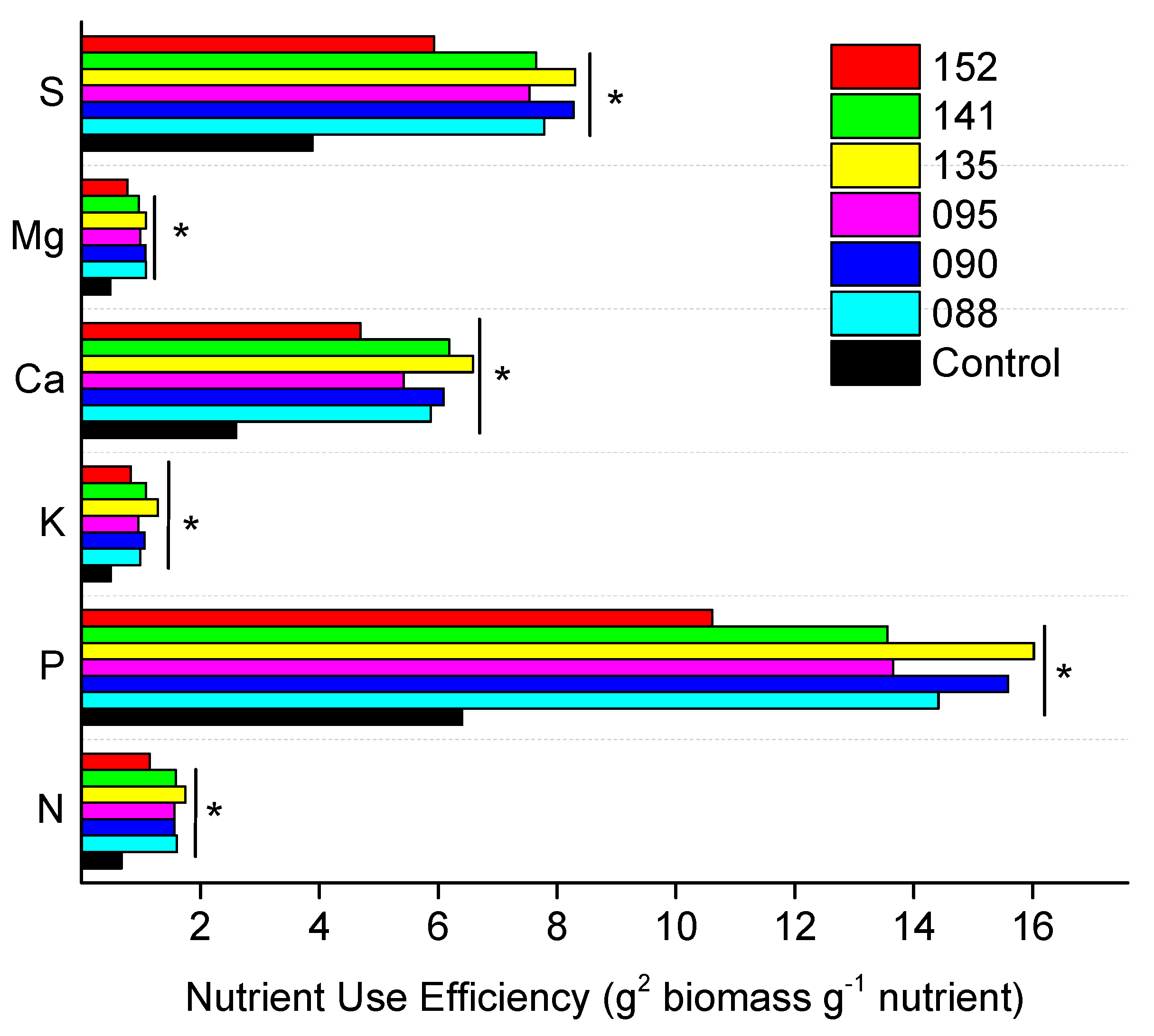
| UEI | Treatments | g2 biomass g−1 nutrient | mg2 biomass g−1 nutrient | ||||||||||||||||||||
| control | 0.1480 | b | 1.4716 | b | 0.0973 | b | 0.5755 | b | 0.9900 | b | 0.9783 | b | 0.0384 | b | 0.0489 | b | 0.3127 | b | 0.0833 | b | 0.0525 | a | |
| IAC-BECa-088 | 0.4211 | a | 3.6949 | a | 0.2476 | a | 1.5051 | a | 2.6804 | a | 2.3511 | a | 0.1096 | a | 0.1143 | a | 0.6745 | a | 0.2037 | a | 0.1309 | a | |
| IAC-BECa-090 | 0.2798 | a | 3.2127 | a | 0.2025 | a | 1.0953 | a | 1.8673 | a | 1.8493 | a | 0.0676 | a | 0.0810 | a | 0.5901 | a | 0.1608 | a | 0.1038 | a | |
| IAC-BECa-095 | 0.3087 | a | 3.3297 | a | 0.2283 | a | 1.1819 | a | 1.9828 | a | 1.9213 | a | 0.0450 | b | 0.0874 | a | 0.6005 | a | 0.1622 | a | 0.1171 | a | |
| IAC-BECa-135 | 0.3763 | a | 3.7250 | a | 0.2620 | a | 1.3904 | a | 2.4004 | a | 2.2113 | a | 0.0839 | a | 0.1112 | a | 0.7395 | a | 0.2283 | a | 0.1358 | a | |
| IAC-BECa-141 | 0.3084 | a | 3.1453 | a | 0.2190 | a | 1.1985 | a | 1.8927 | a | 1.9120 | a | 0.0780 | a | 0.0856 | a | 0.6677 | a | 0.1677 | a | 0.1039 | a | |
| IAC-BECa-152 | 0.2926 | a | 3.7625 | a | 0.2413 | a | 1.3073 | a | 2.0530 | a | 2.0769 | a | 0.0786 | a | 0.0977 | a | 0.6852 | a | 0.1779 | a | 0.1238 | a | |
| CV (%) | 34.51 | 35.53 | 31.80 | 36.22 | 36.64 | 32.52 | 37.84 | 38.72 | 36.52 | 31.19 | 46.70 | ||||||||||||
| Nutrient | N | P | K | Ca | Mg | S | Fe | Mn | Cu | Zn | B | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrient Concentration | Treatments | g kg−1 | mg kg−1 | ||||||||||||||||||||
| control | 18.22 | a * | 1.93 | b | 25.05 | c | 4.70 | a | 2.52 | a | 3.29 | c | 60.11 | b | 43.02 | b | 7.38 | b | 25.26 | b | 17.16 | c | |
| IAC-BECa-088 | 18.60 | a | 1.95 | b | 30.28 | a | 4.78 | a | 5.57 | a | 3.76 | a | 60.28 | b | 50.60 | a | 8.57 | a | 28.01 | a | 23.93 | a | |
| IAC-BECa-090 | 16.49 | b | 1.68 | c | 24.03 | c | 4.07 | b | 2.28 | a | 3.08 | c | 63.53 | a | 43.71 | b | 6.88 | b | 21.92 | b | 19.81 | b | |
| IAC-BECa-095 | 16.79 | b | 1.91 | b | 27.28 | b | 4.83 | a | 2.55 | a | 3.51 | b | 60.90 | b | 52.46 | a | 7.23 | b | 27.63 | a | 24.06 | a | |
| IAC-BECa-135 | 16.28 | b | 1.85 | b | 22.12 | d | 4.53 | a | 2.55 | a | 3.48 | b | 59.84 | b | 52.83 | a | 8.03 | a | 29.68 | a | 16.95 | c | |
| IAC-BECa-141 | 16.08 | b | 1.72 | c | 21.78 | d | 3.92 | b | 2.48 | a | 3.05 | c | 52.02 | b | 4.16 | b | 6.91 | b | 22.70 | c | 20.25 | b | |
| IAC-BECa-152 | 19.03 | a | 2.18 | a | 24.45 | c | 4.23 | b | 2.58 | a | 3.80 | a | 71.20 | a | 53.70 | a | 8.73 | a | 22.99 | c | 21.48 | a | |
| CV (%) | 4.61 | 5.87 | 7.10 | 6.16 | 8.54 | 7.34 | 11.67 | 5.46 | 7.57 | 7.75 | 11.75 | ||||||||||||
| Nutrient Accumulation | Treatments | g plant−1 | mg kg−1 | ||||||||||||||||||||
| control | 0.0633 | c | 0.5206 | b | 0.0863 | d | 0.0162 | c | 0.0087 | c | 0.0114 | c | 0.2254 | b | 0.1483 | c | 0.0256 | d | 0.0847 | c | 0.0593 | c | |
| IAC-BECa-088 | 0.0971 | a | 0.5148 | b | 0.1582 | a | 0.0249 | a | 0.0134 | a | 0.0197 | a | 0.3161 | a | 0.2641 | a | 0.0448 | a | 0.1462 | a | 0.1258 | a | |
| IAC-BECa-090 | 0.0796 | b | 0.6001 | a | 0.1162 | c | 0.0198 | b | 0.0111 | b | 0.1503 | b | 0.3199 | a | 0.2141 | b | 0.0328 | c | 0.1064 | b | 0.0972 | b | |
| IAC-BECa-095 | 0.0845 | b | 0.5248 | b | 0.1377 | b | 0.0241 | a | 0.0128 | a | 0.0175 | a | 0.3055 | a | 0.2612 | a | 0.0360 | b | 0.1360 | a | 0.1213 | a | |
| IAC-BECa-135 | 0.0827 | b | 0.5419 | b | 0.1127 | c | 0.0231 | a | 0.0129 | a | 0.0178 | a | 0.3046 | a | 0.2693 | a | 0.0409 | a | 0.1505 | a | 0.0853 | b | |
| IAC-BECa-141 | 0.0772 | b | 0.5835 | b | 0.1040 | c | 0.0185 | b | 0.0118 | b | 0.0147 | b | 0.2494 | b | 0.2203 | b | 0.3325 | c | 0.1094 | b | 0.0955 | b | |
| IAC-BECa-152 | 0.0845 | b | 0.4610 | c | 0.1088 | c | 0.0189 | b | 0.0115 | b | 0.0170 | a | 0.3154 | a | 0.2391 | b | 0.3832 | b | 0.1021 | b | 0.0938 | b | |
| CV (%) | 11.56 | 8.63 | 13.20 | 10.32 | 11.51 | 14.38 | 18.34 | 12.39 | 12.29 | 11.93 | 16.16 | ||||||||||||
| UEI | Treatments | g2 biomass g−1 nutrient | mg2 biomass g−1 nutrient | ||||||||||||||||||||
| control | 0.6750 | b | 6.4070 | b | 0.4880 | b | 2.6050 | b | 0.4856 | b | 3.8820 | b | 0.1720 | c | 0.2680 | b | 1.5730 | b | 0.4690 | b | 0.6930 | b | |
| IAC-BECa-088 | 1.6050 | a | 14.4150 | a | 0.9880 | a | 5.8750 | a | 1.0817 | a | 7.7800 | a | 0.4620 | a | 0.5680 | a | 3.5540 | a | 1.0850 | a | 1.1920 | b | |
| IAC-BECa-090 | 1.5650 | a | 15.5860 | a | 1.0640 | a | 6.0940 | a | 1.0778 | a | 8.2790 | a | 0.4080 | a | 0.5790 | a | 3.9640 | a | 1.2080 | a | 1.3930 | b | |
| IAC-BECa-095 | 1.5650 | a | 13.6540 | a | 0.9560 | a | 5.4150 | a | 0.9898 | a | 7.5390 | a | 0.4170 | a | 0.5100 | a | 3.7390 | a | 1.0180 | a | 1.0910 | b | |
| IAC-BECa-135 | 1.7420 | a | 16.0180 | a | 1.2870 | a | 6.5820 | a | 1.0806 | a | 8.3050 | a | 0.4890 | a | 0.5680 | a | 3.8180 | a | 1.1350 | a | 1.8800 | b | |
| IAC-BECa-141 | 1.5860 | a | 13.5540 | a | 1.0810 | a | 6.1860 | a | 0.9669 | a | 7.6490 | a | 0.4510 | a | 0.5210 | a | 3.5250 | a | 1.1150 | a | 1.3350 | a | |
| IAC-BECa-152 | 1.1430 | b | 10.6090 | b | 8.3200 | a | 4.6940 | a | 0.7745 | b | 5.9290 | b | 0.3140 | b | 0.4060 | b | 2.1560 | b | 0.8450 | a | 1.0880 | b | |
| CV (%) | 32.01 | 30.79 | 27.89 | 31.73 | 29.27 | 27.69 | 28.30 | 28.21 | 35.49 | 39.11 | 34.95 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipriano, M.A.P.; Freitas-Iório, R.d.P.; Dimitrov, M.R.; de Andrade, S.A.L.; Kuramae, E.E.; Silveira, A.P.D.d. Plant-Growth Endophytic Bacteria Improve Nutrient Use Efficiency and Modulate Foliar N-Metabolites in Sugarcane Seedling. Microorganisms 2021, 9, 479. https://doi.org/10.3390/microorganisms9030479
Cipriano MAP, Freitas-Iório RdP, Dimitrov MR, de Andrade SAL, Kuramae EE, Silveira APDd. Plant-Growth Endophytic Bacteria Improve Nutrient Use Efficiency and Modulate Foliar N-Metabolites in Sugarcane Seedling. Microorganisms. 2021; 9(3):479. https://doi.org/10.3390/microorganisms9030479
Chicago/Turabian StyleCipriano, Matheus Aparecido Pereira, Raquel de Paula Freitas-Iório, Maurício Rocha Dimitrov, Sara Adrián López de Andrade, Eiko Eurya Kuramae, and Adriana Parada Dias da Silveira. 2021. "Plant-Growth Endophytic Bacteria Improve Nutrient Use Efficiency and Modulate Foliar N-Metabolites in Sugarcane Seedling" Microorganisms 9, no. 3: 479. https://doi.org/10.3390/microorganisms9030479
APA StyleCipriano, M. A. P., Freitas-Iório, R. d. P., Dimitrov, M. R., de Andrade, S. A. L., Kuramae, E. E., & Silveira, A. P. D. d. (2021). Plant-Growth Endophytic Bacteria Improve Nutrient Use Efficiency and Modulate Foliar N-Metabolites in Sugarcane Seedling. Microorganisms, 9(3), 479. https://doi.org/10.3390/microorganisms9030479









