Epidemiology and Characterization of CTX-M-55-Type Extended-Spectrum β-Lactamase-Producing Salmonella enterica Serovar Enteritidis Isolated from Patients in Shanghai, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Antibiotic Susceptibility Testing
2.3. Detection of ESBL Phenotypes and Sequencing of ESBL-Encoding Genes
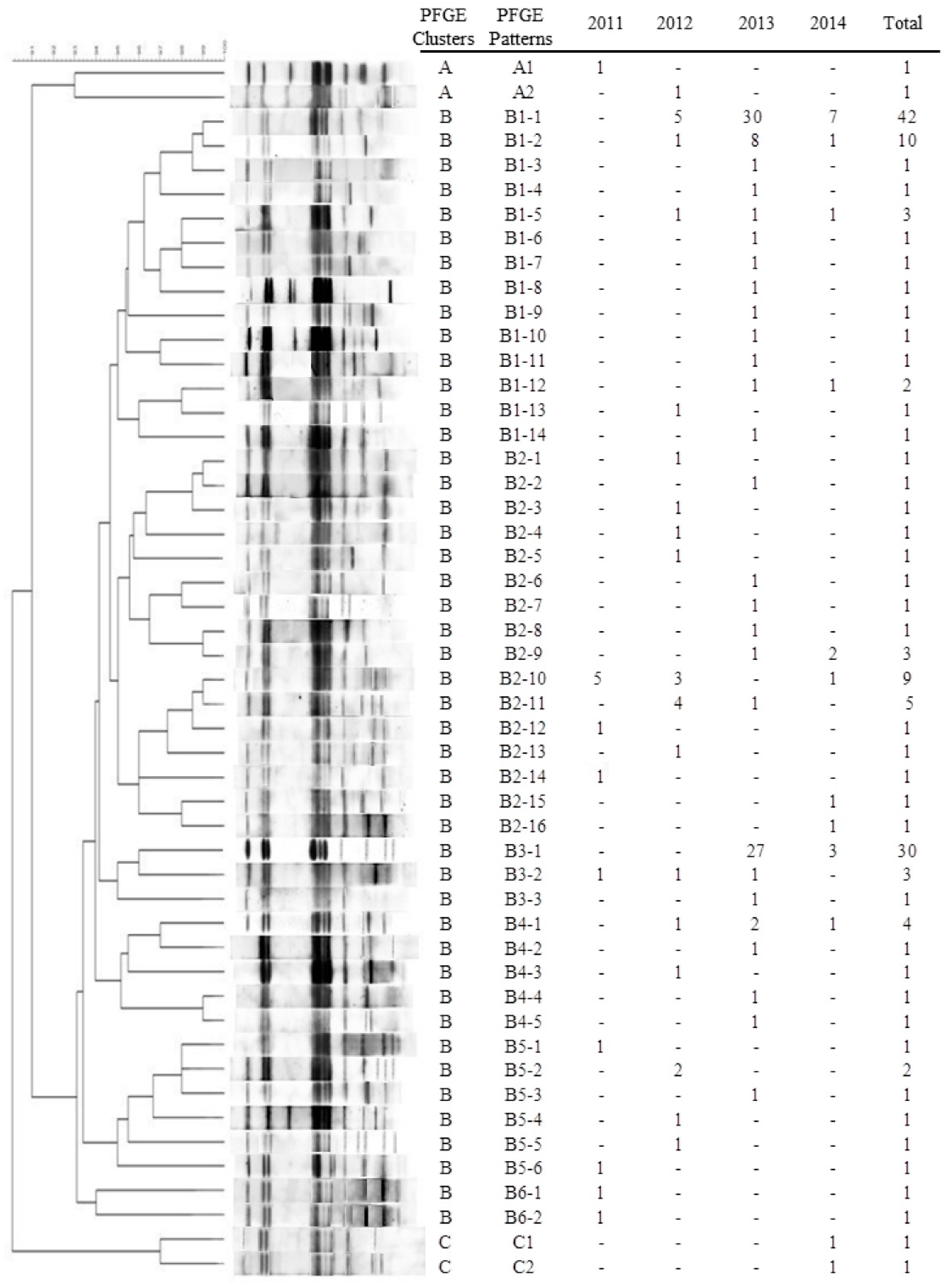
2.4. Pulsed-Field Gel Electrophoresis (PFGE)
2.5. Conjugation Experiment
2.6. Statistical Analysis
3. Results
3.1. Antibiotic Susceptibility
3.2. Characterization of ESBL-SE
3.3. Prevalence of ESBL-Encoding Genes and Description of blaCTX-M-55-Positive ESBL-SE
3.4. PFGE Patterns of blaCTX-M-55-Positive ESBL-SE
3.5. Conjugation of Three blaCTX-M-55-Postitive ESBL-SE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, Y.; Zhou, D. Organoid and Enteroid Modeling of Salmonella Infection. Front. Cell Infect. Microbiol. 2018, 8, 102. [Google Scholar] [CrossRef]
- Gal-Mor, O. Persistent Infection and Long-Term Carriage of Typhoidal and Nontyphoidal Salmonellae. Clin. Microbiol. Rev. 2019, 32, e00088-00018. [Google Scholar] [CrossRef]
- Keestra-Gounder, A.M.; Tsolis, R.M.; Baumler, A.J. Now you see me, now you don’t: The interaction of Salmonella with innate immune receptors. Nat. Rev. Microbiol. 2015, 13, 206–216. [Google Scholar] [CrossRef]
- Shi, C.; Singh, P.; Ranieri, M.L.; Wiedmann, M.; Moreno Switt, A.I. Molecular methods for serovar determination of Salmonella. Crit. Rev. Microbiol. 2015, 41, 309–325. [Google Scholar] [CrossRef]
- Betancor, L.; Pereira, M.; Martinez, A.; Giossa, G.; Fookes, M.; Flores, K.; Barrios, P.; Repiso, V.; Vignoli, R.; Cordeiro, N.; et al. Prevalence of Salmonella enterica in poultry and eggs in Uruguay during an epidemic due to Salmonella enterica serovar Enteritidis. J. Clin. Microbiol. 2010, 48, 2413–2423. [Google Scholar] [CrossRef]
- Taylor, A.J.; Lappi, V.; Wolfgang, W.J.; Lapierre, P.; Palumbo, M.J.; Medus, C.; Boxrud, D. Characterization of Foodborne Outbreaks of Salmonella enterica Serovar Enteritidis with Whole-Genome Sequencing Single Nucleotide Polymorphism-Based Analysis for Surveillance and Outbreak Detection. J. Clin. Microbiol. 2015, 53, 3334–3340. [Google Scholar] [CrossRef]
- Kariuki, S.; Okoro, C.; Kiiru, J.; Njoroge, S.; Omuse, G.; Langridge, G.; Kingsley, R.A.; Dougan, G.; Revathi, G. Ceftriaxone-resistant Salmonella enterica serotype typhimurium sequence type 313 from Kenyan patients is associated with the blaCTX-M-15 gene on a novel IncHI2 plasmid. Antimicrob. Agents Chemother. 2015, 59, 3133–3139. [Google Scholar] [CrossRef][Green Version]
- Dong, N.; Li, Y.; Zhao, J.; Ma, H.; Wang, J.; Liang, B.; Du, X.; Wu, F.; Xia, S.; Yang, X.; et al. The phenotypic and molecular characteristics of antimicrobial resistance of Salmonella enterica subsp. enterica serovar Typhimurium in Henan Province, China. BMC Infect. Dis. 2020, 20, 511. [Google Scholar] [CrossRef]
- Katiyo, S.; Muller-Pebody, B.; Minaji, M.; Powell, D.; Johnson, A.P.; De Pinna, E.; Day, M.; Harris, R.; Godbole, G. Epidemiology and Outcomes of Nontyphoidal Salmonella Bacteremias from England, 2004 to 2015. J. Clin. Microbiol. 2019, 57, e01189-01118. [Google Scholar] [CrossRef]
- Shen, H.; Chen, H.; Ou, Y.; Huang, T.; Chen, S.; Zhou, L.; Zhang, J.; Hu, Q.; Zhou, Y.; Ma, W. Prevalence, serotypes, and antimicrobial resistance of Salmonella isolates from patients with diarrhea in Shenzhen, China. BMC Microbiol. 2020, 20, 197. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Y.; Xiong, Z.; Ma, Y.; Wei, Y.; Qu, X.; Zhang, H.; Zhang, J.; Liao, M. Highly Prevalent Multidrug-Resistant Salmonella From Chicken and Pork Meat at Retail Markets in Guangdong, China. Front. Microbiol. 2018, 9, 2104. [Google Scholar] [CrossRef] [PubMed]
- Monogue, M.L.; Tsuji, M.; Yamano, Y.; Echols, R.; Nicolau, D.P. Efficacy of Humanized Exposures of Cefiderocol (S-649266) against a Diverse Population of Gram-Negative Bacteria in a Murine Thigh Infection Model. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Peirano, G.; Pitout, J.D.D. Extended-Spectrum beta-Lactamase-Producing Enterobacteriaceae: Update on Molecular Epidemiology and Treatment Options. Drugs 2019, 79, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, B.; Hulth, A.; Schwarz, S.; Ji, X.; Nilsson, L.E.; Ma, S.; Sun, Q.; Bi, Z.; Wang, Y.; et al. Genomic analysis of Staphylococcus aureus along a pork production chain and in the community, Shandong Province, China. Int. J. Antimicrob. Agents 2019, 54, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, X.; Zhang, L.; Xiong, Z.; Ma, Y.; Wei, Y.; Chen, Z.; Bai, J.; Liao, M.; Zhang, J. Fourth Generation Cephalosporin Resistance Among Salmonella enterica Serovar Enteritidis Isolates in Shanghai, China Conferred by bla CTX-M-55 Harboring Plasmids. Front. Microbiol. 2020, 11, 910. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Ke, B.; Deng, X.; Liang, J.; Ran, L.; Lu, L.; He, D.; Huang, Q.; Ke, C.; Li, Z.; et al. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal Salmonella from human patients in Guangdong Province, China, 2009-2012. BMC Infect. Dis. 2015, 15, 53. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Zhang, Y.; Zhang, Z.; Lei, L.; Xia, Z. Increasing Prevalence of ESBL-Producing Multidrug Resistance Escherichia coli From Diseased Pets in Beijing, China From 2012 to 2017. Front. Microbiol. 2019, 10, 2852. [Google Scholar] [CrossRef]
- Brown, A.C.; Chen, J.C.; Watkins, L.K.F.; Campbell, D.; Folster, J.P.; Tate, H.; Wasilenko, J.; Van Tubbergen, C.; Friedman, C.R. CTX-M-65 Extended-Spectrum beta-Lactamase-Producing Salmonella enterica Serotype Infantis, United States. Emerg. Infect. Dis. 2018, 24, 2284–2291. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Xu, X.; Fu, Y.; Xiong, Z.; Zhang, L.; Qu, X.; Zhang, H.; Wei, Y.; Zhan, Z.; et al. High-levels of resistance to quinolone and cephalosporin antibiotics in MDR-ACSSuT Salmonella enterica serovar Enteritidis mainly isolated from patients and foods in Shanghai, China. Int. J. Food Microbiol. 2018, 286, 190–196. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, Q.; Alali, W.Q.; Wang, J.; Meng, L.; Xiao, Y.; Yang, H.; Chen, S.; Cui, S.; Yang, B. Characterization of extended-spectrum beta-lactamases (ESBLs)-producing Salmonella in retail raw chicken carcasses. Int. J. Food Microbiol. 2017, 248, 72–81. [Google Scholar] [CrossRef]
- Usha, G.; Chunderika, M.; Prashini, M.; Willem, S.A.; Yusuf, E.S. Characterization of extended-spectrum beta-lactamases in Salmonella spp. at a tertiary hospital in Durban, South Africa. Diagn. Microbiol. Infect. Dis. 2008, 62, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, Y.; Shi, X.; Wang, S.; Ren, H.; Shen, Z.; Wang, Y.; Lin, J.; Wang, S. Rapid rise of the ESBL and mcr-1 genes in Escherichia coli of chicken origin in China, 2008-2014. Emerg. Microbes Infect. 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Harrois, D.; Breurec, S.; Seck, A.; Delaune, A.; Le Hello, S.; Pardos de la Gandara, M.; Sontag, L.; Perrier-Gros-Claude, J.D.; Sire, J.M.; Garin, B.; et al. Prevalence and characterization of extended-spectrum beta-lactamase-producing clinical Salmonella enterica isolates in Dakar, Senegal, from 1999 to 2009. Clin. Microbiol. Infect. 2014, 20, O109–O116. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, H.; Sakatsume, E.; Sekizuka, T.; Yokoyama, H.; Hamada, K.; Etoh, Y.; Carle, Y.; Mizumoto, S.; Hirai, S.; Matsui, M.; et al. Food Workers as a Reservoir of Extended-Spectrum-Cephalosporin-Resistant Salmonella Strains in Japan. Appl. Environ. Microbiol. 2020, 86, e00072-00020. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, Z.L.; Huang, X.Y.; Ma, Z.B.; Guo, Z.W.; Lv, L.C.; Xia, Y.B.; Zeng, L.; Song, Q.H.; Liu, J.H. Evolution and Comparative Genomics of F33:A-:B- Plasmids Carrying blaCTX-M-55 or blaCTX-M-65 in Escherichia coli and Klebsiella pneumoniae Isolated from Animals, Food Products, and Humans in China. mSphere 2018, 3, e00137-00118. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Wang, X.; Bai, X.; Ma, J.; Dang, R.; Xiong, Y.; Fanning, S.; Bai, L.; Yang, Z. Characterization of Five Escherichia coli Isolates Co-expressing ESBL and MCR-1 Resistance Mechanisms From Different Origins in China. Front. Microbiol. 2019, 10, 1994. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; CLSI Document M100-S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Yang, B.; Wang, Q.; Cui, S.; Wang, Y.; Shi, C.; Xia, X.; Xi, M.; Wang, X.; Shi, X.; Wang, D.; et al. Characterization of extended-spectrum beta-lactamases-producing Salmonella strains isolated from retail foods in Shaanxi and Henan Province, China. Food Microbiol. 2014, 42, 14–18. [Google Scholar] [CrossRef]
- Archambault, M.; Petrov, P.; Hendriksen, R.S.; Asseva, G.; Bangtrakulnonth, A.; Hasman, H.; Aarestrup, F.M. Molecular characterization and occurrence of extended-spectrum beta-lactamase resistance genes among Salmonella enterica serovar Corvallis from Thailand, Bulgaria, and Denmark. Microb. Drug Resist. 2006, 12, 192–198. [Google Scholar] [CrossRef]
- Kiratisin, P.; Apisarnthanarak, A.; Laesripa, C.; Saifon, P. Molecular characterization and epidemiology of extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates causing health care-associated infection in Thailand, where the CTX-M family is endemic. Antimicrob. Agents Chemother. 2008, 52, 2818–2824. [Google Scholar] [CrossRef]
- Qiao, J.; Alali, W.Q.; Liu, J.; Wang, Y.; Chen, S.; Cui, S.; Yang, B. Prevalence of Virulence Genes in Extended-Spectrum beta-lactamases (ESBLs)-Producing Salmonella in Retail Raw Chicken in China. J. Food Sci. 2018, 83, 1048–1052. [Google Scholar] [CrossRef]
- Ribot, E.M.; Fair, M.A.; Gautom, R.; Cameron, D.N.; Hunter, S.B.; Swaminathan, B.; Barrett, T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006, 3, 59–67. [Google Scholar] [CrossRef]
- Wang, J.; Sheng, H.; Xu, W.; Huang, J.; Meng, L.; Cao, C.; Zeng, J.; Meng, J.; Yang, B. Diversity of Serotype, Genotype, and Antibiotic Susceptibility of Salmonella Prevalent in Pickled Ready-to-Eat Meat. Front. Microbiol. 2019, 10, 2577. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.; Wu, X.; Wang, L.; Cai, J.; Chan, E.W.; Chen, S.; Zhang, R. Increased prevalence of carbapenem resistant Enterobacteriaceae in hospital setting due to cross-species transmission of the bla NDM-1 element and clonal spread of progenitor resistant strains. Front. Microbiol. 2015, 6, 595. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease ‘Burden of Illness Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Lupo, A.; Saras, E.; Madec, J.Y.; Haenni, M. Emergence of blaCTX-M-55 associated with fosA, rmtB and mcr gene variants in Escherichia coli from various animal species in France. J. Antimicrob. Chemother. 2018, 73, 867–872. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Jin, H.; Hu, J.; Yuan, Z.; Shi, W.; Yang, X.; Meng, J.; Xu, X. Laboratory monitoring of bacterial gastroenteric pathogens Salmonella and Shigella in Shanghai, China 2006–2012. Epidemiol. Infect. 2015, 143, 478–485. [Google Scholar] [CrossRef]
- China, National Health Commission. Antimicrobial Management and Bacterial Resistance in China. Available online: http://www.nhc.gov.cn/yzygj/s3594/201904/1b5a42f0e326487295b260c813da9b0e/files/c4328389c1b2462983fa94da9093cd05.pdf (accessed on 6 June 2020).
- Khosravani, M.; Soltan Dallal, M.M.; Norouzi, M. Phytochemical Composition and Anti-Efflux Pump Activity of Hydroalcoholic, Aqueous, and Hexane Extracts of Artemisia tournefortiana in Ciprofloxacin-Resistant Strains of Salmonella enterica Serotype Enteritidis. Iran. J. Public Health 2020, 49, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Usai, D.; Donadu, M.; Bua, A.; Molicotti, P.; Zanetti, S.; Piras, S.; Corona, P.; Ibba, R.; Carta, A. Enhancement of antimicrobial activity of pump inhibitors associating drugs. J. Infect. Dev. Ctries 2019, 13, 162–164. [Google Scholar] [CrossRef]
- Yamagishi, A.; Nakano, S.; Yamasaki, S.; Nishino, K. An efflux inhibitor of the MacAB pump in Salmonella enterica serovar Typhimurium. Microbiol. Immunol. 2020, 64, 182–188. [Google Scholar] [CrossRef]
- Donadu, M.G.; Trong Le, N.; Viet Ho, D.; Quoc Doan, T.; Tuan Le, A.; Raal, A.; Usai, M.; Marchetti, M.; Sanna, G.; Madeddu, S.; et al. Phytochemical Compositions and Biological Activities of Essential Oils from the Leaves, Rhizomes and Whole Plant of Hornstedtia bella Skornick. Antibiotics 2020, 9, 334. [Google Scholar] [CrossRef]
- Solarte, A.L.; Astorga, R.J.; de Aguiar, F.C.; De Frutos, C.; Barrero-Dominguez, B.; Huerta, B. Susceptibility Distribution to Essential Oils of Salmonella enterica Strains Involved in Animal and Public Health and Comparison of the Typhimurium and Enteritidis Serotypes. J. Med. Food 2018, 21, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Trong Le, N.; Viet Ho, D.; Quoc Doan, T.; Tuan Le, A.; Raal, A.; Usai, D.; Madeddu, S.; Marchetti, M.; Usai, M.; Rappelli, P.; et al. In vitro Antimicrobial Activity of Essential Oil Extracted from Leaves of Leoheo domatiophorus Chaowasku, D.T. Ngo and H.T. Le in Vietnam. Plants 2020, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, L.; Hu, Y.; Dottorini, T.; Fanning, S.; Xu, J.; Li, F. Epidemiological Study on Prevalence, Serovar Diversity, Multidrug Resistance, and CTX-M-Type Extended-Spectrum beta-Lactamases of Salmonella spp. from Patients with Diarrhea, Food of Animal Origin, and Pets in Several Provinces of China. Antimicrob. Agents Chemother. 2020, 64, e00092-00020. [Google Scholar] [CrossRef] [PubMed]
- Nadimpalli, M.; Fabre, L.; Yith, V.; Sem, N.; Gouali, M.; Delarocque-Astagneau, E.; Sreng, N.; Le Hello, S.; The BIRDY Study Group. CTX-M-55-type ESBL-producing Salmonella enterica are emerging among retail meats in Phnom Penh, Cambodia. J. Antimicrob. Chemother. 2019, 74, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Chen, Q.; Yu, X.; Li, Q.; Ding, B.; Yang, L.; Chen, C.; Qin, Z.; Parsons, C.; Zhang, X.; et al. High prevalence of extended-spectrum beta lactamases among Salmonella enterica Typhimurium isolates from pediatric patients with diarrhea in China. PLoS ONE 2011, 6, e16801. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chang, Y.J.; Fang, S.H.; Su, L.H.; Li, H.C.; Yang, H.P.; Yu, M.J.; Chiu, C.H. Emergence and Evolution of High-Level Cephalosporin-Resistant Salmonella Goldcoast in Northern Taiwan. Open Forum. Infect. Dis. 2019, 6, ofz447. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Ding, X.M.; Lin, X.L.; Sun, R.Y.; Lu, Y.W.; Cai, R.M.; Webber, M.A.; Ding, H.Z.; Jiang, H.X. The Emergence of Chromosomally Located bla CTX-M-55 in Salmonella from Foodborne Animals in China. Front. Microbiol. 2019, 10, 1268. [Google Scholar] [CrossRef]
- Adator, E.H.; Walker, M.; Narvaez-Bravo, C.; Zaheer, R.; Goji, N.; Cook, S.R.; Tymensen, L.; Hannon, S.J.; Church, D.; Booker, C.W.; et al. Whole Genome Sequencing Differentiates Presumptive Extended Spectrum Beta-Lactamase Producing Escherichia coli along Segments of the One Health Continuum. Microorganisms 2020, 8, 448. [Google Scholar] [CrossRef]
- Sanjit Singh, A.; Lekshmi, M.; Prakasan, S.; Nayak, B.B.; Kumar, S. Multiple Antibiotic-Resistant, Extended Spectrum-beta-Lactamase (ESBL)-Producing Enterobacteria in Fresh Seafood. Microorganisms 2017, 5, 53. [Google Scholar] [CrossRef]
- Morrissey, I.; Magnet, S.; Hawser, S.; Shapiro, S.; Knechtle, P. In Vitro Activity of Cefepime-Enmetazobactam against Gram-Negative Isolates Collected from U.S. and European Hospitals during 2014-2015. Antimicrob. Agents Chemother. 2019, 63, e00514-00519. [Google Scholar] [CrossRef]
- Luk-In, S.; Chatsuwan, T.; Pulsrikarn, C.; Bangtrakulnonth, A.; Rirerm, U.; Kulwichit, W. High prevalence of ceftriaxone resistance among invasive Salmonella enterica serotype Choleraesuis isolates in Thailand: The emergence and increase of CTX-M-55 in ciprofloxacin-resistant S. Choleraesuis isolates. Int. J. Med. Microbiol. 2018, 308, 447–453. [Google Scholar] [CrossRef] [PubMed]
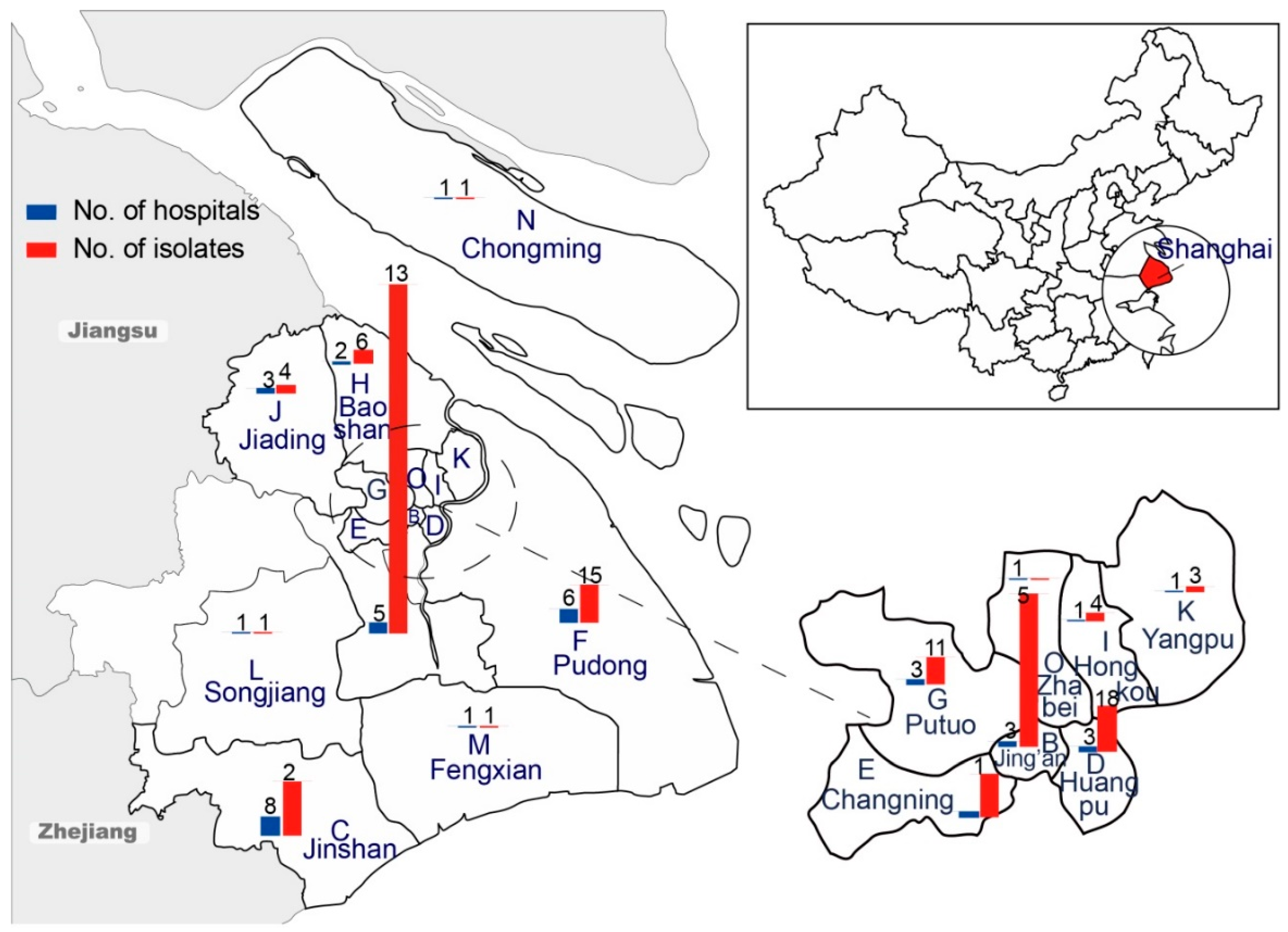
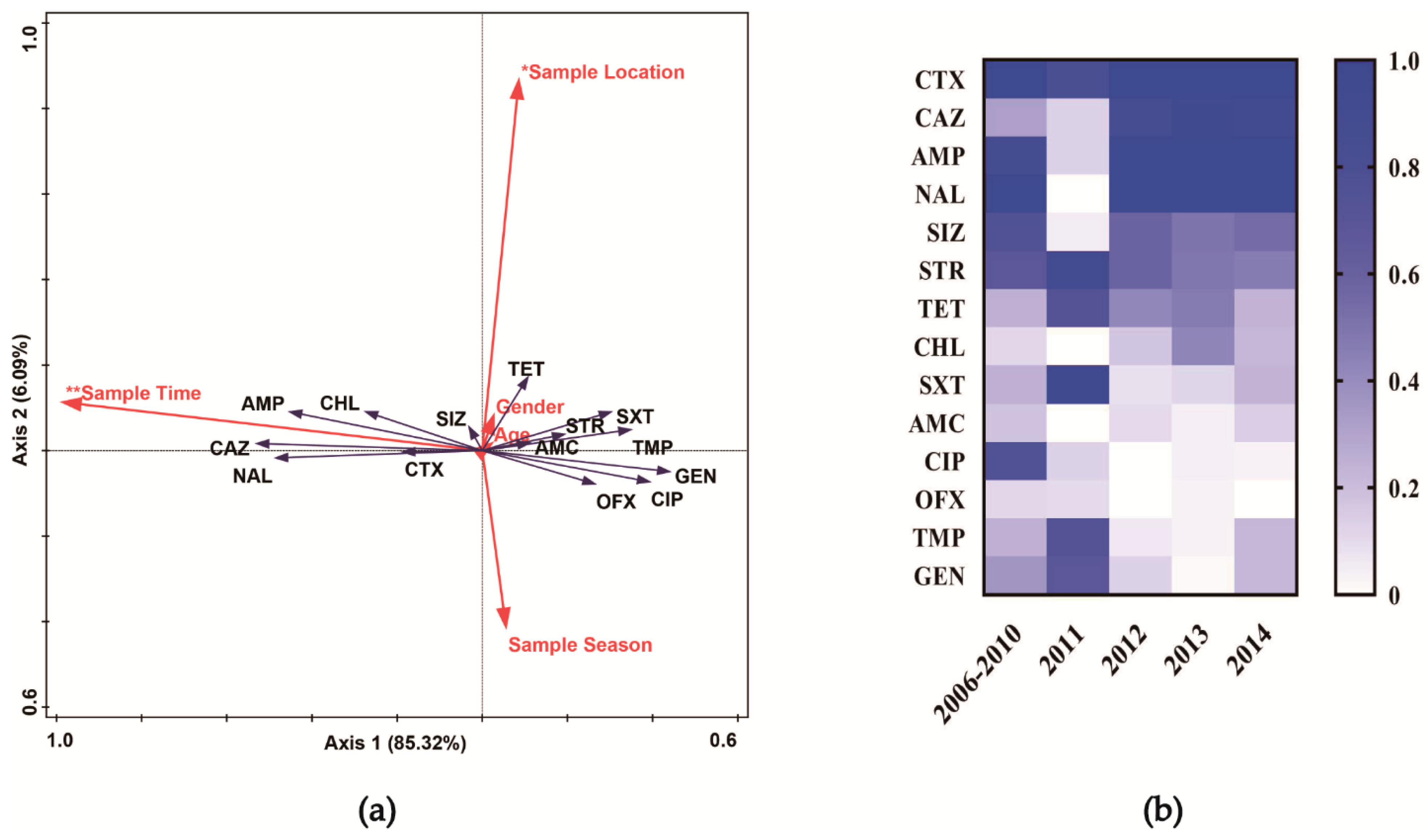
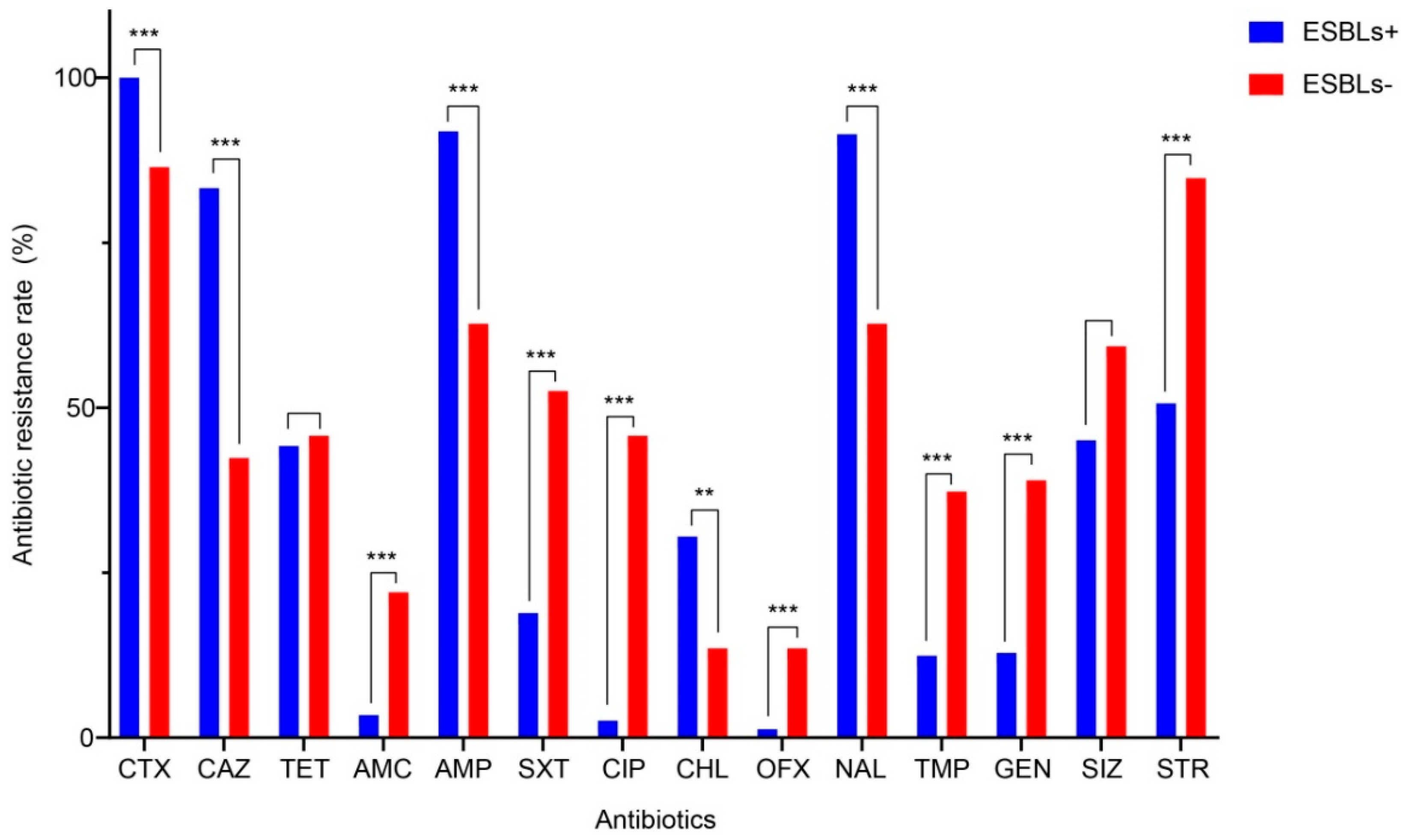
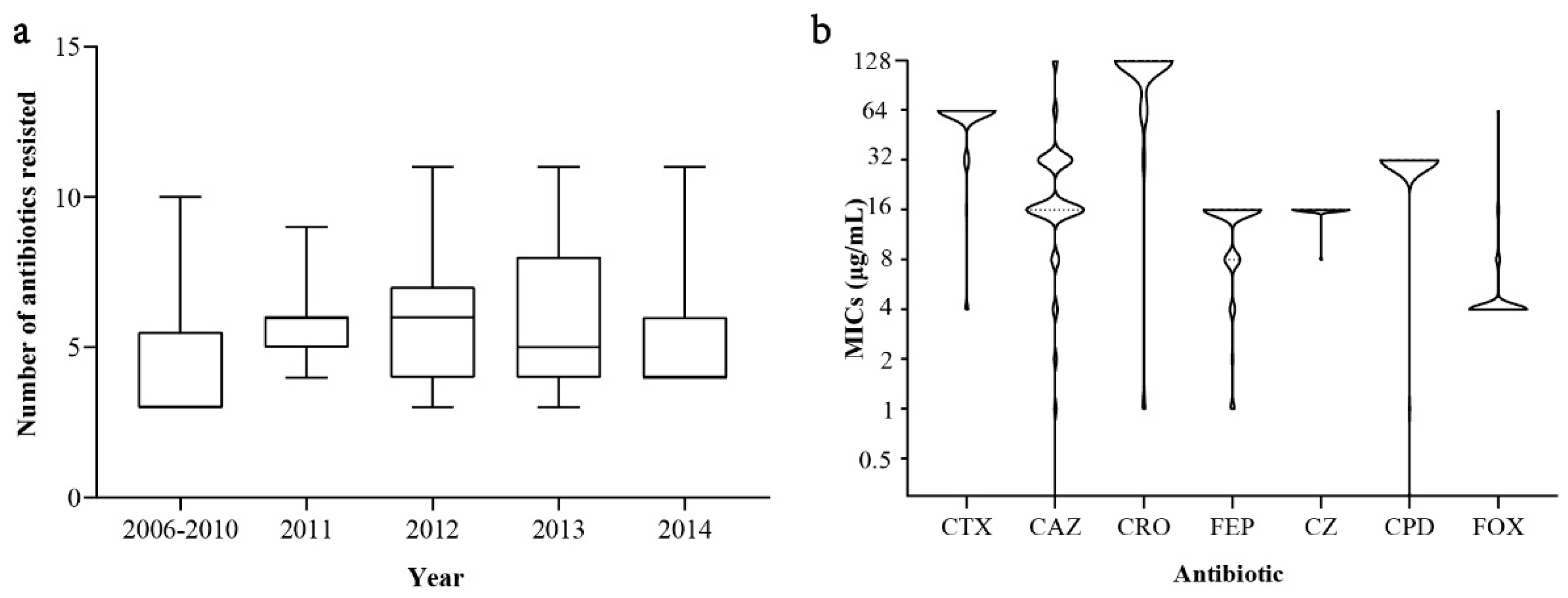
| Characteristic | No. (%) of ESBL-SE Isolates (n = 233) | No. (%) of Non-ESBL-SE Isolates (n = 59) | Unadjusted OR (95% CI) 1 |
|---|---|---|---|
| Year | |||
| 2006–2010 | 6 (2.6) | 22 (37.3) | 1.0 (ref) |
| 2011 | 19 (8.2) | 21 (35.6) | 3.3 (1.1–9.9) |
| 2012 | 46 (19.7) | 2 (3.4) | 84.3 (15.7–452.1) |
| 2013 | 131 (56.2) | 8 (13.6) | 60.0 (19.0–189.8) |
| 2014 | 31 (13.3) | 6 (10.2) | 18.9 (5.4–66.6) |
| Source | |||
| General outpatient | 33 (14.2) | 4 (6.8) | 1.0 (ref) |
| Intestinal outpatient | 132 (56.7) | 47 (79.7) | 0.3 (0.1–1.0) |
| Other outpatient | 45 (19.3) | 8 (13.6) | 0.7 (0.2–2.5) |
| Others | 23 (9.9) | 0 (0.0) | |
| District | |||
| Minhang district | 109 (46.8) | 22 (37.3) | 1.0 (ref) |
| Jing’an district | 53 (22.7) | 5 (8.5) | 2.1 (0.8–6.0) |
| Jinshan district | 14 (6.0) | 7 (11.9) | 0.4 (0.2–1.1) |
| Huangpu district | 8 (3.4) | 10 (16.9) | 0.2 (0.06–0.5) |
| Changning district | 9 (3.9) | 8 (13.6) | 0.2 (0.08–0.7) |
| Others | 40 (17.2) | 7 (11.9) | 1.2 (0.5–2.9) |
| Hospital | |||
| Public hospital | 79 (33.9) | 33 (55.9) | 1.0 (ref) |
| Pediatric hospital | 126 (54.1) | 25 (42.4) | 2.1 (1.2–3.9) |
| Community hospital | 14 (6.0) | 1 (1.7) | 5.9 (0.8–46.9) |
| Others | 14 (6.0) | 0 (0.0) | |
| ESBL-Encoding Gene | No. (%) of Isolates by Year | Total No. (%) (n = 233) | ||||
|---|---|---|---|---|---|---|
| 2006–2010 (n = 6) | 2011 (n = 19) | 2012 (n = 46) | 2013 (n = 131) | 2014 (n = 31) | ||
| blaCTX-M | ||||||
| blaCTX-M-3 | 0 (0.0) | 1 (5.3) | 2 (4.3) | 0 (0.0) | 0 (0.0) | 3 (1.3) |
| blaCTX-M-15 | 0 (0.0) | 0 (0.0) | 1 (2.2) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| blaCTX-M-55 | 0 (0.0) | 13 (68.4) | 28 (60.9) | 90 (68.7) | 21 (67.7) | 152 (65.2) |
| blaCTX-M-64 | 0 (0.0) | 0 (0.0) | 4 (8.7) | 0 (0.0) | 0 (0.0) | 4 (1.7) |
| blaCTX-M-79 | 0 (0.0) | 0 (0.0) | 1 (2.2) | 1 (0.8) | 0 (0.0) | 2 (0.9) |
| blaCTX-M-123 | 1 (16.7) | 1 (5.3) | 1 (2.2) | 0 (0.0) | 1 (3.2) | 4 (1.7) |
| Total | 1 (16.7) | 15 (78.9) | 37 (80.4) | 91 (69.5) | 22 (71.0) | 166 (71.2) |
| blaTEM | ||||||
| blaTEM-1 | 1 (16.7) | 12 (63.2) | 26 (56.5) | 14 (10.7) | 3 (9.7) | 56 (24.0) |
| blaTEM-214 | 0 (0.0) | 3 (15.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.3) |
| Total | 1 (16.7) | 15 (78.9) | 26 (56.5) | 14 (10.7) | 3 (9.7) | 59 (25.3) |
| Cephalosporin | blaCTX-M-55-Positive ESBL-SE (n = 152) | blaCTX-M-55-Negative ESBL-SE (n = 81) | p Value 1 | ||||
|---|---|---|---|---|---|---|---|
| MIC50 (μg/mL) | MIC90 (μg/mL) | Resistance (%) | MIC50 (μg/mL) | MIC90 (μg/mL) | Resistance (%) | ||
| Cefotaxime | 64 | 64 | 100.0 | 64 | 64 | 100.0 | - |
| Ceftazidime | 16 | 32 | 91.5 | 16 | 32 | 67.9 | 0.0 ** |
| Ceftriaxone | 128 | 128 | 98.0 | 128 | 128 | 92.6 | 0.04 * |
| Cefepime | 16 | 16 | 75.0 | 16 | 16 | 58.0 | 0.008 ** |
| Cefazolin | 16 | 16 | 98.0 | 16 | 16 | 92.6 | 0.04 * |
| Cefpodoxime | 32 | 32 | 98.0 | 32 | 32 | 92.6 | 0.04 * |
| Cefoxitin | 4 | 4 | 0.7 | 4 | 8 | 2.5 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, C.; Niu, Q.; Chen, J.; Xu, X.; Sheng, H.; Cui, S.; Liu, B.; Yang, B. Epidemiology and Characterization of CTX-M-55-Type Extended-Spectrum β-Lactamase-Producing Salmonella enterica Serovar Enteritidis Isolated from Patients in Shanghai, China. Microorganisms 2021, 9, 260. https://doi.org/10.3390/microorganisms9020260
Cao C, Niu Q, Chen J, Xu X, Sheng H, Cui S, Liu B, Yang B. Epidemiology and Characterization of CTX-M-55-Type Extended-Spectrum β-Lactamase-Producing Salmonella enterica Serovar Enteritidis Isolated from Patients in Shanghai, China. Microorganisms. 2021; 9(2):260. https://doi.org/10.3390/microorganisms9020260
Chicago/Turabian StyleCao, Chenyang, Qinya Niu, Jia Chen, Xuebin Xu, Huanjing Sheng, Shenghui Cui, Bin Liu, and Baowei Yang. 2021. "Epidemiology and Characterization of CTX-M-55-Type Extended-Spectrum β-Lactamase-Producing Salmonella enterica Serovar Enteritidis Isolated from Patients in Shanghai, China" Microorganisms 9, no. 2: 260. https://doi.org/10.3390/microorganisms9020260
APA StyleCao, C., Niu, Q., Chen, J., Xu, X., Sheng, H., Cui, S., Liu, B., & Yang, B. (2021). Epidemiology and Characterization of CTX-M-55-Type Extended-Spectrum β-Lactamase-Producing Salmonella enterica Serovar Enteritidis Isolated from Patients in Shanghai, China. Microorganisms, 9(2), 260. https://doi.org/10.3390/microorganisms9020260







