Timely Control of Gastrointestinal Eubiosis: A Strategic Pillar of Pig Health
Abstract
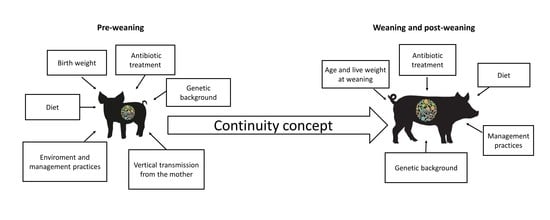
1. Introduction
2. Pre-Weaning
2.1. Birth Body Weight and Physiological Maturation
2.2. Colostrum and Milk—Composition and Intake
2.3. Genetics Background
2.4. Environmental Stressors and Management
2.5. Antimicrobials
2.6. Diet
3. Weaning and Post-Weaning
3.1. Age and Live Weight at Weaning
3.2. Management
3.3. Genetics Background
3.4. Post-Weaning Diet
| Intervention | Quantity | Phase | Analyzed Matrix | Alpha Indices | Beta-Diversity | Difference in Taxa | Direction | Reference |
|---|---|---|---|---|---|---|---|---|
| Probiotics | ||||||||
| Bacillus subtilis KN-42 | 2×109; 4×109; 20×109 CFU/g | Post-weaning period. From weaning for 28 days | Feces | ↑ microbial diversity in 4 × 109 diet | Not tested | Lactobacillus | ↑ in 4 × 109 diet | [142] |
| E. coli | ↓ in 4 × 109 diet | |||||||
| Bacillus subtilis DSM25841 | 1.28 × 106 CFU/g | Post-weaning period. From weaning for 21 days | Cecum | = | = | Enterobacteriaceae | ↓ | [130] |
| Bacillus subtilis DSM 32315 | 5 g/kg of 2 × 109 CFU/g. | Post-weaning period. From weaning for 21 days | Jejunum | = | = | Leucobacter and Cupriavidus | ↑ | [128] |
| Ileum | = | ≠ | Thermus, Coprococcus and Bifidobacterium | ↑ | ||||
| Colon | = | ≠ | Succiniclasticum | ↑ | ||||
| Bacillus subtilis DSM 32540 | 0.5 g/kg (1 × 109 CFU/kg) | Post-weaning period. From weaning for 28 days | Jejunum | = | ≠ | = | - | [129] |
| Ileum | = | ≠ | Lachnospiraceae, Peptostreptococcaceae and Pasteurellaceae | ↓ | ||||
| Colon | = | ≠ | = | = | ||||
| Saccharomyces cerevisiae | 3 g/kg of 4.5 × 106 CFU/g | Post-weaning period. From weaning for 41 days | Feces | = | Not tested | = | = | [143] |
| Saccharomyces cerevisiae (Actisaf Sc 47) | 2.5 × 1010 CFU/piglet | From birth to 28 days post-weaning | Cecum | = | ≠ | Coriobacteriaceae, Halanaerobiaceae, Peptococcaceae, Peptostreptococcaceae, Ruminococcaceae and Turicibacteraceae | ↑ | [132] |
| Colon | = | ≠ | Coriobacteriaceae, Halanaerobiaceae, Peptococcaceae, Peptostreptococcaceae, Ruminococcaceae and Turicibacteraceae | ↑ | ||||
| Lactobacillus plantarum PFM105 | 2 × 107 CFU/g | Post-weaning period. From weaning for 21 days | Colon | ↑ Simpson | = | Bifidobacteriaceae | ↑ | [124] |
| Lactobacillus plantarum | 3·5 × 1010 CFU/g | Post-weaning period. From weaning for 14 days | Feces | Clostridium_sensu_stricto_1, Parabacteroides and Ruminococcus_1 | ↓ | [126] | ||
| Lactobacillus | ↑ | |||||||
| Lactobacilus plantarum (JN560899.1) | 109 CFU/kg | Post-weaning period. From weaning for 28 days | Feces | = | Not tested | Selenomonas and Mitsuokella | ↑ | [125] |
| Organic acids | ||||||||
| Formic acid | 1.4 g/kg or 6.4 g/kg | Post-weaning period. From weaning for 42 days | Jejunum | ↑ Chao1 (6.4 g/kg) | = | Gemella, Lactobacillus, Parvimonas | ↓ (1.4 g/kg) ↓ (6.4 g/kg) | [137] |
| Streptococcus | ↓ (1.4 g/kg) ↑ (6.4 g/kg) | |||||||
| Turicibacter | ↓ (1.4 g/kg) | |||||||
| Acinetobacter, Fusobacterium, Leuconostoc | ↓ (6.4 g/kg) | |||||||
| Formic acid | 0.60 g/kg | Post-weaning period. From weaning for 8 days | Ileum | = | = | = | = | [138] |
| Benzoic acid | 2 g/kg + essential oils | Post-weaning period. From weaning for 28 days | Colon | = | ≠ | Bacteroides, Unclassified S24-7 | ↑ | [140] |
| Prevotella | ↓ | |||||||
| Sodium butyrate (encapsulatded) | 1g/kg + antibiotic | Post-weaning period. From weaning for 28 days | Colon | ↑ Shannon | Not tested | Bacteroidetes, Clostridiaceae, Ruminococcaceae, Lachnospiraceae | ↑ | [139] |
| ↓ Simpson | Lactobacillaceae | ↓ | ||||||
| Ileum | = | Not tested | Clostridiaceae | ↑ | ||||
| Pasteurellaceae, Lactobacillaceae, Enterobacteriaceae | ↓ | |||||||
3.5. Antimicrobials
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Mapping Supply and Demand for Animal-Source Foods to 2030; Robinson, T.P., Pozzi, F., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Steinfeld, H. The livestock revolution—A global veterinary mission. Vet. Parasitol. 2004, 125, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Conchedda, G.; Boeckel, T.P.V.; Cinardi, G.; Linard, C.; Nicolas, G.; Thanapongtharm, W.; D’Aietti, L.; Wint, W.; Newman, S.H.; et al. Income Disparities and the Global Distribution of Intensively Farmed Chicken and Pigs. PLoS ONE 2015, 10, e0133381. [Google Scholar] [CrossRef] [PubMed]
- Eurostat—Data Explorer. Available online: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=apro_mt_lspig&lang=en (accessed on 13 November 2020).
- Raasch, S.; Collineau, L.; Postma, M.; Backhans, A.; Sjölund, M.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Staerk, K.; Dewulf, J. Effectiveness of alternative measures to reduce antimicrobial usage in pig production in four european countries. Porc. Health Manag. 2020, 6, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Buddington, R.K.; Sangild, P.T. Companion animals symposium: Development of the mammalian gastrointestinal tract, the resident microbiota, and the role of diet in early life. J. Anim. Sci. 2011, 89, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; van Bilsen, J.; Cnossen, H.; Houben, G.; Garthoff, J.; Wolterbeek, A. Development of immune organs and functioning in humans and test animals: Implications for immune intervention studies. Reprod. Toxicol. 2016, 64, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Everaert, N.; Van Cruchten, S.; Weström, B.; Bailey, M.; Van Ginneken, C.; Thymann, T.; Pieper, R. A review on early gut maturation and colonization in pigs, including biological and dietary factors affecting gut homeostasis. Anim. Feed Sci. Technol. 2017, 233, 89–103. [Google Scholar] [CrossRef]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the gut microbiota ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Martín-Orúe, S.M.; Castillejos, L. Review: Are we using probiotics correctly in post-weaning piglets? Anim. Int. J. Anim. Biosci. 2018, 12, 2489–2498. [Google Scholar] [CrossRef]
- Gao, J.; Yin, J.; Xu, K.; Li, T.; Yin, Y. What is the impact of diet on nutritional diarrhea associated with gut microbiota in weaning piglets: A system Review. BioMed Res. Int. 2019, 2019, 6916189. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.H.; Suzuki, T.A.; Phifer-Rixey, M.; Nachman, M.W. Transmission modes of the mammalian gut microbiota. Science 2018, 362, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Maradiaga, N.; Zeineldin, M.; Aldridge, B.; Lowe, J. Influence of maternal microbial communities on the mucosal microbiome of neonatal pigs. AASV 2014, 2014, 1–39. [Google Scholar]
- McCormack, U.M.; Curião, T.; Wilkinson, T.; Metzler-Zebeli, B.U.; Reyer, H.; Ryan, T.; Calderon-Diaz, J.A.; Crispie, F.; Cotter, P.D.; Creevey, C.J.; et al. Fecal microbiota transplantation in gestating sows and neonatal offspring alters lifetime intestinal microbiota and growth in offspring. mSystems 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, S.; Jiang, L.; Wang, W.; Li, T.; Zuo, B.; Li, Z.; Wang, J. Differences in the gut microbiota establishment and metabolome characteristics between low- and normal-birth-weight piglets during early-life. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, X.; Zhang, Q.; Mao, L.; Yu, M.; Xu, J. The placental microbiome varies in association with low birth weight in full-term neonates. Nutrients 2015, 7, 6924–6937. [Google Scholar] [CrossRef] [PubMed]
- Gaukroger, C.H.; Stewart, C.J.; Edwards, S.A.; Walshaw, J.; Adams, I.P.; Kyriazakis, I. Changes in faecal microbiota profiles associated with performance and birthweight of piglets. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Jiang, L.; Dai, Z.; Li, T.; Han, D.; Wang, J. Characterization of the early life microbiota development and predominant Lactobacillus species at distinct gut segments of low- and normal-birth-weight piglets. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-Invited Review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Feng, C.; Tao, S.; Li, N.; Zuo, B.; Han, D.; Wang, J. Maternal imprinting of the neonatal microbiota colonization in intrauterine growth restricted piglets: A review. J. Anim. Sci. Biotechnol. 2019, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, C.; Xie, P.; Zhu, Q.; Wang, X.; Yin, Y.; Kong, X. Gut microbiota of newborn piglets with intrauterine growth restriction have lower diversity and different taxonomic abundances. J. Appl. Microbiol. 2019, 127, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; You, J.; Zhang, W.; Zhu, Q.; Blachier, F.; Yin, Y.; Kong, X. Intrauterine growth restriction alters growth performance, plasma hormones, and small intestinal microbial communities in growing-finishing pigs. J. Anim. Sci. Biotechnol. 2020, 11, 86. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Wang, X.; Zhang, X.; Zhang, C.; Zhang, M.; Zhao, Y.; Zhao, L.; Shen, J. The structural alteration of gut microbiota in low-birth-weight mice undergoing accelerated postnatal growth. Sci. Rep. 2016, 6, 27780. [Google Scholar] [CrossRef]
- Wang, W.; Degroote, J.; Ginneken, C.V.; Poucke, M.V.; Vergauwen, H.; Dam, T.M.T.; Vanrompay, D.; Peelman, L.J.; Smet, S.D.; Michiels, J. Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes. FASEB J. 2016, 30, 863–873. [Google Scholar] [CrossRef]
- Dong, L.; Zhong, X.; Ahmad, H.; Li, W.; Wang, Y.; Zhang, L.; Wang, T. Intrauterine growth restriction impairs small intestinal mucosal immunity in neonatal piglets. J. Histochem. Cytochem. 2014, 62, 510–518. [Google Scholar] [CrossRef]
- D’Inca, R.; Kloareg, M.; Gras-Le Guen, C.; Le Huërou-Luron, I. Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs. J. Nutr. 2010, 140, 925–931. [Google Scholar] [CrossRef]
- Morissette, B.; Talbot, G.; Beaulieu, C.; Lessard, M. Growth performance of piglets during the first two weeks of lactation affects the development of the intestinal microbiota. J. Anim. Physiol. Anim. Nutr. 2018, 102, 525–532. [Google Scholar] [CrossRef]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Ren, E.; Su, Y.; Zhu, W. Co-occurrence of early gut colonization in neonatal piglets with microbiota in the maternal and surrounding delivery environments. Anaerobe 2018, 49, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, X.; Fang, S.; Yang, H.; He, M.; Zhao, Y.; Huang, L. Contribution of host genetics to the variation of microbial composition of cecum lumen and feces in pigs. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kwawukume, A.; Moossavi, S.; Sepehri, S.; Nyachoti, M.; Khafipour, E. Time series and correlation network analyses to identify the role of maternal microbiomes on development of piglet gut microbiome and susceptibility to neonatal porcine diarrhea. J. Anim. Sci. 2018, 96, 213. [Google Scholar] [CrossRef]
- Kubasova, T.; Davidova-Gerzova, L.; Merlot, E.; Medvecky, M.; Polansky, O.; Gardan-Salmon, D.; Quesnel, H.; Rychlik, I. Housing systems influence gut microbiota composition of sows but not of their piglets. PLoS ONE 2017, 12, e0170051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mu, C.; He, X.; Su, Y.; Mao, S.; Zhang, J.; Smidt, H.; Zhu, W. Effects of dietary fibre source on microbiota composition in the large intestine of suckling piglets. FEMS Microbiol. Lett. 2016, 363, fnw138. [Google Scholar] [CrossRef]
- De Rodas, B.; Youmans, B.P.; Danzeisen, J.L.; Tran, H.; Johnson, T.J. Microbiome profiling of commercial pigs from farrow to finish. J. Anim. Sci. 2018, 96, 1778–1794. [Google Scholar] [CrossRef]
- Hasan, S.; Junnikkala, S.; Peltoniemi, O.; Paulin, L.; Lyyski, A.; Vuorenmaa, J.; Oliviero, C. Dietary supplementation with yeast hydrolysate in pregnancy influences colostrum yield and gut microbiota of sows and piglets after birth. PLoS ONE 2018, 13, e0197586. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Z.A.; Wang, B.; Jahan, M.; Wang, Z.; Wynn, P.; Du, Y. Characterization of porcine milk oligosaccharides over lactation between primiparous and multiparous female pigs. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Yu, H.; Wang, J.; Zhu, W. Changes in ileal microbial composition and microbial metabolism by an early-life galacto-oligosaccharides intervention in a neonatal porcine model. Nutrients 2019, 11, 1753. [Google Scholar] [CrossRef]
- Salcedo, J.; Frese, S.A.; Mills, D.A.; Barile, D. Characterization of porcine milk oligosaccharides during early lactation and their relation to the fecal microbiome. J. Dairy Sci. 2016, 99, 7733–7743. [Google Scholar] [CrossRef]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, P.; Luise, D.; Won, S.; Salcedo, J.; Bertocchi, M.; Barile, D.; Bosi, P. Variations in porcine colostrum oligosaccharide composition between breeds and in association with sow maternal performance. J. Anim. Sci. Biotechnol. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Bian, G.; Su, Y.; Zhu, W. Differential effects of breed and nursing on early-life colonic microbiota and immune status as revealed in a cross-fostering piglet model. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, S.R.; Smidt, H.; Akkermans, A.D.L.; Casini, L.; Trevisi, P.; Mazzoni, M.; de Filippi, S.; Bosi, P.; de Vos, W.M. Feeding of lactobacillus sobrius reduces Escherichia coli F4 levels in the gut and promotes growth of infected piglets. FEMS Microbiol. Ecol. 2008, 66, 599–607. [Google Scholar] [CrossRef]
- Trevisi, P.; Priori, D.; Jansman, A.J.M.; Luise, D.; Koopmans, S.-J.; Hynönen, U.; Palva, A.; Meulen, J.; van der Bosi, P. molecular networks affected by neonatal microbial colonization in porcine jejunum, luminally perfused with enterotoxigenic Escherichia coli, F4ac fimbria or Lactobacillus amylovorus. PLoS ONE 2018, 13, e0202160. [Google Scholar] [CrossRef]
- Elliott, R.F.; Vander Noot, G.W.; Gilbreath, R.L.; Fisher, H. Effect of dietary protein level on composition changes in sow colostrum and milk. J. Anim. Sci. 1971, 32, 1128–1137. [Google Scholar] [CrossRef]
- Seerley, R.W.; Griffin, F.M.; McCampbell, H.C. Effect of sow’s dietary energy source on sow’s milk and piglet carcass composition. J. Anim. Sci. 1978, 46, 1009–1017. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Ye, H.; Feng, C.; Han, D.; Tao, S.; Pi, Y.; Zhao, J.; Chen, L.; Wang, J. Dietary milk fat globule membrane supplementation during late gestation increased the growth of neonatal piglets by improving their plasma parameters, intestinal barriers, and fecal microbiota. RSC Adv. 2020, 10, 16987–16998. [Google Scholar] [CrossRef]
- Leblois, J.; Massart, S.; Soyeurt, H.; Grelet, C.; Dehareng, F.; Schroyen, M.; Li, B.; Wavreille, J.; Bindelle, J.; Everaert, N. Feeding sows resistant starch during gestation and lactation impacts their faecal microbiota and milk composition but shows limited effects on their progeny. PLoS ONE 2018, 13, e0199568. [Google Scholar] [CrossRef]
- Leblois, J. Early Life Programming of Piglets’ Microbiota and Gut Health by Maternal Dietary Fibre Supplementation. Ph.D. Thesis, Université de Liège, Liège, Belgium, 2018. [Google Scholar]
- Pajarillo, E.A.B.; Chae, J.P.; Balolong, M.P.; Kim, H.B.; Seo, K.-S.; Kang, D.-K. Characterization of the fecal microbial communities of duroc pigs using 16S rRNA gene pyrosequencing. Asian-Australas. J. Anim. Sci. 2015, 28, 584–591. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, K.; Xiang, Y.; Zhou, W.; Gui, G.; Yang, H. The fecal microbiota composition of boar duroc, yorkshire, landrace and hampshire pigs. Asian-Australas. J. Anim. Sci. 2017, 30, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.A.B.; Chae, J.P.; Balolong, M.P.; Kim, H.B.; Seo, K.-S.; Kang, D.-K. Pyrosequencing-based analysis of fecal microbial communities in three purebred pig lines. J. Microbiol. 2014, 52, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Camarinha-Silva, A.; Maushammer, M.; Wellmann, R.; Vital, M.; Preuss, S.; Bennewitz, J. Host genome influence on gut microbial composition and microbial prediction of complex traits in pigs. Genetics 2017, 206, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Ma, S.; Zhu, Z.; Su, Y.; Zoetendal, E.G.; Mackie, R.; Liu, J.; Mu, C.; Huang, R.; Smidt, H.; et al. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ. Microbiol. 2016, 18, 1566–1577. [Google Scholar] [CrossRef]
- Xian, L.; Li, Y.; Jiang, Z.; Ma, J.; Jin, L.; Chen, L.; Zhou, C.; Zhang, J.; Liu, Y.; Zhu, L.; et al. Alterations in cecal microbiota of jinhua piglets fostered by a yorkshire sow. Chin. Sci. Bull. 2014, 59, 4304–4311. [Google Scholar] [CrossRef]
- Martinsen, K.H. Genetic Analyses of Feed Efficiency Traits in Pigs. Ph.D. Thesis, Norwegian University of Life Sciences, Ås, Norway, 2016. [Google Scholar]
- Guy, S.Z.Y.; Thomson, P.C.; Hermesch, S. Selection of pigs for improved coping with health and environmental challenges: Breeding for resistance or tolerance? Front. Genet. 2012, 3. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Zhang, G.; Hou, C.; Li, N.; Yu, H.; Shang, L.; Zhang, X.; Trevisi, P.; Yang, F.; et al. Maternal milk and fecal microbes guide the spatiotemporal development of mucosa-associated microbiota and barrier function in the porcine neonatal gut. BMC Biol. 2019, 17, 106. [Google Scholar] [CrossRef]
- Maradiaga, N.; Aldridge, B.; Zeineldin, M.; Lowe, J. Gastrointestinal microbiota and mucosal immune gene expression in neonatal pigs reared in a cross-fostering model. Microb. Pathog. 2018, 121, 27–39. [Google Scholar] [CrossRef]
- Thompson, C.L.; Wang, B.; Holmes, A.J. The immediate environment during postnatal development has long-term impact on gut community structure in pigs. ISME J. 2008, 2, 739–748. [Google Scholar] [CrossRef]
- Baxter, E.; Rutherford, K.; D’Eath, R.; Arnott, G.; Turner, S.; Sandøe, P.; Moustsen, V.; Thorup, F.; Edwards, S.; Lawrence, A. The welfare implications of large litter size in the domestic pig ii: Management factors. Anim. Welf. 2013, 22, 219–238. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zheng, W.; Tao, C.; Guo, H.; Xue, Y.; Zhao, R.; Yao, W. Heat Stress during Late Gestation disrupts maternal microbial transmission with altered offspring’s gut microbial colonization and serum metabolites in a pig model. Environ. Pollut. 2020. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.-S.R.; de Jonge, N.; Sugiharto, S.; Nielsen, J.L.; Lauridsen, C.; Canibe, N. The microbial community of the gut differs between piglets fed sow milk, milk replacer or bovine colostrum. Br. J. Nutr. 2017, 117, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Laanen, M.; Persoons, D.; Ribbens, S.; de Jong, E.; Callens, B.; Strubbe, M.; Maes, D.; Dewulf, J. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. Vet. J. 2013, 198, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Mulder, I.E.; Musk, C.C.; Aminov, R.I.; Lewis, M.; Stokes, C.R.; Bailey, M.; Prosser, J.I.; Gill, B.P.; Pluske, J.R.; et al. Establishment of normal gut microbiota is compromised under excessive hygiene conditions. PLoS ONE 2011, 6, e28284. [Google Scholar] [CrossRef]
- Sekirov, I.; Tam, N.M.; Jogova, M.; Robertson, M.L.; Li, Y.; Lupp, C.; Finlay, B.B. Antibiotic-Induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect. Immun. 2008, 76, 4726–4736. [Google Scholar] [CrossRef]
- Janczyk, P.; Pieper, R.; Souffrant, W.B.; Bimczok, D.; Rothkötter, H.-J.; Smidt, H. Parenteral long-acting amoxicillin reduces intestinal bacterial community diversity in piglets even 5 weeks after the administration. ISME J. 2007, 1, 180–183. [Google Scholar] [CrossRef]
- Schokker, D.; Zhang, J.; Vastenhouw, S.A.; Heilig, H.G.H.J.; Smidt, H.; Rebel, J.M.J.; Smits, M.A. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PLoS ONE 2015, 10, e0116523. [Google Scholar] [CrossRef]
- Zeineldin, M.; Aldridge, B.; Lowe, J. Antimicrobial effects on swine gastrointestinal microbiota and their accompanying antibiotic resistome. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Jayaraman, B. Evaluation of Standardized Ileal Digestible Threonine to Lysine Ratio and Tryptophan to Lysine Ratio in Weaned Pigs Fed Antibiotic-free Diets and Subjected to Immune Challenge. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2019. Available online: http://hdl.handle.net/1993/34498 (accessed on 29 December 2020).
- Puiman, P.; Stoll, B.; Mølbak, L.; de Bruijn, A.; Schierbeek, H.; Boye, M.; Boehm, G.; Renes, I.; van Goudoever, J.; Burrin, D. Modulation of the gut microbiota with antibiotic treatment suppresses whole body urea production in neonatal pigs. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G300–G310. [Google Scholar] [CrossRef] [PubMed]
- Stokholm, J.; Schjørring, S.; Eskildsen, C.E.; Pedersen, L.; Bischoff, A.L.; Følsgaard, N.; Carson, C.G.; Chawes, B.L.K.; Bønnelykke, K.; Mølgaard, A.; et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin. Microbiol. Infect. 2014, 20, 629–635. [Google Scholar] [CrossRef] [PubMed]
- De Greeff, A.; Schokker, D.; Roubos-van den Hil, P.; Ramaekers, P.; Vastenhouw, S.A.; Harders, F.; Bossers, A.; Smits, M.A.; Rebel, J.M.J. The effect of maternal antibiotic use in sows on intestinal development in offspring. J. Anim. Sci. 2020, 98. [Google Scholar] [CrossRef] [PubMed]
- Arnal, M.-E.; Zhang, J.; Messori, S.; Bosi, P.; Smidt, H.; Lallès, J.-P. Early changes in microbial colonization selectively modulate intestinal enzymes, but not inducible heat shock proteins in young adult swine. PLoS ONE 2014, 9, e87967. [Google Scholar] [CrossRef] [PubMed]
- Arnal, M.-E.; Zhang, J.; Erridge, C.; Smidt, H.; Lallès, J.-P. Maternal antibiotic-induced early changes in microbial colonization selectively modulate colonic permeability and inducible heat shock proteins, and digesta concentrations of alkaline phosphatase and TLR-stimulants in swine offspring. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, P.; Luise, D.; Correa, F.; Messori, S.; Lallès, J.-P.; Bosi, P. Maternal antibiotic treatment affects offspring gastric sensing for umami taste and ghrelin regulation in the pig. J. Anim. Sci. Biotechnol. 2021, in press. [Google Scholar]
- Kelly, D.; Smyth, J.A.; McCracken, K.J. Effect of creep feeding on structural and functional changes of the gut of early weaned pigs. Res. Vet. Sci. 1990, 48, 350–356. [Google Scholar] [CrossRef]
- Aumaitre, A.; Peiniau, J.; Bazer, F. Digestive adaptation after weaning and nutritional consequences in the piglet. Pig News Inf. 1995, 16, 73N–79N. [Google Scholar]
- Mathew, A.G.; Jones, T.; Franklin, M.A. Effect of creep feeding on selected microflora and short-chain fatty acids in the ileum of weanling pigs. J. Anim. Sci. 1994, 72, 3163–3168. [Google Scholar] [CrossRef][Green Version]
- Kelly, D.; O’Brien, J.J.; McCracken, K.J. Effect of creep feeding on the incidence, duration and severity of post-weaning diarrhoea in pigs. Res. Vet. Sci. 1990, 49, 223–228. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, X.; Wang, P.; Yan, Z.; Sun, W.; Zhao, S.; Gun, S. Longitudinal development of the gut microbiota in healthy and diarrheic piglets induced by age-related dietary changes. Microbiol. Open 2019, 8, e923. [Google Scholar] [CrossRef] [PubMed]
- Heo, P.S. Evaluation of Creep Feeding Duration and Creep Feed Quality for Suckling Piglets to Improve Growth Performance during Pre- and Post-Weaning. Ph.D. Thesis, Seoul National University, Seoul, Korea, 2013. [Google Scholar]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; de Wiele, T.V.; Forano, E.; Blanquet-Diot, S. Gut Microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P. Observations on the maternal behaviour of free-ranging domestic pigs. Appl. Anim. Behav. Sci. 1986, 16, 131–142. [Google Scholar] [CrossRef]
- Brooks, P.H.; Tsourgiannis, C.A. Factors affecting the voluntary feed intake of the weaned pig. Weaning Pig Concepts Conseq. 2003, 6, 81–116. [Google Scholar]
- Mulder, I.E.; Schmidt, B.; Stokes, C.R.; Lewis, M.; Bailey, M.; Aminov, R.I.; Prosser, J.I.; Gill, B.P.; Pluske, J.R.; Mayer, C.-D.; et al. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009, 7, 79. [Google Scholar] [CrossRef]
- Metz, J.H.M.; Gonyou, H.W. Effect of age and housing conditions on the behavioural and haemolytic reaction of piglets to weaning. Appl. Anim. Behav. Sci. 1990, 27, 299–309. [Google Scholar] [CrossRef]
- Moeser, A.J.; Ryan, K.A.; Nighot, P.K.; Blikslager, A.T. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G413–G421. [Google Scholar] [CrossRef] [PubMed]
- Leliveld, L.M.C.; Riemensperger, A.V.; Gardiner, G.E.; O’Doherty, J.V.; Lynch, P.B.; Lawlor, P.G. Effect of weaning age and postweaning feeding programme on the growth performance of pigs to 10 weeks of age. Livest. Sci. 2013, 157, 225–233. [Google Scholar] [CrossRef]
- Huting, A.M.S.; Wellock, I.; Tuer, S.; Kyriazakis, I. Weaning age and post-weaning nursery feeding regime are important in improving the performance of lightweight pigs. J. Anim. Sci. 2019, 97, 4834–4844. [Google Scholar] [CrossRef]
- Holman, D.B.; Brunelle, B.W.; Trachsel, J.; Allen, H.K. Meta-analysis to define a core microbiota in the swine gut. mSystems 2017, 2. [Google Scholar] [CrossRef]
- Yang, H.; Yang, M.; Fang, S.; Huang, X.; He, M.; Ke, S.; Gao, J.; Wu, J.; Zhou, Y.; Fu, H.; et al. Evaluating the profound effect of gut microbiome on host appetite in pigs. BMC Microbiol. 2018, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Tiezzi, F.; Schillebeeckx, C.; McNulty, N.P.; Schwab, C.; Shull, C.; Maltecca, C. Host contributes to longitudinal diversity of fecal microbiota in swine selected for lean growth. Microbiome 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Fang, S.; He, M.; Huang, X.; Yang, H.; Yang, B.; Chen, C.; Huang, L. Age-based dynamic changes of phylogenetic composition and interaction networks of health pig gut microbiome feeding in a uniformed condition. BMC Vet. Res. 2019, 15, 172. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Maltecca, C.; Schillebeeckx, C.; McNulty, N.P.; Schwab, C.; Shull, C.; Fix, J.; Tiezzi, F. Heritability and genome-wide association of swine gut microbiome features with growth and fatness parameters. Sci. Rep. 2020, 10, 10134. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Morrow, J.L.; Edrington, T.S.; Genovese, K.J.; Dowd, S.; Carroll, J.; Dailey, J.W.; Harvey, R.B.; Poole, T.L.; Anderson, R.C.; et al. Social stress increases fecal shedding of Salmonella typhimurium by early weaned piglets. Curr. Issues Intest. Microbiol. 2006, 7, 65–71. [Google Scholar] [PubMed]
- Luise, D.; Lauridsen, C.; Bosi, P.; Trevisi, P. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J. Anim. Sci. Biotechnol. 2019, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Spinelli, E.; Correa, F.; Salvarani, C.; Bosi, P.; Trevisi, P. Effects of E. coli bivalent vaccine and of host genetic susceptibility to E. coli on the growth performance and faecal microbial profile of weaned pigs. Livest. Sci. 2020, 241, 104247. [Google Scholar] [CrossRef]
- Luise, D.; Motta, V.; Bertocchi, M.; Salvarani, C.; Clavenzani, P.; Fanelli, F.; Pagotto, U.; Bosi, P.; Trevisi, P. Effect of mucine 4 and fucosyltransferase 1 genetic variants on gut homoeostasis of growing healthy pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 801–812. [Google Scholar] [CrossRef]
- Poulsen, A.-S.R.; Luise, D.; Curtasu, M.V.; Sugiharto, S.; Canibe, N.; Trevisi, P.; Lauridsen, C. Effects of Alpha-(1,2)-Fucosyltransferase genotype variants on plasma metabolome, immune responses and gastrointestinal bacterial enumeration of pigs pre- and post-weaning. PLoS ONE 2018, 13, e0202970. [Google Scholar] [CrossRef]
- Hesselager, M.O.; Everest-Dass, A.V.; Thaysen-Andersen, M.; Bendixen, E.; Packer, N.H. FUT1 genetic variants impact protein glycosylation of porcine intestinal mucosa. Glycobiology 2016, 26, 607–622. [Google Scholar] [CrossRef]
- Tao, X.; Xu, Z.; Wan, J. Intestinal Microbiota Diversity and Expression of Pattern Recognition Receptors in Newly Weaned Piglets. Anaerobe 2015, 32, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yan, H.; Diao, H.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Mao, X.; Luo, Y.; Chen, D. Early Gut microbiota intervention suppresses DSS-Induced inflammatory responses by deactivating TLR/NLR Signalling in Pigs. Sci. Rep. 2017, 7, 3224. [Google Scholar] [CrossRef] [PubMed]
- Estellé, J.; Mach, N.; Ramayo-Caldas, Y.; Levenez, F.; Lemonnier, G.; Denis, C.; Doré, J.; Larzul, C.; Lepage, P.; Rogel-Gaillard, C. The influence of host’s genetics on the gut microbiota composition in pigs and its links with immunity traits. In Proceedings of the 10th World Congress of Genetics Applied to Livestock Production, Vancouver, BC, Canada, 17–22 August 2014. [Google Scholar]
- Rist, V.T.S.; Weiss, E.; Sauer, N.; Mosenthin, R.; Eklund, M. Effect of dietary protein supply originating from soybean meal or casein on the intestinal microbiota of piglets. Anaerobe 2014, 25, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Zhang, L.; He, X.; Smidt, H.; Zhu, W. Dietary fibres modulate the composition and activity of butyrate-producing bacteria in the large intestine of suckling piglets. Antonie Van Leeuwenhoek 2017, 110, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Aumiller, T.; Mosenthin, R.; Weiss, E. Potential of cereal grains and grain legumes in modulating pigs׳ intestinal microbiota—A Review. Livest. Sci. 2015, 172, 16–32. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.F.D. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A Review. Anim. Feed Sci. Technol. 2016, 212, 18–26. [Google Scholar] [CrossRef]
- Wan, K.; Li, Y.; Sun, W.; An, R.; Tang, Z.; Wu, L.; Chen, H.; Sun, Z. Effects of dietary calcium pyruvate on gastrointestinal tract development, intestinal health and growth performance of newly weaned piglets fed low-protein diets. J. Appl. Microbiol. 2020, 128, 355–365. [Google Scholar] [CrossRef]
- Yu, D.; Zhu, W.; Hang, S. Effects of low-protein diet on the intestinal morphology, digestive enzyme activity, blood urea nitrogen, and gut microbiota and metabolites in weaned pigs. Arch. Anim. Nutr. 2019, 73, 287–305. [Google Scholar] [CrossRef]
- Chen, H.; Mao, X.; He, J.; Yu, B.; Huang, Z.; Yu, J.; Zheng, P.; Chen, D. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 2013, 110, 1837–1848. [Google Scholar] [CrossRef]
- Williams, B.A.; Mikkelsen, D.; Flanagan, B.M.; Gidley, M.J. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J. Anim. Sci. Biotechnol. 2019, 10, 45. [Google Scholar] [CrossRef]
- Molist, F.; de Segura, A.G.; Gasa, J.; Hermes, R.G.; Manzanilla, E.G.; Anguita, M.; Pérez, J.F. Effects of the insoluble and soluble dietary fibre on the physicochemical properties of digesta and the microbial activity in early weaned piglets. Anim. Feed Sci. Technol. 2009, 149, 346–353. [Google Scholar] [CrossRef]
- Gerritsen, R.; van der Aar, P.; Molist, F. Insoluble nonstarch polysaccharides in diets for weaned piglets. J. Anim. Sci. 2012, 90, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Luo, J.; et al. Soluble fiber and insoluble fiber regulate colonic microbiota and barrier function in a piglet model. BioMed Res. Int. 2019, 2019, 7809171. [Google Scholar] [CrossRef] [PubMed]
- Barba-Vidal, E.; Martín-Orúe, S.M.; Castillejos, L. Practical aspects of the use of probiotics in pig production: A Review. Livest. Sci. 2019, 223, 84–96. [Google Scholar] [CrossRef]
- Cameron, A.; McAllister, T.A. Could probiotics be the panacea alternative to the use of antimicrobials in livestock diets? Benef. Microbes 2019, 10, 773–799. [Google Scholar] [CrossRef]
- Buntyn, J.O.; Schmidt, T.B.; Nisbet, D.J.; Callaway, T.R. The role of direct-fed microbials in conventional livestock production. Annu. Rev. Anim. Biosci. 2016, 4, 335–355. [Google Scholar] [CrossRef]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhang, J.; Dong, E.; Zhang, X.; Tao, Y.; Zhong, J. Lactobacillus plantarum PFM 105 promotes intestinal development through modulation of gut microbiota in weaning piglets. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Cui, K.; Wang, Q.; Wang, S.; Diao, Q.; Zhang, N. The facilitating effect of tartary buckwheat flavonoids and Lactobacillus plantarum on the growth performance, nutrient digestibility, antioxidant capacity, and fecal microbiota of weaned piglets. Animals 2019, 9, 986. [Google Scholar] [CrossRef]
- Lin, S.; Yang, X.; Long, Y.; Zhong, H.; Wang, P.; Yuan, P.; Zhang, X.; Che, L.; Feng, B.; Li, J.; et al. Dietary supplementation with Lactobacillus plantarum modified gut microbiota, bile acid profile and glucose homoeostasis in weaning piglets. Br. J. Nutr. 2020, 124, 797–808. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus Probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, X.; Ma, C.; Gao, Q.; Yin, Y.; Kong, X.; He, J. Dietary Supplementation with Bacillus subtilis DSM 32315 alters the intestinal microbiota and metabolites in weaned piglets. J. Appl. Microbiol. 2021, 130, 217–232. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jinno, C.; Kim, K.; Wu, Z.; Tan, B.; Li, X.; Whelan, R.; Liu, Y. Dietary Bacillus spp. enhanced growth and disease resistance of weaned pigs by modulating intestinal microbiota and systemic immunity. J. Anim. Sci. Biotechnol. 2020, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Bertocchi, M.; Motta, V.; Salvarani, C.; Bosi, P.; Luppi, A.; Fanelli, F.; Mazzoni, M.; Archetti, I.; Maiorano, G.; et al. Bacillus Sp. probiotic supplementation diminish the Escherichia coli F4ac infection in susceptible weaned pigs by influencing the intestinal immune response, intestinal microbiota and blood metabolomics. J. Anim. Sci. Biotechnol. 2019, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Ishaq, S.L.; Rodriguez-Herrera, M.V.; Garcia-Hernandez, C.A.; Kawas, J.R.; Nagaraja, T.G. Review: Are there indigenous saccharomyces in the digestive tract of livestock animal species? implications for health, nutrition and productivity traits. Anim. Int. J. Anim. Biosci. 2020, 14, 22–30. [Google Scholar] [CrossRef]
- Kiros, T.G.; Derakhshani, H.; Pinloche, E.; D’Inca, R.; Marshall, J.; Auclair, E.; Khafipour, E.; Van Kessel, A. Effect of live yeast Saccharomyces cerevisiae (actisaf sc 47) supplementation on the performance and hindgut microbiota composition of weanling pigs. Sci. Rep. 2018, 8, 5315. [Google Scholar] [CrossRef]
- Partanen, K.H.; Mroz, Z. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 1999, 12, 117–145. [Google Scholar] [CrossRef]
- Ferronato, G.; Prandini, A. Dietary supplementation of inorganic, organic, and fatty acids in pig: A review. Animals 2020, 10, 1740. [Google Scholar] [CrossRef]
- Luise, D.; Correa, F.; Bosi, P.; Trevisi, P. A review of the effect of formic acid and its salts on the gastrointestinal microbiota and performance of pigs. Animals 2020, 10, 887. [Google Scholar] [CrossRef]
- Beasley, D.E.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R.R. The evolution of stomach acidity and its relevance to the human microbiome. PLoS ONE 2015, 10, e0134116. [Google Scholar] [CrossRef]
- Luise, D.; Motta, V.; Salvarani, C.; Chiappelli, M.; Fusco, L.; Bertocchi, M.; Mazzoni, M.; Maiorano, G.; Costa, L.N.; Van Milgen, J.; et al. Long-Term Administration of formic acid to weaners: Influence on intestinal microbiota, immunity parameters and growth performance. Anim. Feed Sci. Technol. 2017, 232, 160–168. [Google Scholar] [CrossRef]
- Ren, C.; Wang, Y.; Lin, X.; Song, H.; Zhou, Q.; Xu, W.; Shi, K.; Chen, J.; Song, J.; Chen, F.; et al. A combination of formic acid and monolaurin attenuates enterotoxigenic Escherichia coli induced intestinal inflammation in piglets by inhibiting the NF-ΚB/MAPK pathways with modulation of gut microbiota. J. Agric. Food Chem. 2020, 68, 4155–4165. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Luo, Y.; Ren, W.; Schyns, G.; Guggenbuhl, P. The effects of benzoic acid and essential oils on growth performance, nutrient digestibility, and colonic microbiota in nursery pigs. Anim. Feed Sci. Technol. 2020, 262, 114426. [Google Scholar] [CrossRef]
- Roselli, M.; Pieper, R.; Rogel-Gaillard, C.; de Vries, H.; Bailey, M.; Smidt, H.; Lauridsen, C. Immunomodulating effects of probiotics for microbiota modulation, gut health and disease resistance in pigs. Anim. Feed Sci. Technol. 2017, 233, 104–119. [Google Scholar] [CrossRef]
- Hu, Y.; Dun, Y.; Li, S.; Zhao, S.; Peng, N.; Liang, Y. Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. Asian-Australas. J. Anim. Sci. 2014, 27, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Lv, X.; Diao, Q.; Zhang, N. Effects of dietary supplementation with Bacillus subtilis and yeast culture on growth performance, nutrient digestibility, serum indices and faeces microbiota of weaned piglets. J. Anim. Feed Sci. 2019, 28, 328–336. [Google Scholar] [CrossRef]
- Massacci, F.R.; Tofani, S.; Forte, C.; Bertocchi, M.; Lovito, C.; Orsini, S.; Tentellini, M.; Marchi, L.; Lemonnier, G.; Luise, D.; et al. Host genotype and amoxicillin administration affect the incidence of diarrhoea and faecal microbiota of weaned piglets during a natural multiresistant ETEC infection. J. Anim. Breed. Genet. 2019, 137, 60–72. [Google Scholar] [CrossRef]
- Thymann, T.; Sørensen, K.U.; Hedemann, M.S.; Elnif, J.; Jensen, B.B.; Banga-Mboko, H.; Leser, T.D.; Sangild, P.T. Antimicrobial treatment reduces intestinal microflora and improves protein digestive capacity without changes in villous structure in weanling pigs. Br. J. Nutr. 2007, 97, 1128–1137. [Google Scholar] [CrossRef]
- Connelly, S.; Subramanian, P.; Hasan, N.A.; Colwell, R.R.; Kaleko, M. Distinct consequences of amoxicillin and ertapenem exposure in the porcine gut microbiome. Anaerobe 2018, 53, 82–93. [Google Scholar] [CrossRef]
- Holman, D.B.; Chénier, M.R. Impact of subtherapeutic administration of tylosin and chlortetracycline on antimicrobial resistance in farrow-to-finish swine. FEMS Microbiol. Ecol. 2013, 85, 1–13. [Google Scholar] [CrossRef]
- Rettedal, E.; Vilain, S.; Lindblom, S.; Lehnert, K.; Scofield, C.; George, S.; Clay, S.; Kaushik, R.S.; Rosa, A.J.M.; Francis, D.; et al. Alteration of the ileal microbiota of weanling piglets by the growth-promoting antibiotic chlortetracycline. Appl. Environ. Microbiol. 2009, 75, 5489–5495. [Google Scholar] [CrossRef] [PubMed]
- Looft, T.; Allen, H.K.; Cantarel, B.L.; Levine, U.Y.; Bayles, D.O.; Alt, D.P.; Henrissat, B.; Stanton, T.B. Bacteria, phages and pigs: The effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. 2014, 8, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Bosi, P.; Merialdi, G.; Scandurra, S.; Messori, S.; Bardasi, L.; Nisi, I.; Russo, D.; Casini, L.; Trevisi, P. Feed supplemented with 3 different antibiotics improved food intake and decreased the activation of the humoral immune response in healthy weaned pigs but had differing effects on intestinal microbiota. J. Anim. Sci. 2011, 89, 4043–4053. [Google Scholar] [CrossRef] [PubMed]
- Kalmokoff, M.; Waddington, L.M.; Thomas, M.; Liang, K.-L.; Ma, C.; Topp, E.; Dandurand, U.D.; Letellier, A.; Matias, F.; Brooks, S.P.J. Continuous feeding of antimicrobial growth promoters to commercial swine during the growing/finishing phase does not modify faecal community erythromycin resistance or community structure. J. Appl. Microbiol. 2011, 110, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Poole, T.L.; Suchodolski, J.S.; Callaway, T.R.; Farrow, R.L.; Loneragan, G.H.; Nisbet, D.J. The effect of chlortetracycline on faecal microbial populations in growing swine. J. Glob. Antimicrob. Resist. 2013, 1, 171–174. [Google Scholar] [CrossRef]
- Holman, D.B.; Chénier, M.R. Temporal changes and the effect of subtherapeutic concentrations of antibiotics in the gut microbiota of swine. FEMS Microbiol. Ecol. 2014, 90, 599–608. [Google Scholar] [CrossRef]
- Allen, H.K.; Looft, T.; Bayles, D.O.; Humphrey, S.; Levine, U.Y.; Alt, D.; Stanton, T.B. Antibiotics in feed induce prophages in swine fecal microbiomes. mBio 2011, 2. [Google Scholar] [CrossRef]
- Sun, W.; Sun, J.; Li, M.; Xu, Q.; Zhang, X.; Tang, Z.; Chen, J.; Zhen, J.; Sun, Z. The effects of dietary sodium butyrate supplementation on the growth performance, carcass traits and intestinal microbiota of growing-finishing pigs. J. Appl. Microbiol. 2020, 128, 1613–1623. [Google Scholar] [CrossRef]
- Holman, D.B.; Chénier, M.R. Antimicrobial use in swine production and its Effect on the swine gut microbiota and antimicrobial resistance. Can. J. Microbiol. 2015. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trevisi, P.; Luise, D.; Correa, F.; Bosi, P. Timely Control of Gastrointestinal Eubiosis: A Strategic Pillar of Pig Health. Microorganisms 2021, 9, 313. https://doi.org/10.3390/microorganisms9020313
Trevisi P, Luise D, Correa F, Bosi P. Timely Control of Gastrointestinal Eubiosis: A Strategic Pillar of Pig Health. Microorganisms. 2021; 9(2):313. https://doi.org/10.3390/microorganisms9020313
Chicago/Turabian StyleTrevisi, Paolo, Diana Luise, Federico Correa, and Paolo Bosi. 2021. "Timely Control of Gastrointestinal Eubiosis: A Strategic Pillar of Pig Health" Microorganisms 9, no. 2: 313. https://doi.org/10.3390/microorganisms9020313
APA StyleTrevisi, P., Luise, D., Correa, F., & Bosi, P. (2021). Timely Control of Gastrointestinal Eubiosis: A Strategic Pillar of Pig Health. Microorganisms, 9(2), 313. https://doi.org/10.3390/microorganisms9020313