Pseudodesulfovibrio cashew sp. Nov., a Novel Deep-Sea Sulfate-Reducing Bacterium, Linking Heavy Metal Resistance and Sulfur Cycle
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
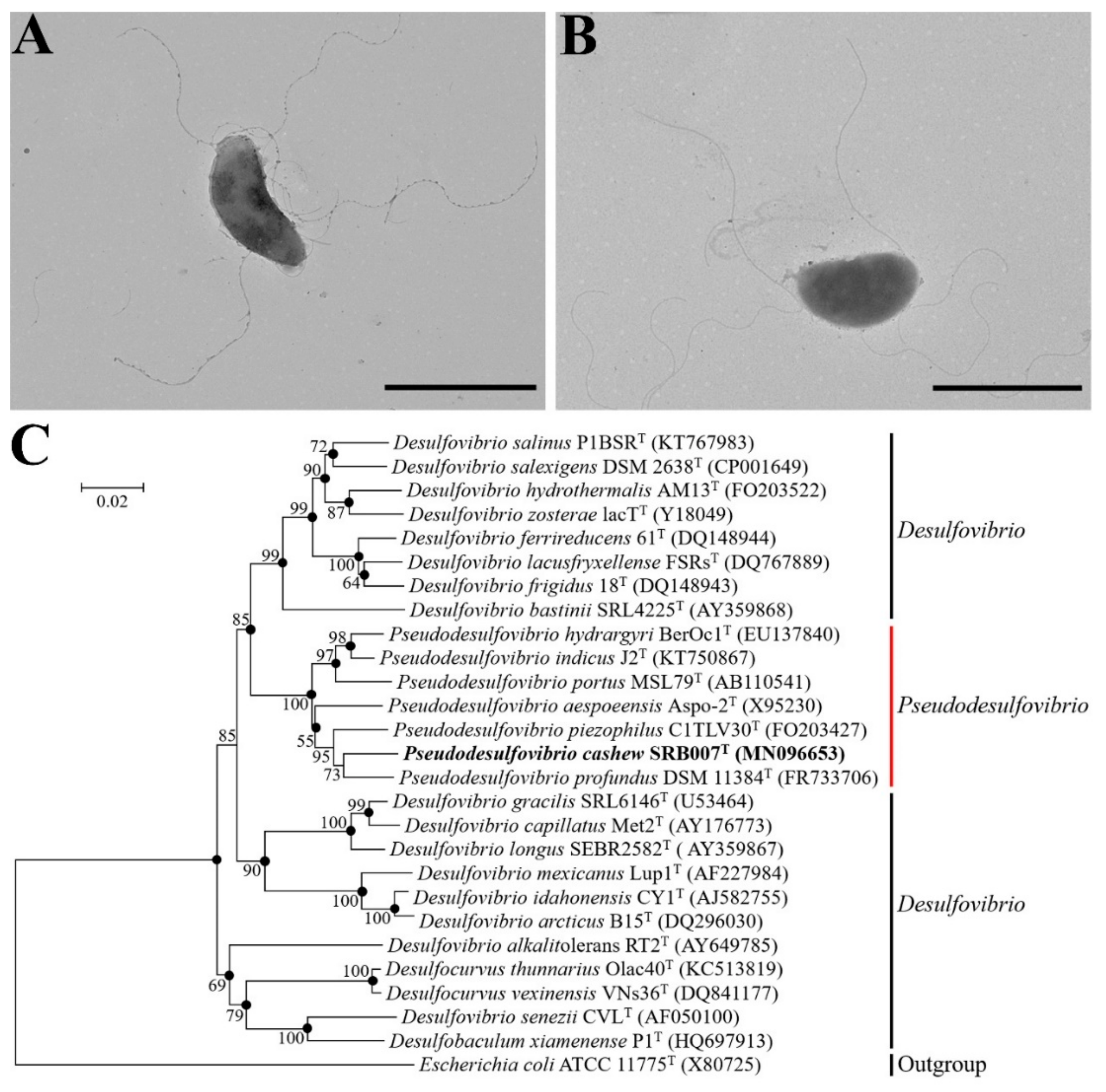
2.2. Transmission Electron Microscopy (TEM) Observation
2.3. Genomic Sequencing and Analysis
2.4. Phylogenetic Analysis
2.5. Physiological and Chemotaxonomic Assays of P. cashew SRB007
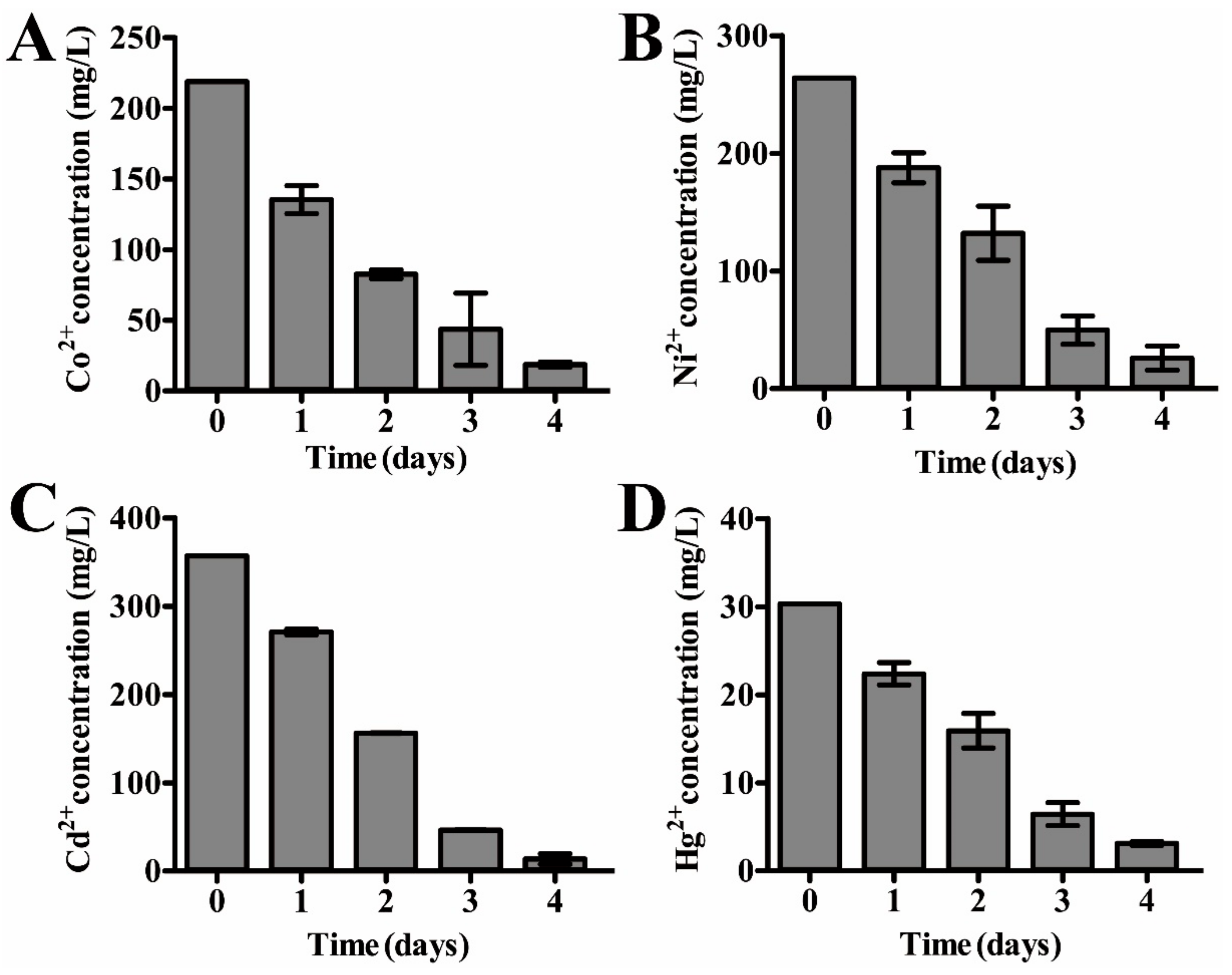
2.6. Heavy Metal Removal Assay and Qualitative Energy-Dispersive Spectrometry (EDS) Analysis
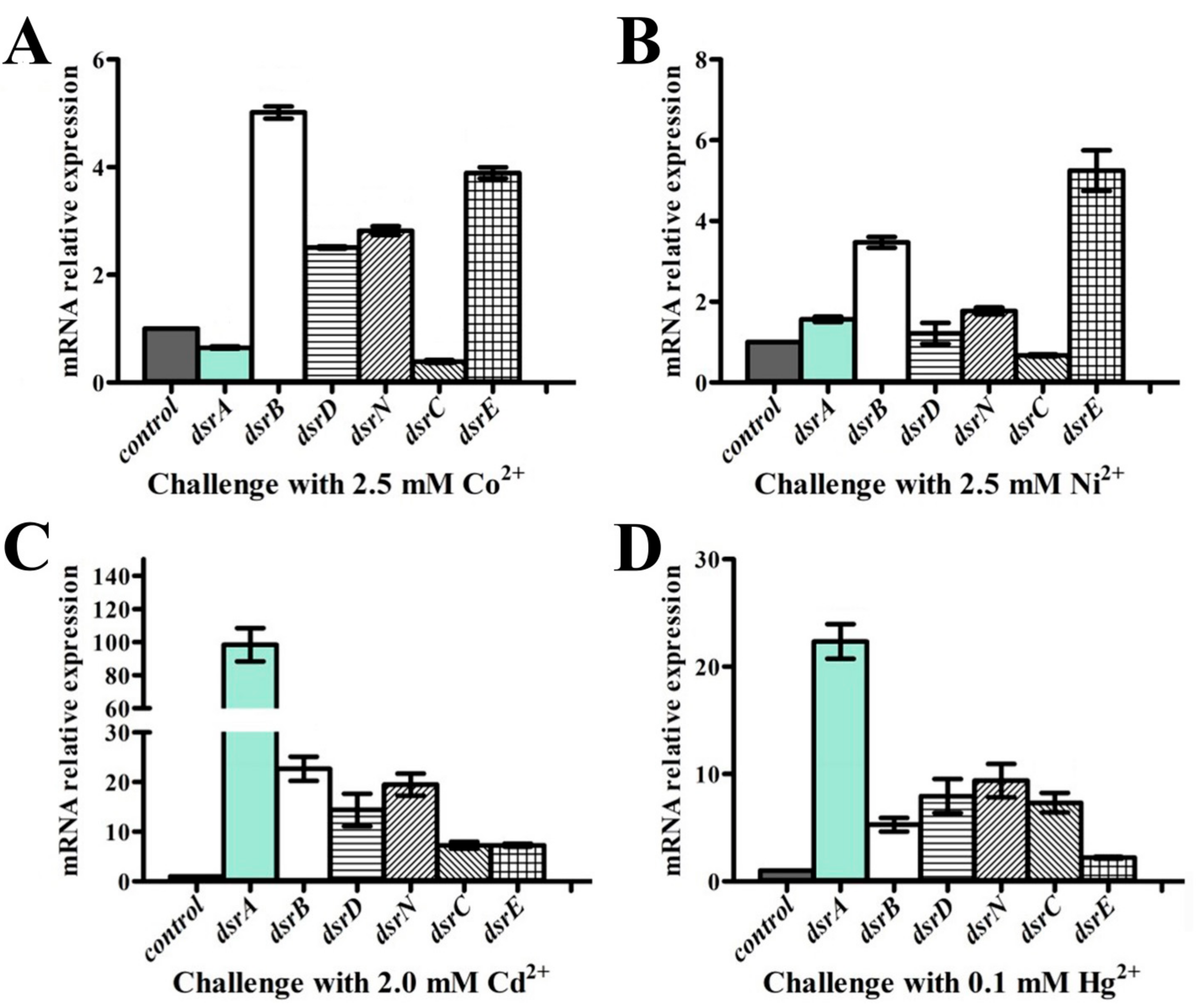
2.7. RNA Extraction, Reverse Transcription and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Data Deposit
3. Results
3.1. Isolation and Identification of a Novel Deep-Sea Sulfate-Reducing Bacterium P. cashew SRB007
3.2. Physiological and Chemotaxonomic Characteristics of P. cashew SRB007
3.3. Description of Pseudodesulfovibrio Cashew sp. Nov.
3.4. Dissimilatory Sulfate Reduction-Related Genes Existing in the Genome of P. cashew SRB007
3.5. Dissimilatory Sulfate Reduction-Related Genes Contribute to the Prominent Capability of P. cashew SRB007 against Different Heavy Metals
3.6. Proposed Lifestyle of P. cashew SRB007
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jørgensen, B.B.; Kasten, S. Sulfur cycling and methane oxidation. In Marine Geochemistry; Schulz, H.D., Zabel, M., Eds.; Springer: Berlin, Germany, 2006; pp. 271–309. [Google Scholar] [CrossRef]
- Wasmund, K.; Mußmann, M.; Loy, A. The life sulfuric: Microbial ecology of sulfur cycling in marine sediments. Environ. Microbiol. Rep. 2017, 9, 323–344. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Xi, S.C.; Cai, R.N.; Zhang, X.; Sun, C.M. A novel bacterial thiosulfate oxidation pathway provides a new clue about the formation of zero-valent sulfur in deep sea. ISME J. 2020, 14, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.A.; Paris, G.; Katsev, S.; Jones, C.; Kim, S.-T.; Zerkle, A.L.; Nomosatryo, S.; Fowle, D.A.; Adkins, J.F.; Sessions, A.L. Sulfate was a trace constituent of Archean seawater. Science 2014, 346, 735–739. [Google Scholar] [CrossRef]
- Peck, H.D., Jr.; LeGall, J. Biochemistry of dissimilatory sulphate reduction. Philos. Trans. R. Soc. B 1982, 298, 443–466. [Google Scholar]
- Fitz, R.M.; Cypionka, H. A study on electron transport-driven proton translocation in Desulfovibrio desulfuricans. Arch. Microbiol. 1989, 152, 369–376. [Google Scholar] [CrossRef]
- Wu, Z.D.; Zheng, R.K.; Liu, G.; Liu, R.; Wu, S.M.; Sun, C.M. Calcium protects bacteria against cadmium stress via reducing nitric oxide production and increasing iron acquisition. Environ. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.K.; Wu, S.M.; Sun, C.M. MerF is a novel regulator of deep-sea Pseudomonas stutzeri flagellum biogenesis and motility. Environ. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kiran, M.G.; Pakshirajan, K.; Das, G. Heavy metal removal from multicomponent system by sulfate reducing bacteria: Mechanism and cell surface characterization. J. Hazard. Mater. 2017, 324, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kieu, H.T.; Müller, E.; Horn, H. Heavy metal removal in anaerobic semi-continuous stirred tank reactors by a consortium of sulfate-reducing bacteria. Water Res. 2011, 45, 3863–3870. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.B.; Gentry, D.M.; Rapp-Giles, B.J.; Casalot, L.; Wall, J.D. Uranium reduction by Desulfovibrio desulfuricans strain G20 and a cytochrome c3 mutant. Appl. Environ. Microbiol. 2002, 68, 3129–3132. [Google Scholar] [CrossRef]
- Jørgensen, B.B. A thiosulfate shunt in the sulfur cycle of marine sediments. Science 1990, 249, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Thamdrup, B.; Finster, K.; Hansen, J.W.; Bak, F. Bacterial disproportionation of elemental sulfur coupled to chemical reduction of iron or manganese. Appl. Environ. Microbiol. 1993, 59, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Van Cappellen, P.; Wang, Y. Cycling of iron and manganese in surface sediments; a general theory for the coupled transport and reaction of carbon, oxygen, nitrogen, sulfur, iron, and manganese. Am. J. Sci. 1996, 296, 197. [Google Scholar] [CrossRef]
- Cao, J.; Gayet, N.; Zeng, X.; Shao, Z.; Jebbar, M.; Alain, K. Pseudodesulfovibrio indicus gen. nov., sp. nov., a piezophilic sulfate-reducing bacterium from the Indian Ocean and reclassification of four species of the genus Desulfovibrio. Int. J. Syst. Evol. Microbiol. 2016, 66, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.; Goodman, K.; Rochelle, P.A.; Marchesi, J.R.; Fry, J.C.; Weightman, A.J.; Parkes, R.J. Desulfovibrio profundus sp. nov., a novel barophilic sulfate-reducing bacterium from deep sediment layers in the Japan Sea. Int. J. Syst. Evol. Microbiol. 1997, 47, 515–521. [Google Scholar] [CrossRef]
- Ollivier, B.; Guyot, F. Sulfate-reducing bacteria: A deep biosphere-early life connection. Environ. Microbiol. Rep. 2009, 1, 14–16. [Google Scholar]
- Khelaifia, S.; Fardeau, M.L.; Pradel, N.; Aussignargues, C.; Garel, M.; Tamburini, C.; Cayol, J.L.; Gaudron, S.; Gaill, F.; Ollivier, B. Desulfovibrio piezophilus sp. nov., a piezophilic, sulfate-reducing bacterium isolated from wood falls in the Mediterranean Sea. Int. J. Syst. Evol. Microbiol. 2011, 61, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Sha, Z.L.; Sun, C.M. Formation of cadmium sulfide nanoparticles mediates cadmium resistance and light utilization of the deep-sea bacterium Idiomarina sp.OT37-5b. Environ. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fardeau, M.L.; Ollivier, B.; Patel, B.K.; Magot, M.; Thomas, P.; Rimbault, A.; Rocchiccioli, F.; Garcia, J.L. Thermotoga hypogea sp. nov., a xylanolytic, thermophilic bacterium from an oil-producing well. Int. J. Syst. Bacteriol 1997, 47, 1013–1019. [Google Scholar] [CrossRef]
- Han, Z.; Yan, H.; Hui, Z.; Zhou, S.; Xu, L. Bio-precipitation of calcite with preferential orientation induced by Synechocystis sp. PCC6803. Geomicrobiol. J. 2014, 31, 884–899. [Google Scholar] [CrossRef]
- Loman, N.J.; Quinlan, A.R. Poretools: A toolkit for analyzing nanopore sequence data. Bioinformatics 2014, 30, 3399–3401. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA-sequences—A maximum-likelihood approach. J. Mol Evol 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Rzhetsky, A.; Nei, M. A simple method for estimating and testing minimum-evolution trees. Mol. Biol. Evol. 1992, 9, 945–967. [Google Scholar]
- Ben Ali Gam, Z.; Oueslati, R.; Abdelkafi, S.; Casalot, L.; Tholozan, J.L.; Labat, M. Desulfovibrio tunisiensis sp. nov., a novel weakly halotolerant, sulfate-reducing bacterium isolated from exhaust water of a Tunisian oil refinery. Int. J. Syst. Evol. Microbiol. 2009, 59, 1059–1063. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Haouari, O.; Fardeau, M.-L.; Casalot, L.; Tholozan, J.-L.; Hamdi, M.; Ollivier, B. Isolation of sulfate-reducing bacteria from Tunisian marine sediments and description of Desulfovibrio bizertensis sp. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 2909–2913. [Google Scholar] [CrossRef]
- Könneke, M.; Kuever, J.; Galushko, A.; Jørgensen, B. Desulfoconvexum algidum gen. nov., sp. nov., a psychrophilic sulfate-reducing bacterium isolated from a permanently cold marine sediment. Int. J. Syst. Evol. Microbiol. 2012, 63. [Google Scholar] [CrossRef] [PubMed]
- Takii, S.; Hanada, S.; Hase, Y.; Tamaki, H.; Uyeno, Y.; Sekiguchi, Y.; Matsuura, K. Desulfovibrio marinisediminis sp. nov., a novel sulfate-reducing bacterium isolated from coastal marine sediment via enrichment with Casamino acids. Int. J. Syst. Evol. Microbiol. 2008, 58, 2433–2438. [Google Scholar] [CrossRef]
- Takii, S.; Hanada, S.; Tamaki, H.; Ueno, Y.; Sekiguchi, Y.; Ibe, A.; Matsuura, K. Dethiosulfatibacter aminovorans gen. nov., sp. nov., a novel thiosulfate-reducing bacterium isolated from coastal marine sediment via sulfate-reducing enrichment with Casamino acids. Int. J. Syst. Evol. Microbiol. 2007, 57, 2320–2326. [Google Scholar] [CrossRef]
- Audiffrin, C.; Cayol, J.-L.; Joulian, C.; Casalot, L.; Thomas, P.; Garcia, J.-L.; Ollivier, B. Desulfonauticus submarinus gen. nov., sp nov., a novel sulfate-reducing bacterium isolated from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2003, 53, 1585–1590. [Google Scholar] [CrossRef]
- Nunoura, T.; Oida, H.; Miyazaki, M.; Suzuki, Y.; Takai, K.; Horikoshi, K. Desulfothermus okinawensis sp. nov., a thermophilic and heterotrophic sulfate-reducing bacterium isolated from a deep-sea hydrothermal field. Int. J. Syst. Evol. Microbiol. 2007, 57, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Fuse, H.; Takimura, O.; Yamaoka, Y. Effects of growth pressure and temperature on Fatty Acid composition of a barotolerant deep-sea bacterium. Appl. Environ. Microbiol. 1993, 59, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Venceslau, S.S.; Stockdreher, Y.; Dahl, C.; Pereira, I.A. The “bacterial heterodisulfide” DsrC is a key protein in dissimilatory sulfur metabolism. Biochim. Biophys. Acta 2014, 1837, 1148–1164. [Google Scholar] [CrossRef]
- Jiang, L.J.; Liu, X.W.; Dong, C.M.; Huang, Z.B.; Cambon-Bonavita, M.A.; Alain, K.; Gu, L.; Wang, S.S.; Shao, Z.Z. “Candidatus Desulfobulbus rimicarensis,” an uncultivated Deltaproteobacterial epibiont from the deep-sea hydrothermal vent shrimp Rimicaris exoculata. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Prieto, A.; Barquera, B.; Juarez, O. Origin and evolution of the sodium-pumping NADH: Ubiquinone oxidoreductase. PLoS ONE 2014, 9, e96696. [Google Scholar]
- Holmkvist, L.; Ferdelman, T.G.; Jørgensen, B.B. A cryptic sulfur cycle driven by iron in the methane zone of marine sediment (Aarhus Bay, Denmark). Geochim. Cosmochim. Acta 2011, 75, 3581–3599. [Google Scholar] [CrossRef]
- Leloup, J.; Loy, A.; Knab, N.J.; Borowski, C.; Wagner, M.; Jørgensen, B.B. Diversity and abundance of sulfate-reducing microorganisms in the sulfate and methane zones of a marine sediment, Black Sea. Environ. Microbiol. 2007, 9, 131–142. [Google Scholar] [CrossRef]
- Cabrera, G.; Pérez, R.; Gomez, J.M.; Abalos, A.; Cantero, D. Toxic effects of dissolved heavy metals on Desulfovibrio vulgaris and Desulfovibrio sp. strains. J. Hazard. Mater. 2006, 135, 1–3. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Azapagic, A. Developing a framework for sustainable development indicators for the mining and minerals industry. J. Clean. Prod. 2004, 12, 639–662. [Google Scholar] [CrossRef]



| Characteristic | 1 | 2 |
|---|---|---|
| Temperature range | ||
| for growth (°C) | 16–45 | 20–45 |
| Optimum | 30 | 28 |
| pH range for growth | 5.5–8.5 | 6.0–8.0 |
| Optimum | 7.0 | 7.0 |
| NaCl range for growth (%) | 0–10.0 | 2.0–10.0 |
| Optimum | 5.0 | 6.0 |
| Electron donors | ||
| Fumarate | + | − |
| Malate | + | + |
| Lactate | + | + |
| Methanol | + | − |
| Ethanol | + | − |
| Formate | + | − |
| Succinate | + | − |
| Electron acceptors | ||
| Sulfate | + | + |
| Sulfite | + | − |
| Thiosulfate | + | − |
| Nitrate | + | − |
| Nitrite | + | − |
| Polar lipids Major fatty acids (>10%) DNA G+C content (%) | phosphatidylethanolamine (PE), diphosphatidylglycerol (DPG), phosphatidylglycerol (PG), unidentified glycolipid (GL), unknown aminoglycolipid (AGL) iso-C15:0, C16:0, iso-C17:0 59.94 | NR iso-C15:0, anteiso-C15:0 NR |
| Isolation source | Deep-sea sediments | Marine sediments |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, R.; Wu, S.; Sun, C. Pseudodesulfovibrio cashew sp. Nov., a Novel Deep-Sea Sulfate-Reducing Bacterium, Linking Heavy Metal Resistance and Sulfur Cycle. Microorganisms 2021, 9, 429. https://doi.org/10.3390/microorganisms9020429
Zheng R, Wu S, Sun C. Pseudodesulfovibrio cashew sp. Nov., a Novel Deep-Sea Sulfate-Reducing Bacterium, Linking Heavy Metal Resistance and Sulfur Cycle. Microorganisms. 2021; 9(2):429. https://doi.org/10.3390/microorganisms9020429
Chicago/Turabian StyleZheng, Rikuan, Shimei Wu, and Chaomin Sun. 2021. "Pseudodesulfovibrio cashew sp. Nov., a Novel Deep-Sea Sulfate-Reducing Bacterium, Linking Heavy Metal Resistance and Sulfur Cycle" Microorganisms 9, no. 2: 429. https://doi.org/10.3390/microorganisms9020429
APA StyleZheng, R., Wu, S., & Sun, C. (2021). Pseudodesulfovibrio cashew sp. Nov., a Novel Deep-Sea Sulfate-Reducing Bacterium, Linking Heavy Metal Resistance and Sulfur Cycle. Microorganisms, 9(2), 429. https://doi.org/10.3390/microorganisms9020429






