Microbial Response to Phytostabilization in Mining Impacted Soils Using Maize in Conjunction with Biochar and Compost
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description, Soil and Biochar Preparation
2.2. Experimental Design
2.3. Soil Analysis
2.4. Phospholipid Fatty Acid (PLFA) Analysis
2.5. Soil Enzyme Assays
2.6. Statistics
3. Results and Discussion
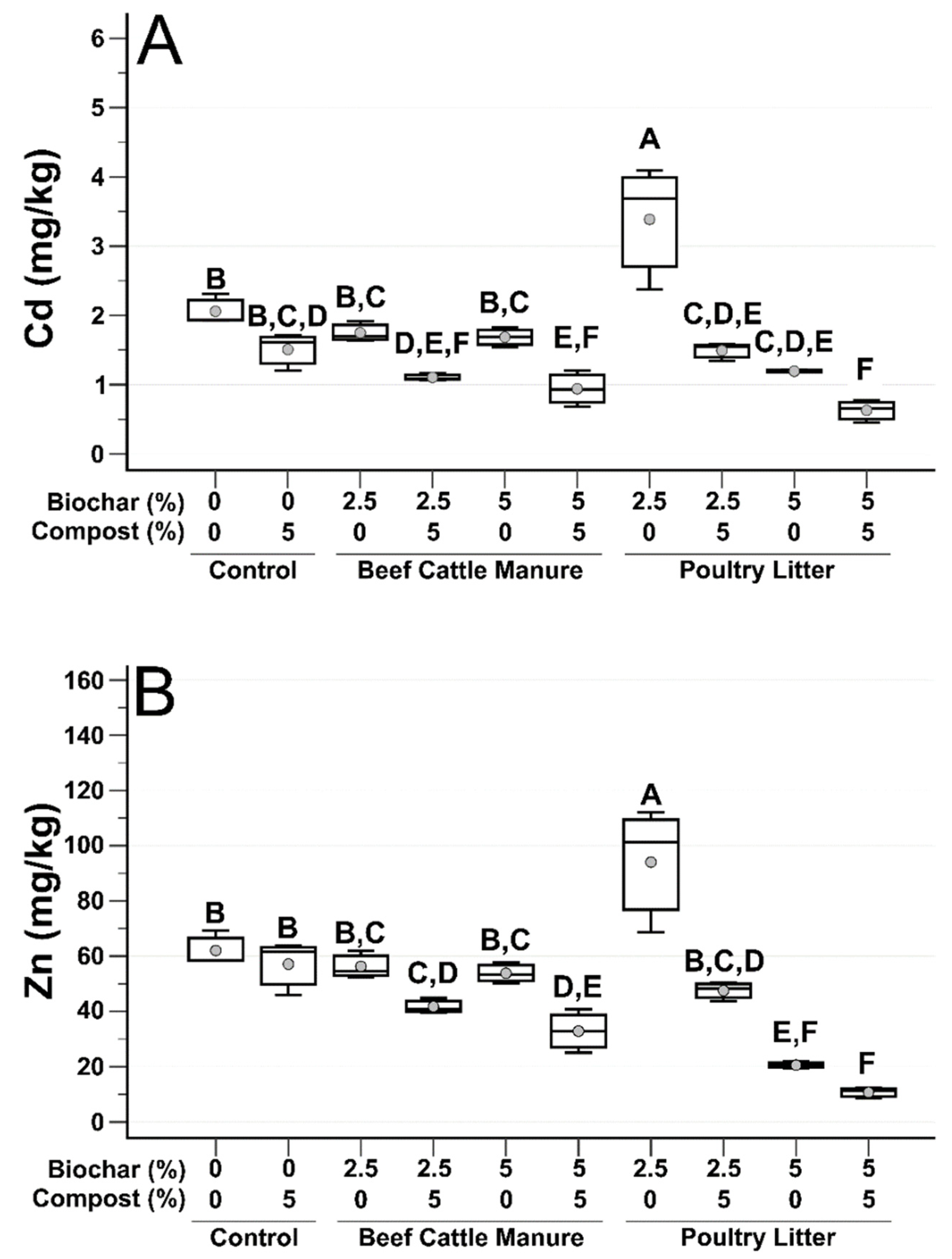
3.1. Post-Treatment Soil Analysis
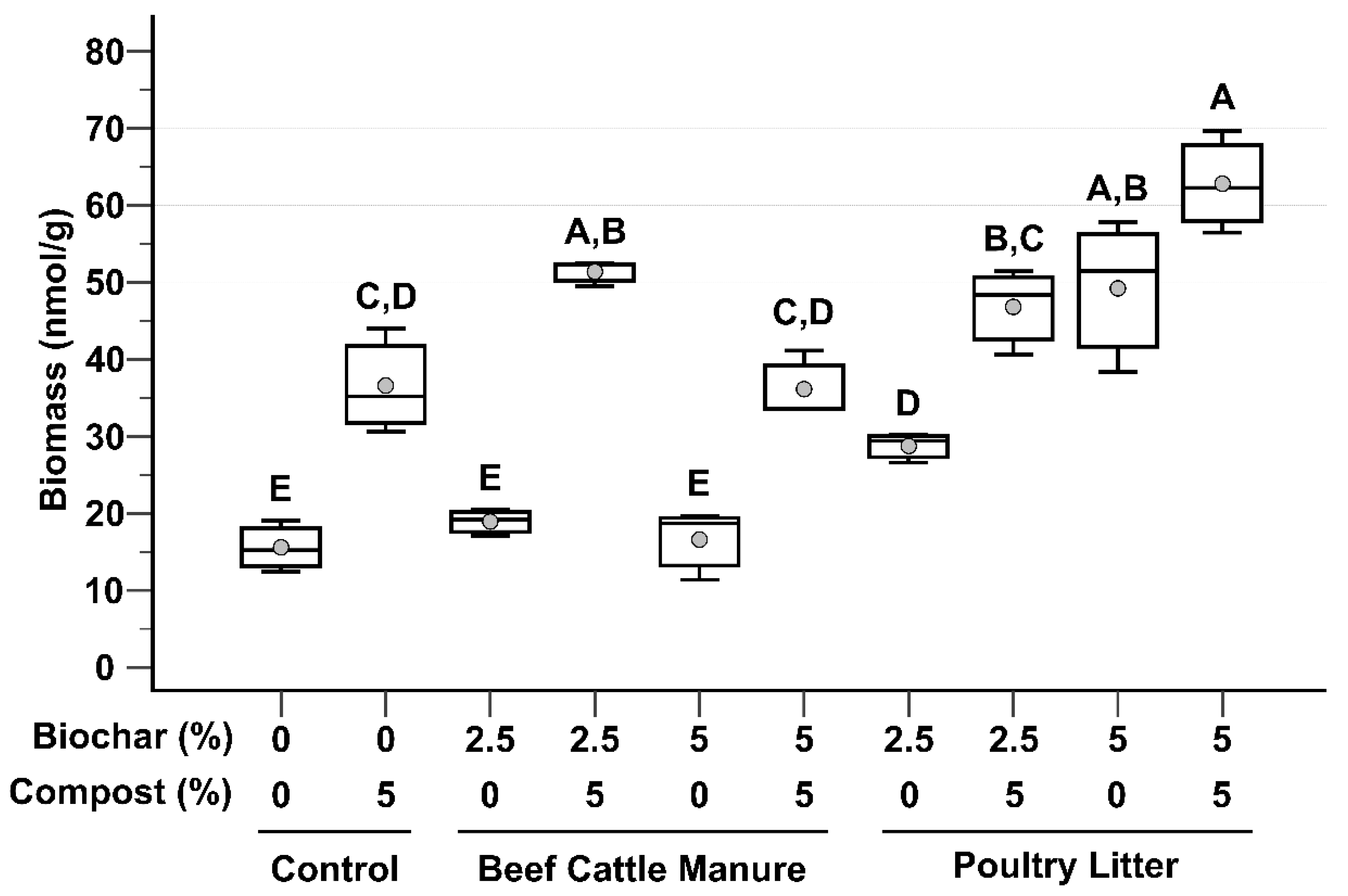
3.2. Microbial Biomass
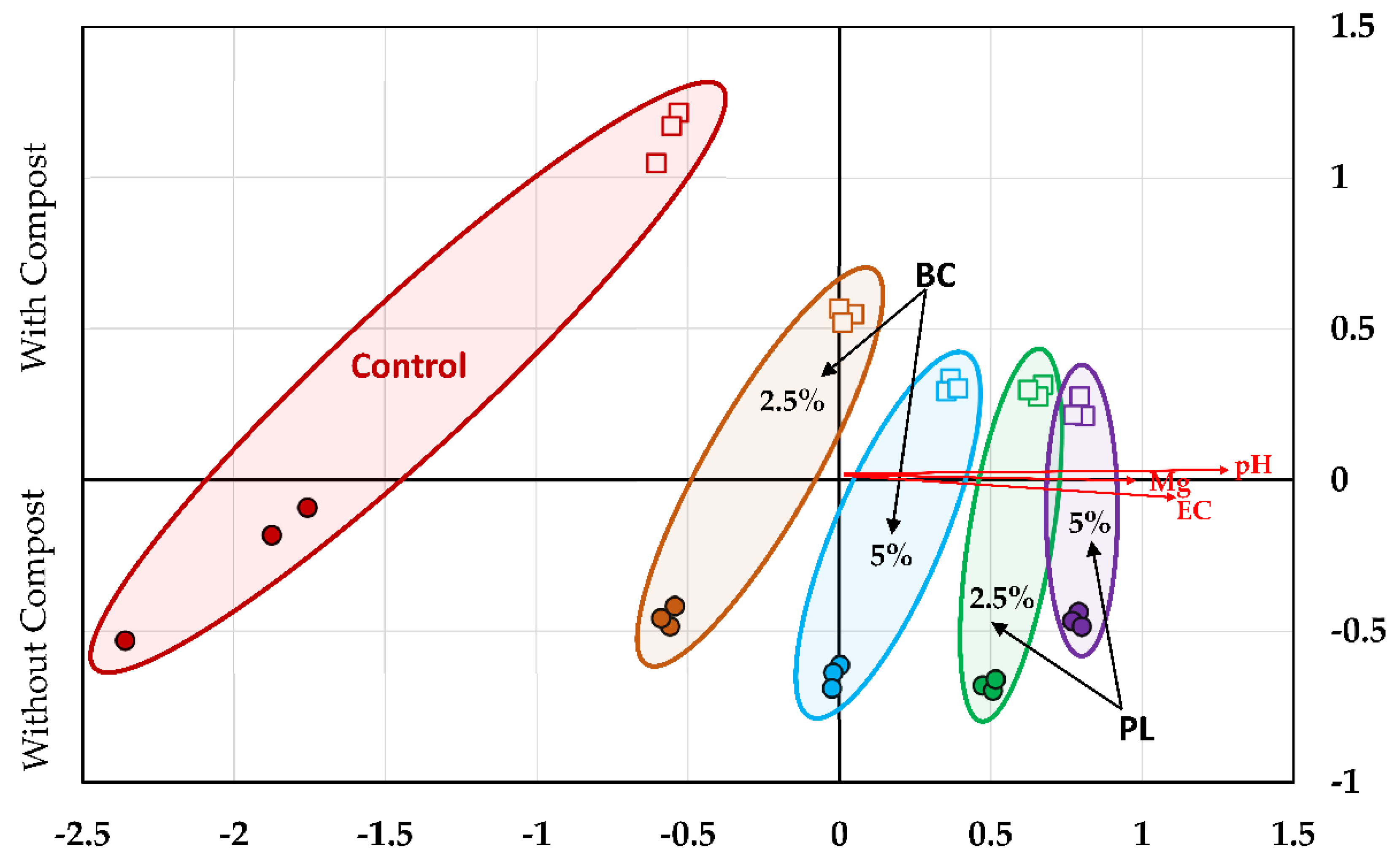
3.3. Soil Microbial Community Structure
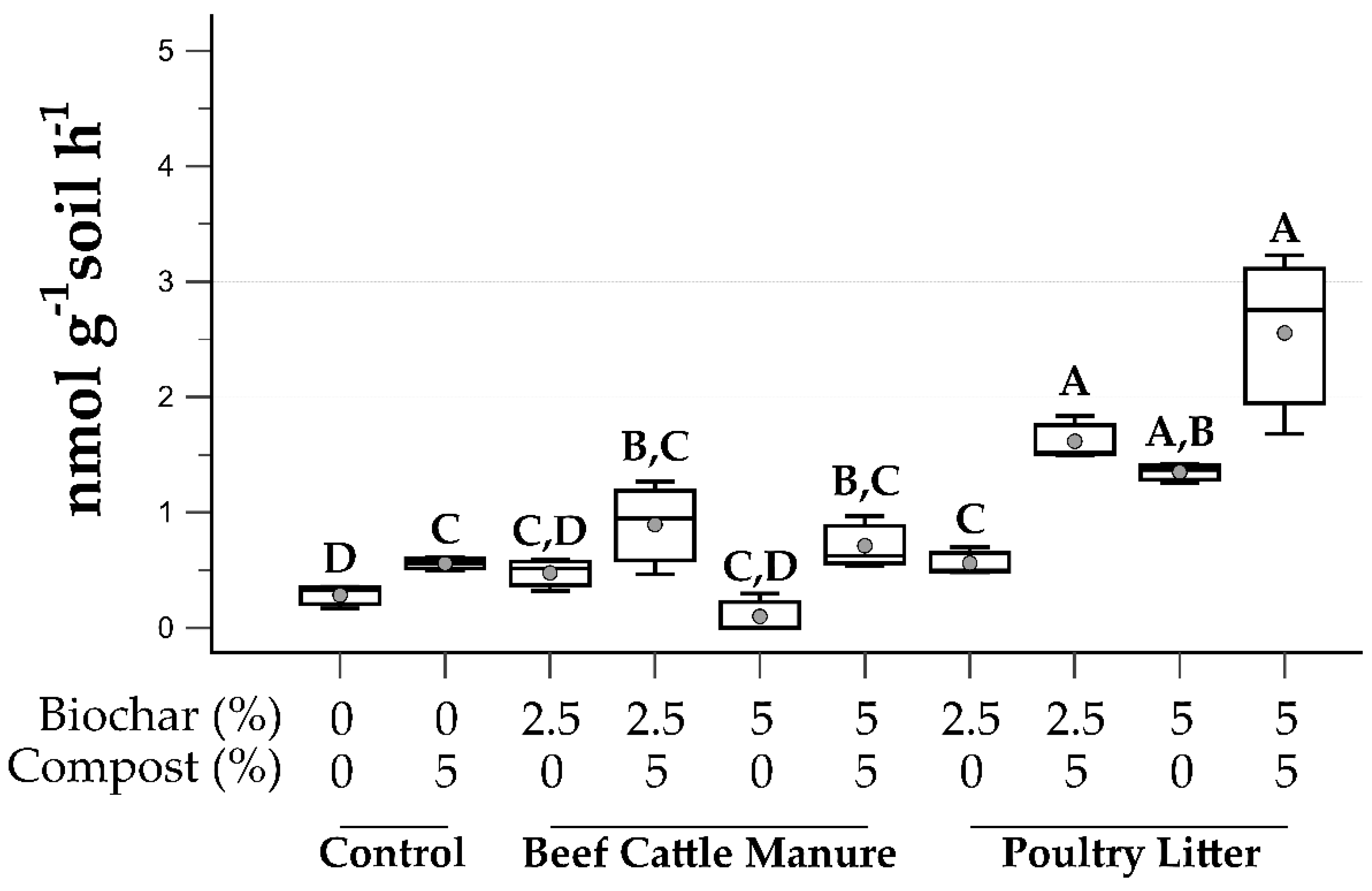
3.4. Soil Microbial Function
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Ghosh, D.; Maiti, S.K. Biochar assisted phytoremediation and biomass disposal in heavy metal contaminated mine soils: A review. Int. J. Phytoremediation 2020, 23, 559–576. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Li, Z.; Wang, Z.; Li, C.; Wei, H. Significance of soil microbe in microbial-assisted phytoremediation: An effective way to enhance phytoremediation of contaminated soil. Int. J. Environ. Sci. Technol. 2020, 17, 2477–2484. [Google Scholar] [CrossRef]
- Singh, S.; Kang, S.H.; Mulchandani, A.; Chen, W. Bioremediation: Environmental clean-up through pathway engineering. Curr. Opin. Biotechnol. 2008, 19, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Glick, B.R. The role of plant growth-promoting bacteria in metal phytoremediation. Adv. Microb. Physiol. 2017, 71, 97–132. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, A.A.M.; Jais, H.M. Interaction between arbuscular mycorrhiza and heavy metals in the rhizosphere and roots of Juniperus procera. Int. J. Agric. Biol. 2012, 14, 69–74. [Google Scholar]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Chander, K.; Brookes, P.; Harding, S. Microbial biomass dynamics following addition of metal-enriched sewage sludges to a sandy loam. Soil Biol. Biochem. 1995, 27, 1409–1421. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, J.; Lu, J.; Sun, Y. Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shen, Q.; Wang, L.; Qiu, G.; Shi, J.; Xu, J.; Brookes, P.C.; Liu, X. Effects of Cd, Cu, Zn and their combined action on microbial biomass and bacterial community structure. Environ. Pollut. 2018, 243, 510–518. [Google Scholar] [CrossRef]
- Gremion, F.; Chatzinotas, A.; Kaufmann, K.; Sigler, W.V.; Harms, H. Impacts of heavy metal contamination and phytoremediation on a microbial community during a twelve-month microcosm experiment. FEMS Microbiol. Ecol. 2004, 48, 273–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bünemann, E.K.; Schwenke, G.; Van Zwieten, L. Impact of agricultural inputs on soil organisms—A review. Soil Res. 2006, 44, 379–406. [Google Scholar] [CrossRef] [Green Version]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Fornes, F.; García-De-La-Fuente, R.; Belda, R.M.; Abad, M. ‘Alperujo’ compost amendment of contaminated calcareous and acidic soils: Effects on growth and trace element uptake by five Brassica species. Bioresour. Technol. 2009, 100, 3982–3990. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Cantrell, K.B.; Watts, D.W.; Busscher, W.J.; Johnson, M.G. Designing relevant biochars as soil amendments using lignocellulosic-based and manure-based feedstocks. J. Soils Sediments 2014, 14, 330–343. [Google Scholar] [CrossRef]
- Ducey, T.F.; Novak, J.M.; Johnson, M.G. Effects of biochar blends on microbial community composition in two Coastal Plain soils. Agriculture 2015, 5, 1060–1075. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.W.; Gutiérrez, M.; Gouzie, D.; McAliley, L.R. State of remediation and metal toxicity in the Tri-State Mining District, USA. Chemosphere 2016, 144, 1132–1141. [Google Scholar] [CrossRef]
- Novak, J.M.; Ippolito, J.A.; Watts, D.W.; Sigua, G.C.; Ducey, T.F.; Johnson, M.G. Biochar compost blends facilitate switchgrass growth in mine soils by reducing Cd and Zn bioavailability. Biochar 2019, 1, 97–114. [Google Scholar] [CrossRef] [Green Version]
- Ducey, T.F.; Novak, J.M.; Sigua, G.C.; Ippolito, J.A.; Rushmiller, H.C.; Watts, D.W.; Trippe, K.M.; Spokas, K.A.; Stone, K.C.; Johnson, M.G. Microbial response to designer biochar and compost treatments for mining impacted soils. Biochar 2021, 3, 299–314. [Google Scholar] [CrossRef]
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Ippolito, J.A.; Ducey, T.F.; Johnson, M.G.; Spokas, K.A. Phytostabilization of Zn and Cd in mine soil using corn in combination with biochars and manure-based compost. Environments 2019, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Novak, J.M.; Ippolito, J.A.; Ducey, T.; Watts, D.W.; Spokas, K.A.; Trippe, K.M.; Sigua, G.C.; Johnson, M.G. Remediation of an acidic mine spoil: Miscanthus biochar and lime amendment affects metal availability, plant growth, and soil enzyme activity. Chemosphere 2018, 205, 709–718. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 14. [Google Scholar]
- Bai, S.H.; Trueman, S.J.; Nevenimo, T.; Hannet, G.; Bapiwai, P.; Poienou, M.; Wallace, H.M. Effects of shade-tree species and spacing on soil and leaf nutrient concentrations in cocoa plantations at 8 years after establishment. Agric. Ecosyst. Environ. 2017, 246, 134–143. [Google Scholar] [CrossRef]
- Veum, K.S.; Lorenz, T.; Kremer, R.J. Phospholipid fatty acid profiles of soils under variable handling and storage conditions. Agron. J. 2019, 111, 1090–1096. [Google Scholar] [CrossRef] [Green Version]
- Buyer, J.S.; Sasser, M. High throughput phospholipid fatty acid analysis of soils. Appl. Soil Ecol. 2012, 61, 127–130. [Google Scholar] [CrossRef]
- Drijber, R.; Doran, J.; Parkhurst, A.; Lyon, D. Changes in soil microbial community structure with tillage under long-term wheat-fallow management. Soil Biol. Biochem. 2000, 32, 1419–1430. [Google Scholar] [CrossRef] [Green Version]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur. J. Soil Sci. 2013, 65, 28–39. [Google Scholar] [CrossRef]
- Deng, S.; Popova, I.E.; Dick, L.; Dick, R. Bench scale and microplate format assay of soil enzyme activities using spectroscopic and fluorometric approaches. Appl. Soil Ecol. 2013, 64, 84–90. [Google Scholar] [CrossRef]
- Deng, S.; Kang, H.; Freeman, C. Microplate Fluorimetric Assay of Soil Enzymes. In SSSA Book Series; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 9, pp. 311–318. [Google Scholar]
- Ghallab, A.; Usman, A.R.A. Effect of sodium chloride-induced salinity on phyto-availability and speciation of Cd in soil solution. Water Air Soil Pollut. 2007, 185, 43–51. [Google Scholar] [CrossRef]
- Jahiruddin, M.; Livesey, N.; Cresser, M. Observations on the effect of soil pH upon zinc absorption by soils. Commun. Soil Sci. Plant Anal. 1985, 16, 909–922. [Google Scholar] [CrossRef]
- Clemente, R.; Ángeles, E.; Bernal, M.P. Heavy metals fractionation and organic matter mineralisation in contaminated calcareous soil amended with organic materials. Bioresour. Technol. 2006, 97, 1894–1901. [Google Scholar] [CrossRef]
- Irfan, M.; Hussain, Q.; Khan, K.S.; Akmal, M.; Ijaz, S.S.; Hayat, R.; Khalid, A.; Azeem, M.; Rashid, M. Response of soil microbial biomass and enzymatic activity to biochar amendment in the organic carbon deficient arid soil: A 2-year field study. Arab. J. Geosci. 2019, 12, 95. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Gabhane, J.W.; Bhange, V.P.; Patil, P.; Bankar, S.T.; Kumar, S. Recent trends in biochar production methods and its application as a soil health conditioner: A review. SN Appl. Sci. 2020, 2, 1307. [Google Scholar] [CrossRef]
- Kumar, S.; Masto, R.E.; Ram, L.C.; Sarkar, P.; George, J.; Selvi, V.A. Biochar preparation from Parthenium hysterophorus and its potential use in soil application. Ecol. Eng. 2013, 55, 67–72. [Google Scholar] [CrossRef]
- Masto, R.E.; Kumar, S.; Rout, T.; Sarkar, P.; George, J.; Ram, L. Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Ghosh, D.; Maiti, S.K. Can biochar reclaim coal mine spoil? J. Environ. Manag. 2020, 272, 111097. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Sparling, G.P. Soil microbial biomass activity and nutrient cycling, as indicators of soil health. In Biological Indicators of Soil Health; Panchurts, C.E., Doube, B.M., Gupta, V.S.R., Eds.; Cab International: New York, NY, USA, 1997; pp. 97–119. [Google Scholar]
- Zhalnina, K.; Dias, R.; De Quadros, P.D.; Davis-Richardson, A.; Camargo, F.A.O.; Clark, I.M.; McGrath, S.P.; Hirsch, P.R.; Triplett, E.W. Soil pH determines microbial diversity and composition in the Park Grass Experiment. Microb. Ecol. 2015, 69, 395–406. [Google Scholar] [CrossRef]
- Kashem, M.A.; Singh, B.R. Metal availability in contaminated soils: I. Effects of flooding and organic matter on changes in Eh, pH and solubility of Cd, Ni and Zn. Nutr. Cycl. Agroecosys. 2001, 61, 247–255. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.-W.; Su, Z.-C.; Zhang, C.-G. Soil microbial characteristics under long-term heavy metal stress: A case study in Zhangshi Wastewater Irrigation Area, Shenyang. Pedosphere 2008, 18, 1–10. [Google Scholar] [CrossRef]
- Johnson, M.; Lee, K.; Scow, K. DNA fingerprinting reveals links among agricultural crops, soil properties, and the composition of soil microbial communities. Geoderma 2003, 114, 279–303. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. BioMetals 2002, 15, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cheng, X.; Li, J.; Dong, Y. Magnesium and nitrogen drive soil bacterial community structure under long-term apple orchard cultivation systems. Appl. Soil Ecol. 2021, 167, 104103. [Google Scholar] [CrossRef]
- Souza, R.C.; Cantão, M.E.; Nogueira, M.A.; Vasconcelos, A.T.; Hungria, M. Outstanding impact of soil tillage on the abundance of soil hydrolases revealed by a metagenomic approach. Braz. J. Microbiol. 2018, 49, 723–730. [Google Scholar] [CrossRef]
- Lin, S.; Wang, S.; Si, Y.; Yang, W.; Zhu, S.; Ni, W. Variations in eco-enzymatic stoichiometric and microbial characteristics in paddy soil as affected by long-term integrated organic-inorganic fertilization. PLoS ONE 2017, 12, e0189908. [Google Scholar] [CrossRef] [PubMed]
- Olander, L.P.; Vitousek, P.M. Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 2000, 49, 175–190. [Google Scholar] [CrossRef]
- Lagomarsino, A.; De Meo, I.; Agnelli, A.E.; Paletto, A.; Mazza, G.; Bianchetto, E.; Pastorelli, R. Decomposition of black pine (Pinus nigra JF Arnold) deadwood and its impact on forest soil components. Sci. Tot. Environ. 2021, 754. [Google Scholar] [CrossRef]
- Steven, B.; Gallegos-Graves, L.V.; Belnap, J.; Kuske, C.R. Dryland soil microbial communities display spatial biogeographic patterns associated with soil depth and soil parent material. FEMS Microbiol. Ecol. 2013, 86, 101–113. [Google Scholar] [CrossRef]
- Acea, M.; Carballas, T. Principal components analysis of the soil microbial population of humid zone of Galicia (Spain). Soil Biol. Biochem. 1990, 22, 749–759. [Google Scholar] [CrossRef]
- Van Leeuwen, J.P.; Djukic, I.; Bloem, J.; Lehtinen, T.; Hemerik, L.; De Ruiter, P.; Lair, G. Effects of land use on soil microbial biomass, activity and community structure at different soil depths in the Danube floodplain. Eur. J. Soil Biol. 2017, 79, 14–20. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J.B. The variation of soil microbial respiration with depth in relation to soil carbon composition. Plant Soil 2005, 268, 243–253. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar] [CrossRef]
- Stone, M.; DeForest, J.; Plante, A. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Crump, B.C.; Amaral-Zettler, L.A.; Kling, G. Microbial diversity in arctic freshwaters is structured by inoculation of microbes from soils. ISME J. 2012, 6, 1629–1639. [Google Scholar] [CrossRef]
- Mony, C.; Vandenkoornhuyse, P.; Bohannan, B.J.M.; Peay, K.; Leibold, M.A. A landscape of opportunities for microbial ecology research. Front. Microbiol. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Fawole, O.B. Microbial inoculants-assisted phytoremediation for sustainable soil management. In Immobilization of Enzymes and Cells; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–17. [Google Scholar]
- Trippe, K.M.; Manning, V.A.; Reardon, C.L.; Klein, A.M.; Weidman, C.; Ducey, T.F.; Novak, J.M.; Watts, D.W.; Rushmiller, H.; Spokas, K.A.; et al. Phytostabilization of acidic mine tailings with biochar, biosolids, lime, and locally-sourced microbial inoculum: Do amendment mixtures influence plant growth, tailing chemistry, and microbial composition? Appl. Soil Ecol. 2021, 165, 103962. [Google Scholar] [CrossRef]

| Biochar 1 | Biochar Rate (%) | Compost Rate (%) | pH 2 | EC 2,3 (mS/m) |
|---|---|---|---|---|
| None | 0 | 0 | 4.4 ± 0.1 g | 269.7 ± 55.2 e |
| None | 0 | 5 | 5.1 ± 0.0 e,f | 397.9 ± 85.9 d |
| BC | 2.5 | 0 | 5.1 ± 0.1f | 262.2 ± 39.9 e |
| BC | 2.5 | 5 | 5.3 ± 0.1 d,e | 473.9 ± 35.1 c,d |
| BC | 5 | 0 | 5.3 ± 0.2 d | 437.1 ± 24.6 c,d |
| BC | 5 | 5 | 5.9 ± 0.1 c | 559.6 ± 72.4 c |
| PL | 2.5 | 0 | 5.5 ± 0.2 d | 1425.7 ± 281.7 b |
| PL | 2.5 | 5 | 5.9 ± 0.0 c | 1577.0 ± 419.4 b |
| PL | 5 | 0 | 6.3 ± 0.0 b | 2767.0 ± 600.5 a |
| PL | 5 | 5 | 6.6 ± 0.0 a | 2765.7 ± 322.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ducey, T.F.; Sigua, G.C.; Novak, J.M.; Ippolito, J.A.; Spokas, K.A.; Johnson, M.G. Microbial Response to Phytostabilization in Mining Impacted Soils Using Maize in Conjunction with Biochar and Compost. Microorganisms 2021, 9, 2545. https://doi.org/10.3390/microorganisms9122545
Ducey TF, Sigua GC, Novak JM, Ippolito JA, Spokas KA, Johnson MG. Microbial Response to Phytostabilization in Mining Impacted Soils Using Maize in Conjunction with Biochar and Compost. Microorganisms. 2021; 9(12):2545. https://doi.org/10.3390/microorganisms9122545
Chicago/Turabian StyleDucey, Thomas F., Gilbert C. Sigua, Jeffrey M. Novak, James A. Ippolito, Kurt A. Spokas, and Mark G. Johnson. 2021. "Microbial Response to Phytostabilization in Mining Impacted Soils Using Maize in Conjunction with Biochar and Compost" Microorganisms 9, no. 12: 2545. https://doi.org/10.3390/microorganisms9122545
APA StyleDucey, T. F., Sigua, G. C., Novak, J. M., Ippolito, J. A., Spokas, K. A., & Johnson, M. G. (2021). Microbial Response to Phytostabilization in Mining Impacted Soils Using Maize in Conjunction with Biochar and Compost. Microorganisms, 9(12), 2545. https://doi.org/10.3390/microorganisms9122545








