Influence on Secondary Metabolism of Piper nigrum L. by Co-Inoculation with Arbuscular Mycorrhizal Fungi and Fusarium solani f. sp. piperis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Arbuscular Mycorrhizal Fungal Spore Inoculum and Inoculation
2.3. Acquisition, Isolation, and Culture of Fusarium solani f. sp. piperis Phytopathogen
2.4. Experimental Design and Material Collection
2.5. Inoculation, Observation of Symptoms and Recovery of Fusarium solani f. sp. piperis
2.6. Extraction and Volatile Compounds
2.7. Lipoxygenase (LOX) Activity
2.8. Phenylalanine Ammonia Lyase (PAL) Activity
2.9. Total Phenolics Determination
2.10. Statistical Analyses
3. Results
3.1. Aspects of Infection
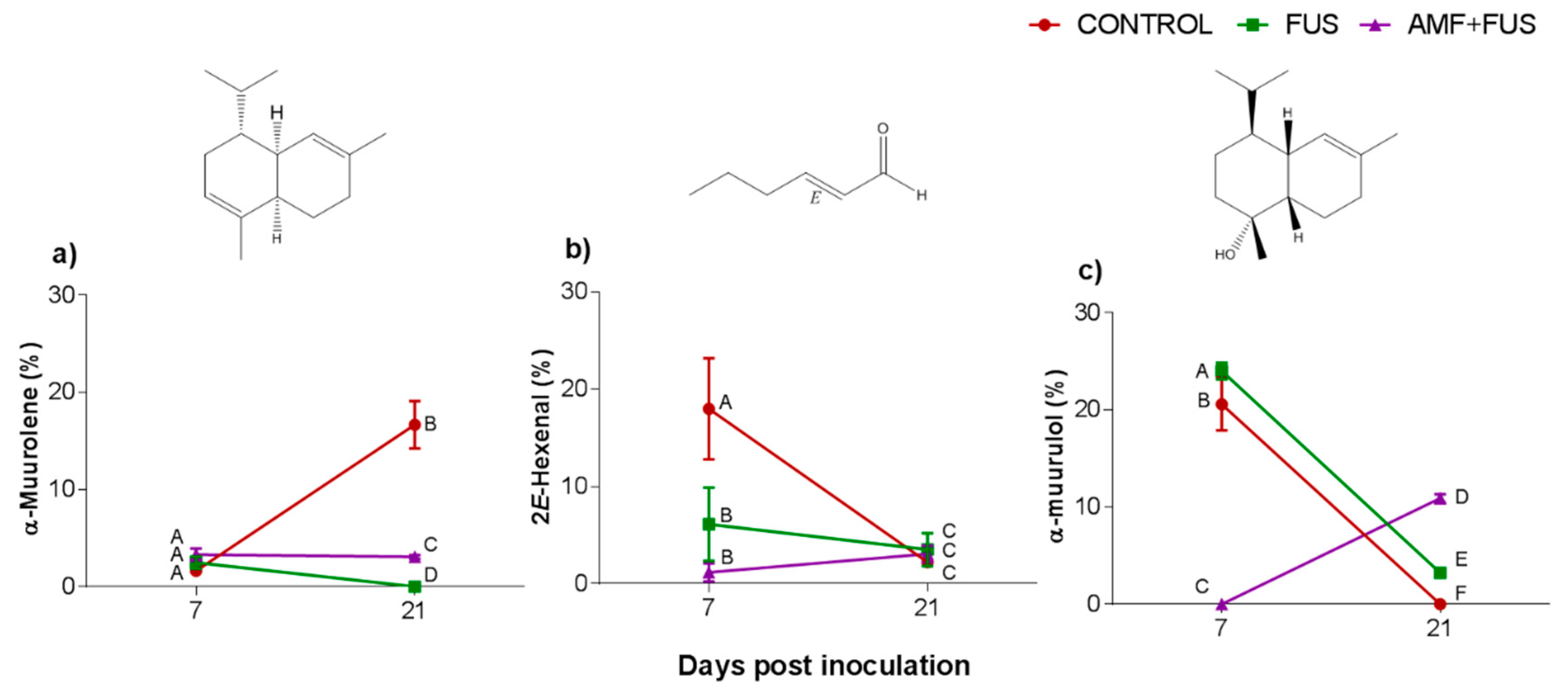
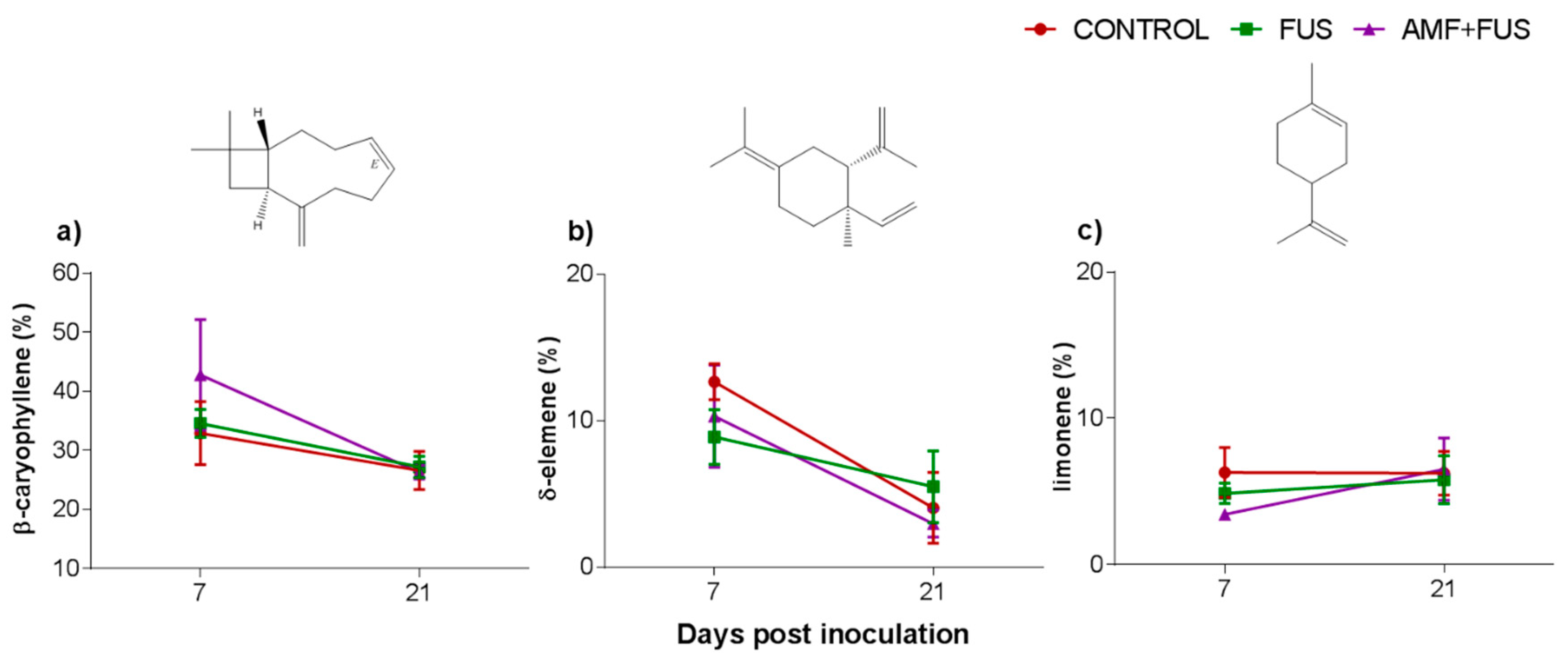
3.2. Oil Essential Composition
3.3. Total Phenolic Content
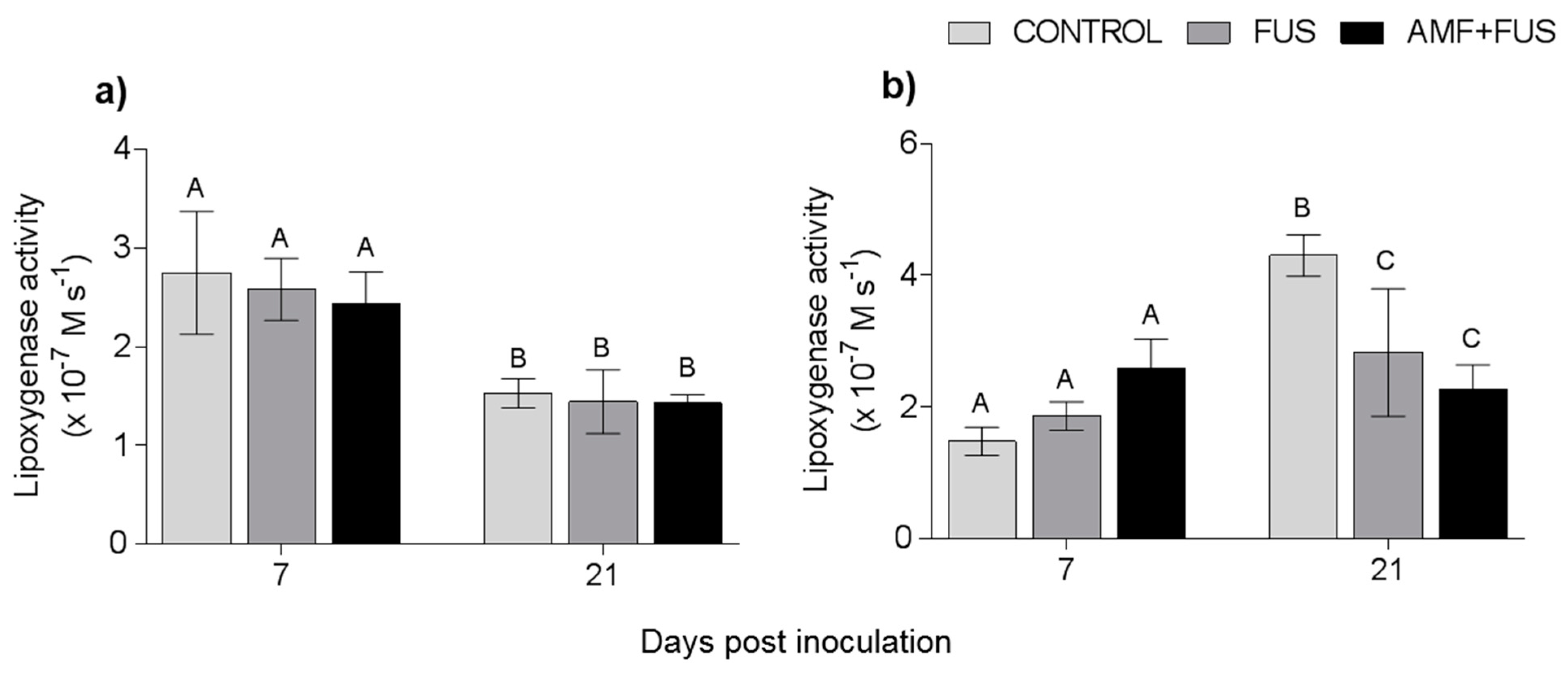
3.4. Enzymatic Activity
3.4.1. Lipoxygenase Enzyme
3.4.2. Phenylalanine Ammonia-Lyase Enzymatic Activity
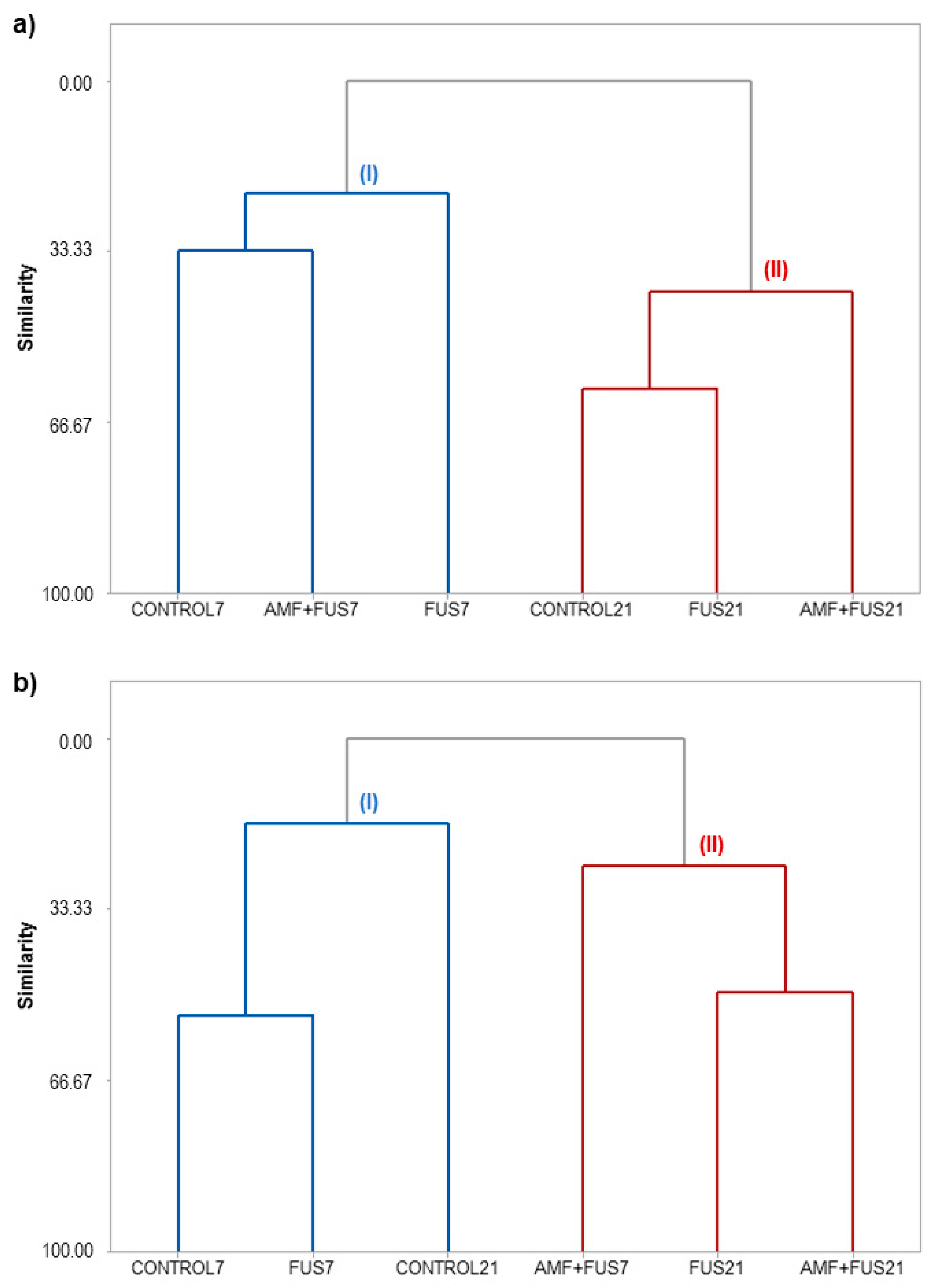
3.5. Hierarchical Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trindade, D.R.; Poltronieri, L.C. Doenças da Pimenta-do-reino. In Manual de Fitopatologia Volume 2: Doença das Plantas Cultivadas; Ltda, Editora AgronômicaCeres: São Paulo, Brazil, 1997; Volume 2, pp. 536–540. ISBN 8531800080. Available online: https://ppgfito.ufersa.edu.br/wp-content/uploads/sites/45/2015/02/Livro-Manual-de-Fitopatologia-vol.2.pdf (accessed on 1 December 2020).
- de Lemos, O.F. Conservação e Melhoramento Genético da Pimenteira-Do-Reino (Piper nigrum L.) em Associação com as Técnicas de Biotecnologia; 1a; Embrapa Amazônia Oriental: Belém, Brazil, 2011; ISBN 1983-0513. [Google Scholar]
- Tremacoldi, C. Principais Doenças Fúngicas da Pimenteira-do-Reino no Estado do Pará e Recomendações de Controle; Embrapa Amazônia Oriental: Belém, Brazil, 2010; pp. 10–14. ISBN 85-318-0008-0. [Google Scholar]
- da Luz, S.F.M.; de Reis, L.A.; de Lemos, O.F.; Maia, J.G.S.; de Mello, A.H.; Ramos, A.R.; da Silva, J.K.R. Effect of arbuscular mycorrhizal fungi on the essential oil composition and antioxidant activity of black pepper (Piper nigrum L.). Int. J. Appl. Res. Nat. Prod. 2016, 9, 10–17. [Google Scholar]
- Benchimol, R.L.; Chu, E.Y.; Muto, R.Y.; Dias-Filho, M.B. Controle da fusariose em plantas de pimenta-do-reino com bactérias endofíticas: Sobrevivêncla e respostas morfofisiológicas. Pesqui. Agropecu. Bras. 2000, 35, 1343–1348. [Google Scholar] [CrossRef]
- Albuquerque, F.C.; Duarte, M.L.R. Inoculação de mudas de cultivares, clones e espécies de Piper com Fusarium solani f. sp. piperis e Phytophthora palmivora. Terra 1995, 14, 1–7. [Google Scholar] [CrossRef]
- Mousumi Das, M.; Haridas, M.; Sabu, A. Biological control of black pepper and ginger pathogens, Fusarium oxysporum, Rhizoctonia solani and Phytophthora capsici, using Trichoderma spp. Biocatal. Agric. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Kollakkodan, N.; Anith, K.N.; Radhakrishnan, N.V. Diversity of endophytic bacteria from Piper spp. with antagonistic property against phytophthora capsici causing foot rot disease in black pepper (Piper nigrum L.). J. Trop. Agric. 2017, 55, 63–70. [Google Scholar]
- Duarte, M. de L.R. Cultivo da Pimenteira-do-reino na Região Norte; 1a; Embrapa Amazônia Oriental: Belém, Brazil, 2004; ISBN 3807-0043. [Google Scholar]
- Thanuja, T.V.; Hegde, R.V.; Sreenivasa, M.N. Induction of rooting and root growth in black pepper cuttings (Piper nigrum L.) with the inoculation of arbuscular mycorrhizae. Sci. Hortic. 2002. [Google Scholar] [CrossRef]
- da Trindade, R.; Almeida, L.; Xavier, L.; Lins, A.L.; Andrade, E.H.; Maia, J.G.; Mello, A.; Setzer, W.N.; Ramos, A.; da Silva, J.K. Arbuscular Mycorrhizal Fungi Colonization Promotes Changes in the Volatile Compounds and Enzymatic Activity of Lipoxygenase and Phenylalanine Ammonia Lyase in Piper nigrum L. ‘Bragantina’. Plants 2019, 8, 442. [Google Scholar] [CrossRef]
- Eke, P.; Chatue Chatue, G.; Wakam, L.N.; Kouipou, R.M.T.; Fokou, P.V.T.; Boyom, F.F. Mycorrhiza consortia suppress the fusarium root rot (Fusarium solani f. sp. Phaseoli) in common bean (Phaseolus vulgaris L.). Biol. Control 2016. [Google Scholar] [CrossRef]
- Pozo, M.J.; Jung, S.C.; López-Ráez, J.A.; Azcón-Aguilar, C. Impact of Arbuscular Mycorrhizal Symbiosis on Plant Response to Biotic Stress: The Role of Plant Defence Mechanisms. In Arbuscular Mycorrhizas: Physiology and Function; Springer: Dordrecht, The Netherlands, 2010; pp. 193–207. [Google Scholar]
- Ganugi, P.; Masoni, A.; Pietramellara, G.; Benedettelli, S. A review of studies from the last twenty years on plant–arbuscular mycorrhizal fungi associations and their uses for wheat crops. Agronomy 2019, 9, 840. [Google Scholar] [CrossRef]
- Schouteden, N.; de Waele, D.; Panis, B.; Vos, C.M. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tahat, M.M.; Kamaruzaman, S.; Othman, R. Mycorrhizal Fungi as a Biocontrol Agent. Plant Pathol. J. 2010. [Google Scholar] [CrossRef]
- French, K.E. Engineering Mycorrhizal Symbioses to Alter Plant Metabolism and Improve Crop Health. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Chandra, P. Microbial Volatiles as Chemical Weapons against Pathogenic Fungi. In Volatiles and Food Security; Springer: Singapore, 2017; pp. 227–254. [Google Scholar]
- Santos Filho, H.P.; Carollo, E.M. Técnicas Fitopatológicas. In Manual Básico de Técnicas Fitopatológicas: Laboratório de Fitopatologia Embrapa Mandioca e Fruticultura; Embrapa Mandioca e Fruticultura: Cruz das Almas, Bahia, 2016; pp. 85–94. [Google Scholar]
- da Luz, S.F.M.; Yamaguchi, L.F.; Kato, M.J.; de Lemos, O.F.; Xavier, L.P.; Maia, J.G.S.; de Ramos, A.R.; Setzer, W.N.; da Silva, J.K.D.R. Secondary metabolic profiles of two cultivars of Piper nigrum (black pepper) resulting from infection by Fusarium solani f. sp. piperis. Int. J. Mol. Sci. 2017, 18, 2434. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Corporation, A.P., Ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- Nist 11 National Institute of Standard and Technology. Available online: https://webbook.nist.gov/ (accessed on 28 May 2019).
- Luigi, M. Flavors and Fragrances of Natural and Synthetic Compounds 2, 2nd ed.; Wiley–Blackwell, Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2011; ISBN 1118145836. [Google Scholar]
- Vaganan, M.M.; Ravi, I.; Nandakumar, A.; Sarumathi, S.; Sundararaju, P.; Mustaffa, M.M. Phenylpropanoid enzymes, phenolic polymers and metabolites as chemical defenses to infection of Pratylenchus coffeae in roots of resistant and susceptible bananas (Musa spp.). Indian J. Exp. Biol. 2014, 52, 252–260. [Google Scholar] [PubMed]
- Sousa, C.M.D.M.; Silva, H.R.E.; Vieira, G.M.; Ayres, M.C.C.; Da Costa, C.L.S.; Araújo, D.S.; Cavalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.D.M.; Brandão, M.S.; et al. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim. Nova 2007, 30, 351–355. [Google Scholar] [CrossRef]
- Garcia-Garrido, J.M. Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. J. Exp. Bot. 2002, 53, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.E.; Grayston, S.J.; Helmisaari, H.-S.; Kaštovská, E.; Körner, C.; Lambers, H.; Meier, I.C.; Millard, P.; Ostonen, I. Surplus Carbon Drives Allocation and Plant–Soil Interactions. Trends Ecol. Evol. 2020, 35, 1110–1118. [Google Scholar] [CrossRef]
- Erisléia-Meireles, N.; Luciana-Xavier, P.; Alessandra-Ramos, R.; José-Guilherme, M.S.; William-Setzer, N.; Kelly-da-Silva, J.R. Phenylpropanoids Produced by Piper divaricatum, A Resistant Species to Infection by Fusarium solani f. sp. piperis, the Pathogenic Agent of Fusariosis in Black Pepper. J. Plant Pathol. Microbiol. 2016. [Google Scholar] [CrossRef]
- Sharifi, R.; Lee, S.M.; Ryu, C.M. Microbe-induced plant volatiles. New Phytol. 2017. [Google Scholar] [CrossRef]
- Rana, A.; Sahgal, M.; Johri, B.N. Fusarium oxysporum: Genomics, Diversity and Plant–Host Interaction. In Developments in Fungal Biology and Applied Mycology; Springer: Singapore, 2017; pp. 159–199. [Google Scholar]
- Cruz, A.F.; Hamel, C.; Yang, C.; Matsubara, T.; Gan, Y.; Singh, A.K.; Kuwada, K.; Ishii, T. Phytochemicals to suppress Fusarium head blight in wheat-chickpea rotation. Phytochemistry 2012, 78, 72–80. [Google Scholar] [CrossRef]
- Effah, E.; Holopainen, J.K.; McCormick, A.C. Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 58–63. [Google Scholar] [CrossRef]
- Fantaye, C.A.; Köpke, D.; Gershenzon, J.; Degenhardt, J. Restoring (E)-β-Caryophyllene Production in a Non-producing Maize Line Compromises its Resistance against the Fungus Colletotrichum graminicola. J. Chem. Ecol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Woranoot, K.; Buaruang, R.; Aranyakanon, K.; Ratanasut, K.; Kongbangkerd, A.; Jannoey, P.; Nangngam, P.; Choopayak, C. Fusarium solani Upregulated Sesquiterpene Synthase Expression, Sesquiterpene Production and Allelopathic Activity in Piper betle L. Rice Sci. 2019. [Google Scholar] [CrossRef]
- Gao, X.; Lu, X.; Wu, M.; Zhang, H.; Pan, R.; Tian, J.; Li, S.; Liao, H. Co-inoculation with rhizobia and AMF inhibited soybean red crown rot: From field study to plant defense-related gene expression analysis. PLoS ONE 2012, 7, e33977. [Google Scholar] [CrossRef] [PubMed]
- Mapope, N.; Dakora, F.D. Role of Flavonoid and Isoflavonoid Molecules in Symbiotic Functioning and Host-Plant Defence in the Leguminosae. In Chemistry for Sustainable Development in Africa; Gurib-Fakim, A., Eloff, J.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 33–48. ISBN 978-3-642-29641-3. [Google Scholar]
- Sikes, B.A. When do arbuscular mycorrhizal fungi protect plant roots from pathogens? Plant Signal. Behav. 2010. [Google Scholar] [CrossRef]
- Sparkman, O.D. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Robert, P.A., Ed.; Allured Publishing Corporation: NewYork, NY, USA, 2005; Volume 16, ISBN 0931710855. [Google Scholar]
- de Quadros, F.M.; Garcés-Fiallos, F.R.; de Borba, M.C.; de Freitas, M.B.; Stadnik, M.J. Fusarium oxysporum affects differently the hydrogen peroxide levels and oxidative metabolism in susceptible and resistant bean roots. Physiol. Mol. Plant Pathol. 2019, 106, 1–6. [Google Scholar] [CrossRef]
- Kong, J.-Q. Phenylalanine ammonia-lyase, a key component used for phenylpropanoids production by metabolic engineering. RSC Adv. 2015, 5, 62587–62603. [Google Scholar] [CrossRef]
- Ma, L.-J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium Pathogenomics. Annu. Rev. Microbiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R. Induced resistance in mycorrhizal tomato is correlated to concentration of jasmonic acid. Online J. Biol. Sci. 2008, 8, 49–56. [Google Scholar] [CrossRef]
- Falahian, F.; Oraghi Ardebili, Z.; Fahimi, F.; Khavarinejad, R. Effect of mycorrhizal fungi on some defense enzymes against Gaeumannomyces gaminis in wheat. Pakistan J. Biol. Sci. 2007. [Google Scholar] [CrossRef]
- Koeck, M.; Hardham, A.R.; Dodds, P.N. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell. Microbiol. 2011, 13, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Zhou, J.-M. Phytopathogen Effectors Subverting Host Immunity: Different Foes, Similar Battleground. Cell Host Microbe 2012, 12, 484–495. [Google Scholar] [CrossRef]




| Compounds | RIa | RIb | 7 dpi | 21 dpi | ||||
|---|---|---|---|---|---|---|---|---|
| CONTROL | FUS | AMF + FUS | CONTROL | FUS | AMF + FUS | |||
| 2E-hexenal | 846 | 846 | 17.98 ± 5.20 A | 6.11 ± 3.77 B | 1.16 ± 0.92 B | 2.21 ± 0.58 C | 3.50 ± 1.68 C | 3.06 ± 0.83 C |
| δ-elemene | 1335 | 1339 | 2.92 ± 0.14 | 5.20 ± 0.34 | 5.15 ± 0.82 | 4.11 ± 0.18 | 4.02 ± 0.66 | 3.42 ± 0.11 |
| α-cubebene | 1345 | 1352 | 2.70 ± 0.19 | 4.17 ± 0.77 | 3.42 ± 0.30 | 2.45 ± 0.32 | 2.86 ± 0.14 | 2.87 ± 0.09 |
| α-copaene | 1374 | 1378 | 1.95 ± 0.45 | 6.32 ± 2.68 | 6.16 ± 0.90 | 4.11 ± 0.39 | 3.67 ± 0.34 | 4.46 ± 0.22 |
| β-cubebene | 1387 | 1392 | 3.32 ± 0.55 | 2.93 ± 0.81 | 0.0 | 1.08 ± 0.06 | 0.89 ± 0.03 | 1.10 ± 0.13 |
| α-gurjunene | 1409 | 1413 | 0.97 ± 0.23 | 4.78 ± 2.43 | 3.94 ± 0.20 | 3.46 ± 0.14 | 3.89 ± 0.32 | 3.62 ± 0.09 |
| β-caryophyllene | 1417 | 1423 | 2.76 ± 0.63 | 5.32 ± 1.15 | 4.73 ± 0.23 | 4.07 ± 0.33 | 6.54 ± 1.78 | 3.80 ± 0.27 |
| β-selinene | 1489 | 1491 | 1.94 ± 0.49 | 5.26 ± 0.89 | 3.86 ± 0.48 | 4.40 ± 0.28 | 4.36 ± 0.42 | 3.57 ± 0.16 |
| Z-β-guaiene | 1492 | 1500 | 0.0 | 0.0 | 0.0 | 6.59 | 0.0 | 0.0 |
| viridiflorene | 1496 | 1500 | 5.36 ± 0.13 | 5.78 ± 0.88 | 0.0 | 0.0 | 0.0 | 0.0 |
| bicyclogermacrene | 1500 | 1502 | 4.60 ± 0.48 | 4.12 ± 3.15 | 11.82 ± 4.75 | 5.20 ± 0.28 | 4.21 ± 0.79 | 7.13 ± 2.17 |
| α-muurolene | 1500 | 1505 | 1.63 ± 0.32 A | 2.49 ± 0.63 A | 3.31 ± 0.62 A | 16.68 ± 2.44 B | 0.0 C | 3.08 ± 0.18 D |
| E-β-guaiene | 1502 | 1522 | 0.0 | 4.77 ± 0.84 | 0.0 | 0.0 | 0.0 | 0.0 |
| δ-amorphene | 1511 | 1512 | 0.0 | 2.18 ± 1.93 | 0.0 | 0.0 | 0.0 | 0.52 ± 0.12 |
| γ-cadinene | 1513 | 1521 | 6.78 ± 0.70 | 8.82 ± 0.40 | 0.0 | 0.0 | 0.0 | 5.45 ± 0.14 |
| δ-cadinene | 1513 | 1522 | 3.630.57 | 3.92 ± 0.39 | 4.16 ± 0.87 | 10.60 ± 0.57 | 10.07 ± 0.55 | 5.23 ± 0.69 |
| E-nerolidol | 1561 | 1567 | 2.42 ± 0.70 | 1.11 ± 0.56 | 1.94 ± 0.79 | 2.96 ± 0.62 | 2.08 ± 0.28 | 1.04 ± 0.12 |
| caryophyllene oxide | 1582 | 1589 | 1.33 ± 0.29 | 1.02 ± 0.23 | 1.50 ± 0.09 | 2.03 ± 0.26 | 1.38 ± 0.17 | 1.52 ± 0.33 |
| α-epi-muurolol | 1640 | 1647 | 3.05 ± 0.84 | 3.97 ± 0.78 | 0.0 | 0.0 | 0.0 | 0.0 |
| α-muurolol | 1644 | 1651 | 20.59 ± 2.71 A | 24.52 ± 0.83 B | 0.0 C | 0.0 D | 3.23 ± 0.27 E | 10.91 ± 0.44 F |
| cubenol | 1645 | 1653 | 0.0 | 14.20 ± 4.73 | 18.97 ± 2.02 | 3.15 ± 0.08 | 9.07 ± 0.34 | 1.79 |
| α-cadinol | 1652 | 1659 | 0.86 ± 0.29 | 1.44 ± 0.47 | 0.56 ± 0.06 | 0.0 | 0.0 | 4.39 ± 0.36 |
| Monoterpene hydrocarbons | 0.27 ± 0.43 | 0.0 | 0.0 | 0.76 ± 0.09 | 1.90 ± 0.51 | 3.64 ± 2.12 | ||
| Oxygenated monoterpenes | 0.72 ± 0.20 | 1.05 ± 0.58 | 0.0 | 1.40 ± 0.22 | 1.49 ± 0.24 | 1.59 ± 0.31 | ||
| Sesquiterpene hydrocarbons | 45.09 ± 7.54 A | 78.86 ± 20.67 A | 56.25 ± 10.41 A | 72.92 ± 6.86 A | 51.93 ± 6.80 A | 55.20 ± 6.40 A | ||
| Oxygenated Sesquiterpenes | 29.96 ± 5.70 A | 50.04 ± 9.39 A,B | 28.64 ± 4.64 A,C | 15.35 ± 2.90 A | 21.69 ± 2.98 A | 24.63 ± 3.82 A | ||
| Phenylpropanoids | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Others | 18.36 ± 5.40 | 6.29 ± 3.83 | 2.48 ± 1.18 | 3.24 ± 1.15 | 4.97 ± 2.01 | 5.09 ± 1.94 | ||
| Total | 94.40 ± 19.27 | 136.25 ± 34.48 | 87.37 ± 16.23 | 93.67 ± 11.21 | 81.98 ± 12.53 | 90.15 ± 14.58 | ||
| Compounds | RIa | RIb | 7 dpi | 21 dpi | ||||
|---|---|---|---|---|---|---|---|---|
| CONTROL | FUS | AMF + FUS | CONTROL | FUS | AMF + FUS | |||
| n-octane | 800 | 775 | 0.0 | 0.0 | 7.72 ± 3.30 | 0.0 | 0.0 | 0.0 |
| n-nonane | 900 | 900 | 3.53 ± 0.84 | 4.02 ± 0.47 | 3.79 ± 1.61 | 4.70 ± 0.71 | 8.24 ± 1.25 | 7.23 ± 0.95 |
| α-pinene | 932 | 927 | 2.28 ± 0.41 | 2.76 ± 0.24 | 1.75 ± 0.26 | 3.42 ± 0.76 | 3.58 ± 0.43 | 3.44 ± 1.07 |
| canfene | 946 | 945 | 1.64 ± 0.93 | 3.95 ± 1.30 | 1.46 ± 0.53 | 8.17 ± 0.34 | 6.36 ± 1.25 | 4.50 ± 1.95 |
| β-pinene | 974 | 971 | 4.05 ± 1.38 | 3.11 ± 0.46 | 2.16 ± 0.20 | 3.42 ± 1.10 | 3.98 ± 0.76 | 3.71 ± 1.57 |
| limonene | 1024 | 1022 | 6.31 ± 1.70 | 4.88 ± 0.69 | 3.44 ± 0.30 | 6.25 ± 1.49 | 5.81 ± 1.64 | 6.53 ± 2.12 |
| camphor | 1141 | 1146 | 1.82 ± 0.49 | 3.17 ± 0.56 | 0.87 ± 0.46 | 1.57 ± 0.38 | 1.36 ± 0.59 | 0.46 ± 0.14 |
| isoborneol | 1155 | 1158 | 1.62 ± 0.45 | 0.54 ± 0.13 | 0.44 ± 0.19 | 2.31 ± 0.36 | 1.60 ± 0.35 | 0.62 ± 0.10 |
| δ-elemene | 1335 | 1337 | 12.68 ± 1.23 | 8.90 ± 1.88 | 10.33 ± 3.50 | 4.06 ± 2.41 | 5.50 ± 2.46 | 2.98 ± 0.92 |
| β-caryophyllene | 1417 | 1423 | 32.93 ± 5.34 | 34.58 ± 2.38 | 42.79 ± 9.36 | 26.60 ± 3.21 | 27.21 ± 1.80 | 26.32 ± 1.16 |
| Monoterpene hydrocarbons | 15.37 ± 4.86 | 15.74 ± 3.17 | 15.37 ± 4.86 | 22.63 ± 4.19 | 21.10 ± 4.37 | 19.75 ± 7.13 | ||
| Oxygenated monoterpenes | 3.44 ± 0.95 | 3.71 ± 0.68 | 1.31 ± 0.65 | 3.88 ± 0.74 A,B | 2.96 ± 0.93 A | 1.08 ± 0.24 A,C | ||
| Sesquiterpene hydrocarbons | 51.80 ± 7.81 | 49.61 ± 5.34 | 51.80 ± 7.81 | 35.37 ± 7.23 | 37.07 ± 5.67 | 32.91 ± 3.06 | ||
| Oxygenated Sesquiterpenes | 2.18 ± 0.84 | 3.48 ± 0.83 | 2.42 ± 0.18 | 2.42 ± 1.12 | 0.20 ± 0.04 | 0.94 ± 0.60 | ||
| Others | 3.66 ± 0.91 | 4.19 ± 0.49 | 11.62 ± 4.91 | 5.68 ± 0.84 | 8.70 ± 1.29 | 7.61 ± 0.99 | ||
| Total | 76.44 ± 15.37 | 76.74 ± 10.51 | 76.44 ± 15.37 | 69.98 ± 14.11 | 70.03 ± 12.30 | 62.29 ± 12.02 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trindade, R.; Almeida, L.; Xavier, L.; Andrade, E.H.; Maia, J.G.; Mello, A.; Setzer, W.N.; Ramos, A.; da Silva, J.K.R. Influence on Secondary Metabolism of Piper nigrum L. by Co-Inoculation with Arbuscular Mycorrhizal Fungi and Fusarium solani f. sp. piperis. Microorganisms 2021, 9, 484. https://doi.org/10.3390/microorganisms9030484
Trindade R, Almeida L, Xavier L, Andrade EH, Maia JG, Mello A, Setzer WN, Ramos A, da Silva JKR. Influence on Secondary Metabolism of Piper nigrum L. by Co-Inoculation with Arbuscular Mycorrhizal Fungi and Fusarium solani f. sp. piperis. Microorganisms. 2021; 9(3):484. https://doi.org/10.3390/microorganisms9030484
Chicago/Turabian StyleTrindade, Rafaela, Laís Almeida, Luciana Xavier, Eloisa Helena Andrade, José Guilherme Maia, Andréa Mello, William N. Setzer, Alessandra Ramos, and Joyce Kelly R. da Silva. 2021. "Influence on Secondary Metabolism of Piper nigrum L. by Co-Inoculation with Arbuscular Mycorrhizal Fungi and Fusarium solani f. sp. piperis" Microorganisms 9, no. 3: 484. https://doi.org/10.3390/microorganisms9030484
APA StyleTrindade, R., Almeida, L., Xavier, L., Andrade, E. H., Maia, J. G., Mello, A., Setzer, W. N., Ramos, A., & da Silva, J. K. R. (2021). Influence on Secondary Metabolism of Piper nigrum L. by Co-Inoculation with Arbuscular Mycorrhizal Fungi and Fusarium solani f. sp. piperis. Microorganisms, 9(3), 484. https://doi.org/10.3390/microorganisms9030484









