Identification and Characterization of Four c-di-GMP-Metabolizing Enzymes from Streptomyces ghanaensis ATCC14672 Involved in the Regulation of Morphogenesis and Moenomycin A Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatic Analysis
2.2. Strains, Plasmids, and Growth Conditions
2.3. DNA Manipulation and Intergeneric Conjugation
2.4. Proteins Production and Purification
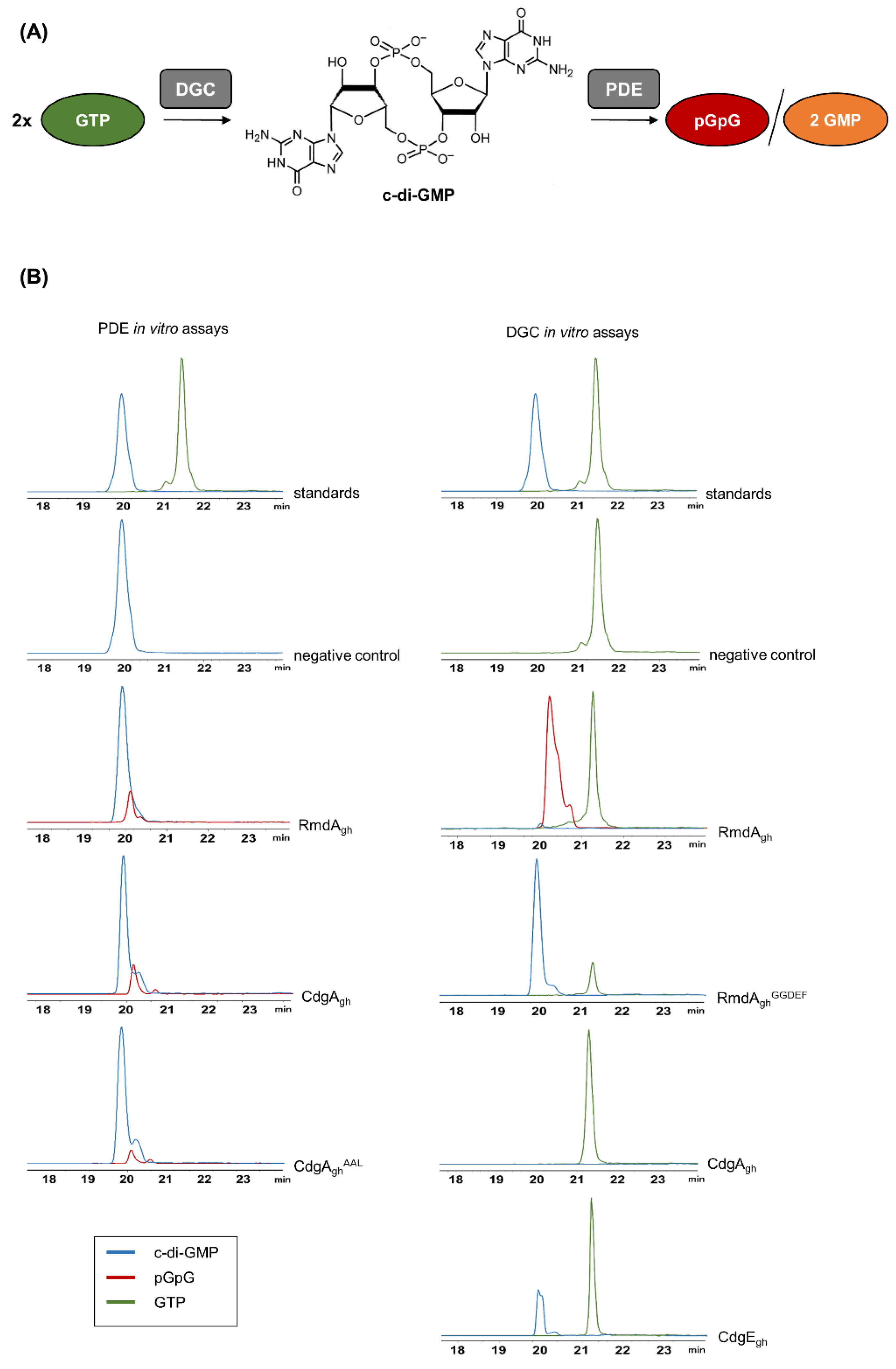
2.5. Diguanylate Cyclase and Phosphodiesterase Enzymatic In Vitro Assays
2.6. Gene Overexpression Experiments
2.7. Gene Deletion and Complementation Experiments
2.8. Quantitative Analysis of MmA Accumulation
3. Results
3.1. Bioinformatic Analysis of CdgEgh, CdgDgh, RmdAgh and CdgAgh, Four Putative c-di-GMP-Metabolizing Enzymes from S. ghanaensis
3.2. In Vitro Determination of RmdAgh, CdgAgh and CdgEgh Enzymatic Activities
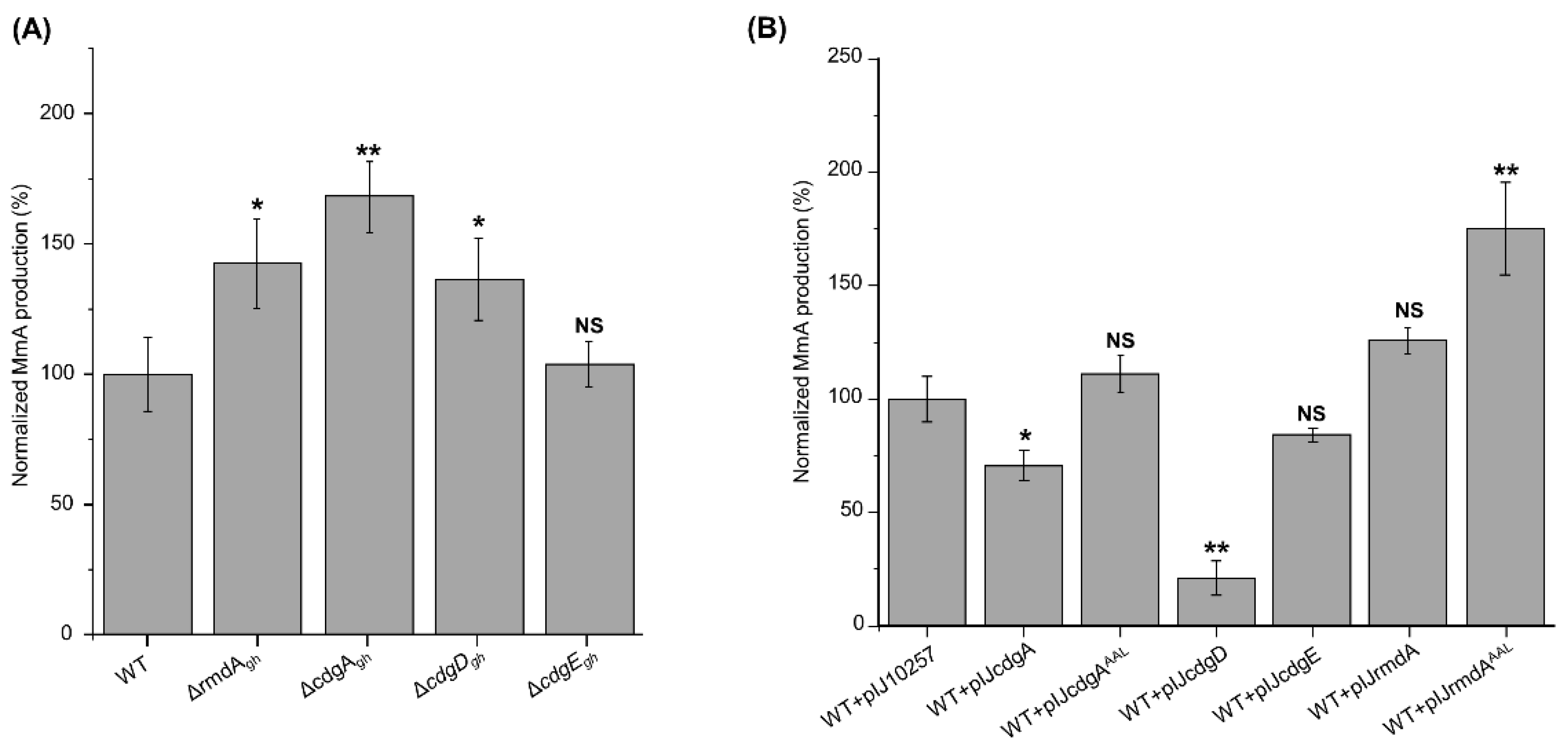
3.3. Overexpression of cdgEgh, cdgDgh, rmdAgh and cdgAgh Remarkably Alters Morphogenesis in S. ghanaensis
3.4. Genetic Manipulation of c-di-GMP-Metabolizing Enzymes Leads to Variations in MmA Accumulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flärdh, K.; Buttner, M.J. Streptomyces morphogenetics: Dissecting differentiation in a filamentous bacterium. Nat. Rev. Microbiol. 2009, 7, 36–49. [Google Scholar] [CrossRef]
- McCormick, J.R.; Flärdh, K. Signals and regulators that govern Streptomyces development. FEMS Microbiol. Rev. 2012, 36, 206–231. [Google Scholar] [CrossRef]
- Ostash, B.; Saghatelian, A.; Walker, S. A Streamlined Metabolic Pathway For the Biosynthesis of Moenomycin A. Chem. Biol. 2007, 14, 257–267. [Google Scholar] [CrossRef][Green Version]
- Ostash, B.; Walker, S. Moenomycin family antibiotics: Chemical synthesis, biosynthesis, and biological activity. Nat. Prod. Rep. 2010, 27, 1594–1617. [Google Scholar] [CrossRef]
- Van Wezel, G.P.; McDowall, K.J. The regulation of the secondary metabolism of Streptomyces: New links and experimental advances. Nat. Prod. Rep. 2011, 28, 1311–1333. [Google Scholar] [CrossRef]
- Rabyk, M.; Ostash, B.; Rebets, Y.; Walker, S.; Fedorenko, V. Streptomyces ghanaensis pleiotropic regulatory gene wblA(gh) influences morphogenesis and moenomycin production. Biotechnol. Lett. 2011, 33, 2481–2486. [Google Scholar] [CrossRef]
- Makitrynskyy, R.; Ostash, B.; Tsypik, O.; Rebets, Y.; Doud, E.; Meredith, T.; Luzhetskyy, A.; Bechthold, A.; Walker, S.; Fedorenko, V. Pleiotropic regulatory genes bldA, adpA and absB are implicated in production of phosphoglycolipid antibiotic moenomycin. Open Biol. 2013, 3, 130121. [Google Scholar] [CrossRef]
- Kang, S.-H.; Huang, J.; Lee, H.-N.; Hur, Y.-A.; Cohen, S.N.; Kim, E.-S. Interspecies DNA microarray analysis identifies WblA as a pleiotropic down-regulator of antibiotic biosynthesis in Streptomyces. J. Bacteriol. 2007, 189, 4315–4319. [Google Scholar] [CrossRef]
- Fowler-Goldsworthy, K.; Gust, B.; Mouz, S.; Chandra, G.; Findlay, K.C.; Chater, K.F. The actinobacteria-specific gene wblA controls major developmental transitions in Streptomyces coelicolor A3(2). Microbiology (Reading England) 2011, 157, 1312–1328. [Google Scholar] [CrossRef]
- Higo, A.; Hara, H.; Horinouchi, S.; Ohnishi, Y. Genome-wide distribution of AdpA, a global regulator for secondary metabolism and morphological differentiation in Streptomyces, revealed the extent and complexity of the AdpA regulatory network. DNA Res. 2012, 19, 259–273. [Google Scholar] [CrossRef]
- Rabyk, M.; Yushchuk, O.; Rokytskyy, I.; Anisimova, M.; Ostash, B. Genomic Insights into Evolution of AdpA Family Master Regulators of Morphological Differentiation and Secondary Metabolism in Streptomyces. J. Mol. Evol. 2018, 86, 204–215. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Kameyama, S.; Onaka, H.; Horinouchi, S. The A-factor regulatory cascade leading to streptomycin biosynthesis in Streptomyces griseus: Identification of a target gene of the A-factor receptor. Mol. Microbiol. 1999, 34, 102–111. [Google Scholar] [CrossRef]
- Leskiw, B.K.; Lawlor, E.J.; Fernandez-Abalos, J.M.; Chater, K.F. TTA codons in some genes prevent their expression in a class of developmental, antibiotic-negative, Streptomyces mutants. Proc. Natl. Acad. Sci. USA 1991, 88, 2461–2465. [Google Scholar] [CrossRef]
- Den Hengst, C.D.; Tran, N.T.; Bibb, M.J.; Chandra, G.; Leskiw, B.K.; Buttner, M.J. Genes essential for morphological development and antibiotic production in Streptomyces coelicolor are targets of BldD during vegetative growth. Mol. Microbiol. 2010, 78, 361–379. [Google Scholar] [CrossRef]
- Makitrynskyy, R.; Tsypik, O.; Nuzzo, D.; Paululat, T.; Zechel, D.L.; Bechthold, A. Secondary nucleotide messenger c-di-GMP exerts a global control on natural product biosynthesis in streptomycetes. Nucleic Acids Res. 2020. [Google Scholar] [CrossRef]
- Elliot, M.A.; Bibb, M.J.; Buttner, M.J.; Leskiw, B.K. BldD is a direct regulator of key developmental genes in Streptomyces coelicolor A3(2). Mol. Microbiol. 2001, 40, 257–269. [Google Scholar] [CrossRef]
- Tschowri, N.; Schumacher, M.A.; Schlimpert, S.; Chinnam, N.B.; Findlay, K.C.; Brennan, R.G.; Buttner, M.J. Tetrameric c-di-GMP mediates effective transcription factor dimerization to control Streptomyces development. Cell 2014, 158, 1136–1147. [Google Scholar] [CrossRef]
- Gallagher, K.A.; Schumacher, M.A.; Bush, M.J.; Bibb, M.J.; Chandra, G.; Holmes, N.A.; Zeng, W.; Henderson, M.; Zhang, H.; Findlay, K.C.; et al. c-di-GMP Arms an Anti-σ to Control Progression of Multicellular Differentiation in Streptomyces. Mol. Cell 2020, 77, 586–599.e6. [Google Scholar] [CrossRef]
- Chan, C.; Paul, R.; Samoray, D.; Amiot, N.C.; Giese, B.; Jenal, U.; Schirmer, T. Structural basis of activity and allosteric control of diguanylate cyclase. Proc. Natl. Acad. Sci. USA 2004, 101, 17084–17089. [Google Scholar] [CrossRef]
- Paul, R.; Weiser, S.; Amiot, N.C.; Chan, C.; Schirmer, T.; Giese, B.; Jenal, U. Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 2004, 18, 715–727. [Google Scholar] [CrossRef]
- Christen, M.; Christen, B.; Folcher, M.; Schauerte, A.; Jenal, U. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 2005, 280, 30829–30837. [Google Scholar] [CrossRef]
- Schmidt, A.J.; Ryjenkov, D.A.; Gomelsky, M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: Enzymatically active and inactive EAL domains. J. Bacteriol. 2005, 187, 4774–4781. [Google Scholar] [CrossRef]
- Ryan, R.P.; Fouhy, Y.; Lucey, J.F.; Crossman, L.C.; Spiro, S.; He, Y.-W.; Zhang, L.-H.; Heeb, S.; Cámara, M.; Williams, P.; et al. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc. Natl. Acad. Sci. USA 2006, 103, 6712–6717. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef]
- Tran, N.T.; Den Hengst, C.D.; Gomez-Escribano, J.P.; Buttner, M.J. Identification and characterization of CdgB, a diguanylate cyclase involved in developmental processes in Streptomyces coelicolor. J. Bacteriol. 2011, 193, 3100–3108. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, G.; Wang, G.; Jiang, W.; Li, L.; Lu, Y. Overexpression of the diguanylate cyclase CdgD blocks developmental transitions and antibiotic biosynthesis in Streptomyces Coelicolor. Sci. China Life Sci. 2019, 62, 1492–1505. [Google Scholar] [CrossRef]
- Hull, T.D.; Ryu, M.-H.; Sullivan, M.J.; Johnson, R.C.; Klena, N.T.; Geiger, R.M.; Gomelsky, M.; Bennett, J.A. Cyclic Di-GMP phosphodiesterases RmdA and RmdB are involved in regulating colony morphology and development in Streptomyces coelicolor. J. Bacteriol. 2012, 194, 4642–4651. [Google Scholar] [CrossRef]
- Al-Bassam, M.M.; Haist, J.; Neumann, S.A.; Lindenberg, S.; Tschowri, N. Expression Patterns, Genomic Conservation and Input Into Developmental Regulation of the GGDEF/EAL/HD-GYP Domain Proteins in Streptomyces. Front. Microbiol. 2018, 9, 2524. [Google Scholar] [CrossRef]
- Haist, J.; Neumann, S.A.; Al-Bassam, M.M.; Lindenberg, S.; Elliot, M.A.; Tschowri, N. Specialized and shared functions of diguanylate cyclases and phosphodiesterases in Streptomyces development. Mol. Microbiol. 2020. [Google Scholar] [CrossRef]
- Nuzzo, D.; Makitrynskyy, R.; Tsypik, O.; Bechthold, A. Cyclic di-GMP cyclase SSFG_02181 from Streptomyces ghanaensis ATCC14672 regulates antibiotic biosynthesis and morphological differentiation in streptomycetes. Sci. Rep. 2020, 10, 12021. [Google Scholar] [CrossRef]
- Schultz, J.; Milpetz, F.; Bork, P.; Ponting, C.P. SMART, a simple modular architecture research tool: Identification of signaling domains. Proc. Natl. Acad. Sci. USA 1998, 95, 5857–5864. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989; ISBN 0879693096. [Google Scholar]
- Kieser, T. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000; ISBN 0708406238. [Google Scholar]
- Gust, B.; Chandra, G.; Jakimowicz, D.; Yuqing, T.; Bruton, C.J.; Chater, K.F. Lambda red-mediated genetic manipulation of antibiotic-producing Streptomyces. Adv. Appl. Microbiol. 2004, 54, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Lopatniuk, M.; Ostash, B.; Makitrynskyy, R.; Walker, S.; Luzhetskyy, A.; Fedorenko, V. Testing the utility of site-specific recombinases for manipulations of genome of moenomycin producer Streptomyces ghanaensis ATCC14672. J. Appl. Genet. 2015, 56, 547–550. [Google Scholar] [CrossRef][Green Version]
- Makitrynskyy, R.; Rebets, Y.; Ostash, B.; Zaburannyi, N.; Rabyk, M.; Walker, S.; Fedorenko, V. Genetic factors that influence moenomycin production in streptomycetes. J. Ind. Microbiol. Biotechnol. 2010, 37, 559–566. [Google Scholar] [CrossRef][Green Version]
- Ostash, B.; Doud, E.H.; Lin, C.; Ostash, I.; Perlstein, D.L.; Fuse, S.; Wolpert, M.; Kahne, D.; Walker, S. Complete characterization of the seventeen step moenomycin biosynthetic pathway. Biochemistry 2009, 48, 8830–8841. [Google Scholar] [CrossRef]
- Rao, F.; Yang, Y.; Qi, Y.; Liang, Z.-X. Catalytic mechanism of cyclic di-GMP-specific phosphodiesterase: A study of the EAL domain-containing RocR from Pseudomonas aeruginosa. J. Bacteriol. 2008, 190, 3622–3631. [Google Scholar] [CrossRef]
- Schirmer, T. C-di-GMP Synthesis: Structural Aspects of Evolution, Catalysis and Regulation. J. Mol. Biol. 2016, 428, 3683–3701. [Google Scholar] [CrossRef]
- Wassmann, P.; Chan, C.; Paul, R.; Beck, A.; Heerklotz, H.; Jenal, U.; Schirmer, T. Structure of BeF3−-Modified Response Regulator PleD: Implications for Diguanylate Cyclase Activation, Catalysis, and Feedback Inhibition. Structure 2007, 15, 1155. [Google Scholar] [CrossRef]
- Nesbitt, N.M.; Arora, D.P.; Johnson, R.A.; Boon, E.M. Modification of a bi-functional diguanylate cyclase-phosphodiesterase to efficiently produce cyclic diguanylate monophosphate. Biotechnol. Rep. (Amsterdam) 2015, 7, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Tischler, A.D.; Camilli, A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 2004, 53, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Tchigvintsev, A.; Xu, X.; Singer, A.; Chang, C.; Brown, G.; Proudfoot, M.; Cui, H.; Flick, R.; Anderson, W.F.; Joachimiak, A.; et al. Structural insight into the mechanism of c-di-GMP hydrolysis by EAL domain phosphodiesterases. J. Mol. Biol. 2010, 402, 524–538. [Google Scholar] [CrossRef]
- Lindenberg, S.; Klauck, G.; Pesavento, C.; Klauck, E.; Hengge, R. The EAL domain protein YciR acts as a trigger enzyme in a c-di-GMP signalling cascade in E. coli biofilm control. EMBO J. 2013, 32, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.T.; Crosson, S. Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu. Rev. Microbiol. 2011, 65, 261–286. [Google Scholar] [CrossRef]
- Qi, Y.; Chuah, M.L.C.; Dong, X.; Xie, K.; Luo, Z.; Tang, K.; Liang, Z.-X. Binding of cyclic diguanylate in the non-catalytic EAL domain of FimX induces a long-range conformational change. J. Biol. Chem. 2011, 286, 2910–2917. [Google Scholar] [CrossRef]
- Abel, S.; Bucher, T.; Nicollier, M.; Hug, I.; Kaever, V.; zur Abel Wiesch, P.; Jenal, U. Bi-modal distribution of the second messenger c-di-GMP controls cell fate and asymmetry during the caulobacter cell cycle. PLoS Genet. 2013, 9, e1003744. [Google Scholar] [CrossRef]
- Abel, S.; Chien, P.; Wassmann, P.; Schirmer, T.; Kaever, V.; Laub, M.T.; Baker, T.A.; Jenal, U. Regulatory cohesion of cell cycle and cell differentiation through interlinked phosphorylation and second messenger networks. Mol. Cell 2011, 43, 550–560. [Google Scholar] [CrossRef]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef]
- Ryan, R.P.; McCarthy, Y.; Andrade, M.; Farah, C.S.; Armitage, J.P.; Dow, J.M. Cell-cell signal-dependent dynamic interactions between HD-GYP and GGDEF domain proteins mediate virulence in Xanthomonas campestris. Proc. Natl. Acad. Sci. USA 2010, 107, 5989–5994. [Google Scholar] [CrossRef]
- García, B.; Latasa, C.; Solano, C.; García-del Portillo, F.; Gamazo, C.; Lasa, I. Role of the GGDEF protein family in Salmonella cellulose biosynthesis and biofilm formation. Mol. Microbiol. 2004, 54, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Zeng, W.; Findlay, K.C.; Buttner, M.J.; Brennan, R.G.; Tschowri, N. The Streptomyces master regulator BldD binds c-di-GMP sequentially to create a functional BldD2-(c-di-GMP)4 complex. Nucleic Acids Res. 2017, 45, 6923–6933. [Google Scholar] [CrossRef] [PubMed]
- Som, N.F.; Heine, D.; Holmes, N.A.; Munnoch, J.T.; Chandra, G.; Seipke, R.F.; Hoskisson, P.A.; Wilkinson, B.; Hutchings, M.I. The Conserved Actinobacterial Two-Component System MtrAB Coordinates Chloramphenicol Production with Sporulation in Streptomyces venezuelae NRRL B-65442. Front. Microbiol. 2017, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.J.; Chandra, G.; Al-Bassam, M.M.; Findlay, K.C.; Buttner, M.J. BldC Delays Entry into Development to Produce a Sustained Period of Vegetative Growth in Streptomyces venezuelae. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.J.; Bibb, M.J.; Chandra, G.; Findlay, K.C.; Buttner, M.J. Genes required for aerial growth, cell division, and chromosome segregation are targets of WhiA before sporulation in Streptomyces venezuelae. mBio 2013, 4, e00684-13. [Google Scholar] [CrossRef]


| Gene | Domain Architecture | Orthologs | References |
|---|---|---|---|
| ssfg_00725 (rmdAgh) | PAS-PAC-GGDEF-EAL | sco0928 (rmdA), sven6830 | This work |
| ssfg_02196 (rmdBgh) | 6TM1-GGDEF-EAL | sco5495 (rmdB), sven5165 | [15] |
| ssfg_04551 (cdgAgh) | PAS-GGDEF-EAL | sco2817 (cdgA), sven2604 | This work |
| ssfg_03956 (cdgBgh) | GAF-PAS-PAC-GGDEF | sco4281 (cdgB), sven4034 | [15] |
| ssfg_02181 (cdgCgh) | 9TM-PAS-GGDEF-EAL | sco5511, sven5187 (cdgC) | [30] |
| ssfg_02707 (cdgEgh) | GAF-GGDEF | sco4931, sven4602 (cdgE) | This work |
| ssfg_02343 (cdgDgh) | GGDEF | sco5345 (cdgD), sven3999 | This work |
| ssfg_02459 | 5TM-HD-GYP | sven4873 | |
| ssfg_02460 | 2TM-HD-GYP2 | sven4872 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzzo, D.; Makitrynskyy, R.; Tsypik, O.; Bechthold, A. Identification and Characterization of Four c-di-GMP-Metabolizing Enzymes from Streptomyces ghanaensis ATCC14672 Involved in the Regulation of Morphogenesis and Moenomycin A Biosynthesis. Microorganisms 2021, 9, 284. https://doi.org/10.3390/microorganisms9020284
Nuzzo D, Makitrynskyy R, Tsypik O, Bechthold A. Identification and Characterization of Four c-di-GMP-Metabolizing Enzymes from Streptomyces ghanaensis ATCC14672 Involved in the Regulation of Morphogenesis and Moenomycin A Biosynthesis. Microorganisms. 2021; 9(2):284. https://doi.org/10.3390/microorganisms9020284
Chicago/Turabian StyleNuzzo, Desirèe, Roman Makitrynskyy, Olga Tsypik, and Andreas Bechthold. 2021. "Identification and Characterization of Four c-di-GMP-Metabolizing Enzymes from Streptomyces ghanaensis ATCC14672 Involved in the Regulation of Morphogenesis and Moenomycin A Biosynthesis" Microorganisms 9, no. 2: 284. https://doi.org/10.3390/microorganisms9020284
APA StyleNuzzo, D., Makitrynskyy, R., Tsypik, O., & Bechthold, A. (2021). Identification and Characterization of Four c-di-GMP-Metabolizing Enzymes from Streptomyces ghanaensis ATCC14672 Involved in the Regulation of Morphogenesis and Moenomycin A Biosynthesis. Microorganisms, 9(2), 284. https://doi.org/10.3390/microorganisms9020284






