Arbuscular Mycorrhizal Fungi Increase Pb Uptake of Colonized and Non-Colonized Medicago truncatula Root and Deliver Extra Pb to Colonized Root Segment
Abstract
1. Introduction
2. Materials and Methods
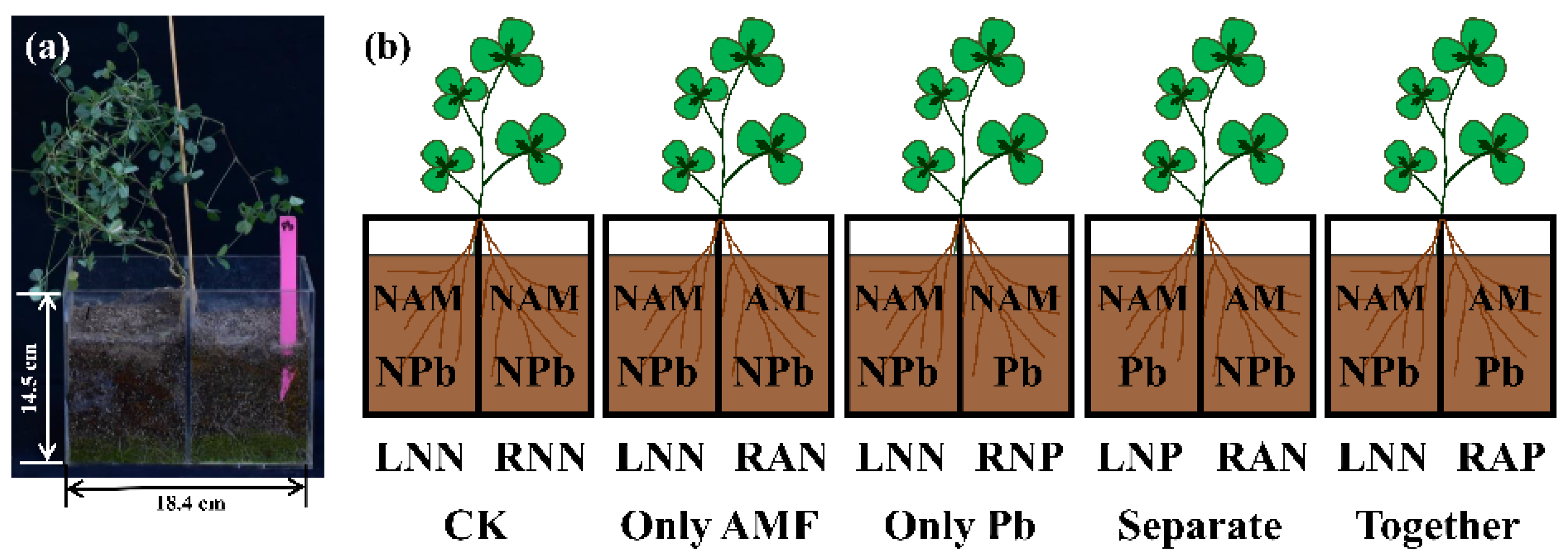
2.1. Plant Material, Split-Root System, Growth Substrate, and AM Fungal Inoculum
2.2. Experimental Design
2.3. Plant Sampling, Biomass, and AM Fungal Colonization
2.4. Concentrations of Pb and P
2.5. Photosynthesis
2.6. Gene Relative Expression
2.7. Statistical Analysis
3. Results
3.1. Biomass and Colonization
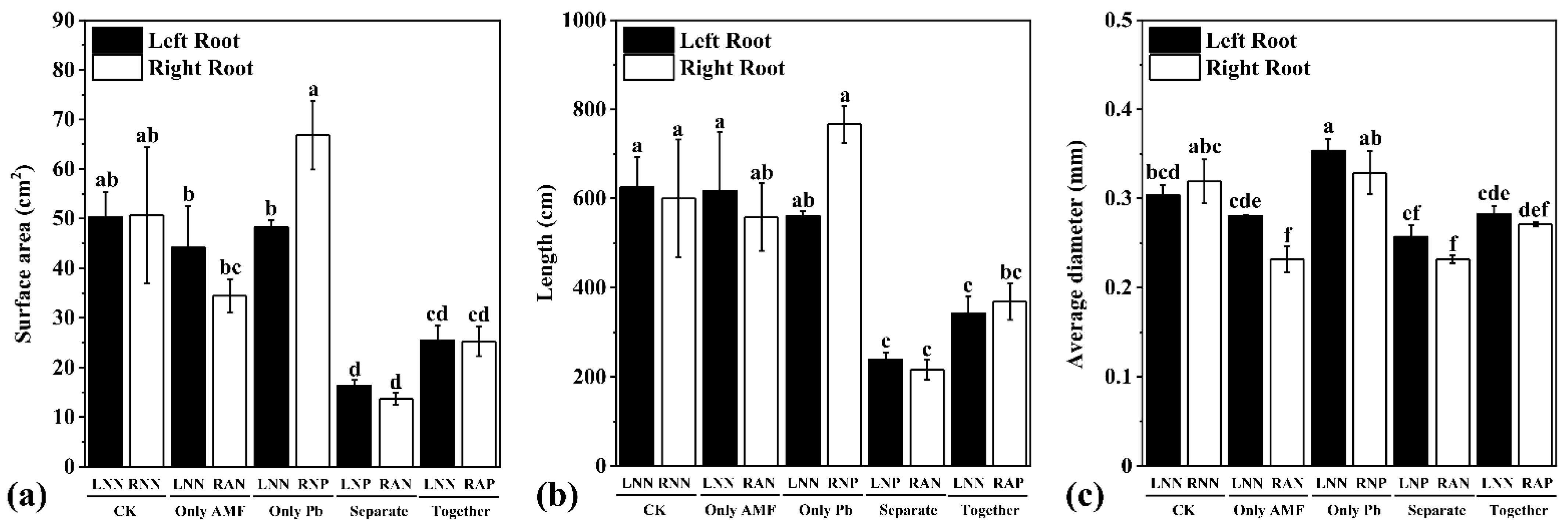
3.2. Root Structure, Pb Concentration, and Content
3.3. Photosynthesis
3.4. Relative Expressions of Aquaporins
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, Y.Z.; Xu, Z.H.; Huang, Y.K.; Wang, T.B.; Zheng, S.K.; De Pasquale, A.; Brueckner, C.; Lei, Y.; Li, B.K. Long-term continuous and real-time in situ monitoring of Pb(II) toxic contaminants in wastewater using solid-state ion selective membrane (S-ISM) Pb and pH auto-correction assembly. J. Hazard. Mater. 2020, 400, 123299. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alcaraz, M.N.; Loureiro, S.; van Gestel, C.A.M. Toxicokinetics of Zn and Cd in the earthworm Eisenia andrei exposed to metal-contaminated soils under different combinations of air temperature and soil moisture content. Chemosphere 2018, 197, 26–32. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Tolerance and hyperaccumulation of cadmium by a wild, unpalatable herb Coronopus didymus (L.) Sm. (Brassicaceae). Ecotoxicol. Environ. Saf. 2017, 135, 209–215. [Google Scholar] [CrossRef]
- Li, Z.Y.; Ma, Z.W.; van der Kuijp, T.J.; Yuan, Z.W.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef]
- Baloch, S.; Kazi, T.G.; Baig, J.A.; Afridi, H.I.; Arain, M.B. Occupational exposure of lead and cadmium on adolescent and adult workers of battery recycling and welding workshops: Adverse impact on health. Sci. Total Environ. 2020, 720, 137549. [Google Scholar] [CrossRef]
- Wang, C.R.; Rong, H.; Zhang, X.B.; Shi, W.J.; Hong, X.; Liu, W.C.; Cao, T.; Yu, X.X.; Yu, Q.F. Effects and mechanisms of foliar application of silicon and selenium composite sols on diminishing cadmium and lead translocation and affiliated physiological and biochemical responses in hybrid rice (Oryza sativa L.) exposed to cadmium and lead. Chemosphere 2020, 251, 126347. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Diao, F.W.; Wang, Q.F.; Pan, L.; Dang, Z.H.; Guo, W. Effects of arbuscular mycorrhizal symbiosis on growth, nutrient and metal uptake by maize seedlings (Zea mays L.) grown in soils spiked with Lanthanum and Cadmium. Environ. Pollut. 2018, 241, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J. Hazard. Mater. 2019, 379, 120813. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, M.C.; Carrillo-Gonzalez, R.; Gutierrez-Castorena, M.C. Natural attenuation in a slag heap contaminated with cadmium: The role of plants and arbuscular mycorrhizal fungi. J. Hazard. Mater. 2009, 161, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Hachani, C.; Lamhamedi, M.S.; Cameselle, C.; Gouveia, S.; Zine El Abidine, A.; Khasa, D.P.; Bejaoui, Z. Effects of ectomycorrhizal fungi and heavy metals (Pb, Zn, and Cd) on growth and mineral nutrition of Pinus halepensis seedlings in North Africa. Microorganisms 2020, 8, 2033. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Parray, J.A. Use of mycorrhiza as metal tolerance strategy in plants. In Approaches to Heavy Metal Tolerance in Plants; Springer: Singapore, 2016; pp. 57–68. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.H.; Liu, T.; Huang, S.P.; Chang, Y.F. Elevated CO2 increases glomalin-related soil protein (GRSP) in the rhizosphere of Robinia pseudoacacia L. seedlings in Pb- and Cd-contaminated soils. Environ. Pollut. 2016, 218, 349–357. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Opik, M.; Adholeya, A.; Ainsaar, L.; Ba, A.; Burla, S.; Diedhiou, A.G.; Hiiesalu, I.; Jairus, T.; et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 2015, 349, 970–973. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, V.; Menoyo, E.; Geml, J.; Kemppainen, M.; Pardo, A.; Salazar, M.J.; Becerra, A.G. Soil lead pollution modifies the structure of arbuscular mycorrhizal fungal communities. Mycorrhiza 2019, 29, 363–373. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Lou, X.; Zhang, H.Q.; Ren, W.; Tang, M. Effects of sodium sulfide application on the growth of Robinia pseudoacacia, heavy metal immobilization, and soil microbial activity in Pb-Zn polluted soil. Ecotoxicol. Environ. Saf. 2020, 197. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, C.H.; Tang, J.J.; Hu, S.J. Arbuscular mycorrhizae enhance metal lead uptake and growth of host plants under a sand culture experiment. Chemosphere 2005, 60, 665–671. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.Q.; Song, Y.Y.; Yang, Y.R.; Chen, H.; Tang, M. Subcellular compartmentalization and chemical forms of lead participate in lead tolerance of Robinia pseudoacacia L. with Funneliformis mosseae. Front. Plant Sci. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.Y.; Zhang, H.Q.; Lou, X.; Tang, M. Mycorrhizal and non-mycorrhizal Medicago truncatula roots exhibit differentially regulated NADPH oxidase and antioxidant response under Pb stress. Environ. Exp. Bot. 2019, 164, 10–19. [Google Scholar] [CrossRef]
- Gavito, M.E.; Jakobsen, I.; Mikkelsen, T.N.; Mora, F. Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytol. 2019, 223, 896–907. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Mortimer, P.E.; Pérez-Fernández, M.A.; Valentine, A.J. The role of arbuscular mycorrhizal colonization in the carbon and nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol. Biochem. 2008, 40, 1019–1027. [Google Scholar] [CrossRef]
- Puschel, D.; Bitterlich, M.; Rydlova, J.; Jansa, J. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: A Gordian knot of roots and hyphae. Mycorrhiza 2020, 30, 299–313. [Google Scholar] [CrossRef]
- Li, G.W.; Santoni, V.; Maurel, C. Plant aquaporins: Roles in plant physiology. Biochim. Biophys. Acta 2014, 1840, 1574–1582. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Cavagnaro, T.R.; Tyerman, S.D. Variable effects of arbuscular mycorrhizal fungal inoculation on physiological and molecular measures of root and stomatal conductance of diverse Medicago truncatula accessions. Plant Cell Environ. 2019, 42, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hijikata, N.; Ohtomo, R.; Handa, Y.; Kawaguchi, M.; Saito, K.; Masuta, C.; Ezawa, T. Aquaporin-mediated long-distance polyphosphate translocation directed towards the host in arbuscular mycorrhizal symbiosis: Application of virus-induced gene silencing. New Phytol. 2016, 211, 1202–1208. [Google Scholar] [CrossRef]
- Sudova, R.; Vosatka, M. Differences in the effects of three arbuscular mycorrhizal fungal strains on P and Pb accumulation by maize plants. Plant Soil 2007, 296, 77–83. [Google Scholar] [CrossRef]
- Yang, Y.R.; Liang, Y.; Han, X.Z.; Chiu, T.Y.; Ghosh, A.; Chen, H.; Tang, M. The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. Sci. Rep. 2016, 6, 20469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhang, H.J.; Zhang, Y.X.; Liu, Y.Q.; Zhang, H.Q.; Tang, M. Arbuscular mycorrhizal fungi alter carbohydrate distribution and amino acid accumulation in Medicago truncatula under lead stress. Environ. Exp. Bot. 2020, 171, 103950. [Google Scholar] [CrossRef]
- Liu, J.Y.; Maldonado-Mendoza, I.; Lopez-Meyer, M.; Cheung, F.; Town, C.D.; Harrison, M.J. Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J. 2007, 50, 529–544. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Franken, P. Comparison of systemic and local interactions between the arbuscular mycorrhizal fungus Funneliformis mosseae and the root pathogen Aphanomyces euteiches in Medicago truncatula. Mycorrhiza 2014, 24, 419–430. [Google Scholar] [CrossRef]
- Dos Anjos, V.E.; Rohwedder, J.R.; Cadore, S.; Abate, G.; Grassi, M.T. Montmorillonite and vermiculite as solid phases for the preconcentration of trace elements in natural waters: Adsorption and desorption studies of As, Ba, Cu, Cd, Co, Cr, Mn, Ni, Pb, Sr, V, and Zn. Appl. Clay Sci. 2014, 99, 289–296. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil, 2nd ed.; California Agricultural Experiment Station: Berkeley, CA, USA, 1950; Volume 347. [Google Scholar] [CrossRef]
- Ma, Y.L.; He, J.L.; Ma, C.F.; Luo, J.; Li, H.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Ectomycorrhizas with Paxillus involutus enhance cadmium uptake and tolerance in Populus x canescens. Plant Cell Environ. 2014, 37, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Chen, X.D.; Zhang, X.L.; Hamel, C.; Cui, X.W.; Chen, J.; Chen, H.; Tang, M. Changes in arbuscular mycorrhizal fungal attributes along a chronosequence of black locust (Robinia pseudoacacia) plantations can be attributed to the plantation-induced variation in soil properties. Sci. Total Environ. 2017, 599, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725. [Google Scholar] [CrossRef]
- Parádi, I.; Tuinen, D.V.; Morandi, D.; Ochatt, S.; Robert, F.; Jacas, L.; Dumas-Gaudot, E. Transcription of two blue copper-binding protein isogenes is highly correlated with arbuscular mycorrhizal development in Medicago truncatula. Mol. Plant Microbe Interact. 2010, 23, 1175–1183. [Google Scholar] [CrossRef][Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T) (-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.Z.; Bourque, G.; Wishart, D.S.; Xia, J.G. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, 486–494. [Google Scholar] [CrossRef]
- Yang, Y.R.; Liang, Y.; Ghosh, A.; Song, Y.Y.; Chen, H.; Tang, M. Assessment of arbuscular mycorrhizal fungi status and heavy metal accumulation characteristics of tree species in a lead-zinc mine area: Potential applications for phytoremediation. Environ. Sci. Pollut. Res. 2015, 22, 13179–13193. [Google Scholar] [CrossRef] [PubMed]
- Dhawi, F.; Datta, R.; Ramakrishna, W. Mycorrhiza and heavy metal resistant bacteria enhance growth, nutrient uptake and alter metabolic profile of sorghum grown in marginal soil. Chemosphere 2016, 157, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Koide, R.T.; Adams, T.S.; DeForest, J.L.; Cheng, L.; Eissenstat, D.M. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl. Acad. Sci. USA 2016, 113, 8741–8746. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Smith, F.A.; Smith, S.E. Phosphorus efficiencies and responses of barley (Hordeum vulgare L.) to arbuscular mycorrhizal fungi grown in highly calcareous soil. Mycorrhiza 2003, 13, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Punamiya, P.; Datta, R.; Sarkar, D.; Barber, S.; Patel, M.; Das, P. Symbiotic role of Glomus mosseae in phytoextraction of lead in vetiver grass [Chrysopogon zizanioides (L.)]. J. Hazard. Mater. 2010, 177, 465–474. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, C.; Shen, Z.; Quan, Y.; Liu, X. Role of extrinsic arbuscular mycorrhizal fungi in heavy metal-contaminated wetlands with various soil moisture levels. Int. J. Phytoremediat. 2015, 17, 208–214. [Google Scholar] [CrossRef]
- Weissenhorn, I.; Leyval, C.; Berthelin, J. Cd-tolerant arbuscular mycorrhizal (Am) fungi from heavy-metal polluted soils. Plant Soil 1993, 157, 247–256. [Google Scholar] [CrossRef]
- Yabe, J.; Nakayama, S.M.M.; Ikenaka, Y.; Yohannes, Y.B.; Bortey-Sam, N.; Kabalo, A.N.; Ntapisha, J.; Mizukawa, H.; Umemura, T.; Ishizuka, M. Lead and cadmium excretion in feces and urine of children from polluted townships near a lead-zinc mine in Kabwe, Zambia. Chemosphere 2018, 202, 48–55. [Google Scholar] [CrossRef]
- Xin, J.L.; Dai, H.W.; Huang, B.F. Assessing the roles of roots and shoots in the accumulation of cadmium in two sweet potato cultivars using split-root and reciprocal grafting systems. Plant Soil 2017, 412, 413–424. [Google Scholar] [CrossRef]
- Weissenhorn, I.; Leyval, C. Root colonization of maize by a cd-sensitive and a cd-tolerant Glomus Mosseae and cadmium uptake in sand culture. Plant Soil 1995, 175, 233–238. [Google Scholar] [CrossRef]
- Ruby, M.V.; Davis, A.; Nicholson, A. In Situ formation of lead phosphates in soils as a method to immobilize lead. Environ. Sci. Technol. 1994, 28, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.J.; Menoyo, E.; Faggioli, V.; Geml, J.; Cabello, M.; Rodriguez, J.H.; Marro, N.; Pardo, A.; Pignata, M.L.; Becerra, A.G. Pb accumulation in spores of arbuscular mycorrhizal fungi. Sci. Total Environ. 2018, 643, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.M.; Tinker, P.B. Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas: IV. Effect of environmental variables on movement of phosphorus. New Phytol. 1981, 88, 327–339. [Google Scholar] [CrossRef]
- Jozefkowicz, C.; Sigaut, L.; Scochera, F.; Soto, G.; Ayub, N.; Pietrasanta, L.I.; Amodeo, G.; Flecha, F.L.G.; Alleva, K. PIP water transport and its pH dependence are regulated by tetramer stoichiometry. Biophys. J. 2016, 110, 1312–1321. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ren, W.; Zheng, Y.; Li, Y.; Zhu, M.; Tang, M. Arbuscular Mycorrhizal Fungi Increase Pb Uptake of Colonized and Non-Colonized Medicago truncatula Root and Deliver Extra Pb to Colonized Root Segment. Microorganisms 2021, 9, 1203. https://doi.org/10.3390/microorganisms9061203
Zhang H, Ren W, Zheng Y, Li Y, Zhu M, Tang M. Arbuscular Mycorrhizal Fungi Increase Pb Uptake of Colonized and Non-Colonized Medicago truncatula Root and Deliver Extra Pb to Colonized Root Segment. Microorganisms. 2021; 9(6):1203. https://doi.org/10.3390/microorganisms9061203
Chicago/Turabian StyleZhang, Haoqiang, Wei Ren, Yaru Zheng, Yanpeng Li, Manzhe Zhu, and Ming Tang. 2021. "Arbuscular Mycorrhizal Fungi Increase Pb Uptake of Colonized and Non-Colonized Medicago truncatula Root and Deliver Extra Pb to Colonized Root Segment" Microorganisms 9, no. 6: 1203. https://doi.org/10.3390/microorganisms9061203
APA StyleZhang, H., Ren, W., Zheng, Y., Li, Y., Zhu, M., & Tang, M. (2021). Arbuscular Mycorrhizal Fungi Increase Pb Uptake of Colonized and Non-Colonized Medicago truncatula Root and Deliver Extra Pb to Colonized Root Segment. Microorganisms, 9(6), 1203. https://doi.org/10.3390/microorganisms9061203





