Impact of O-Acetylation on S. flexneri 1b and 2a O-Antigen Immunogenicity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Generation of Mutants
2.2. GMMA Production and Characterization
2.3. Mouse Studies
2.4. Statistical Analysis
3. Results
3.1. Generation of Differently O-Acetylated S. flexneri 1b and 2a Strains and Characterization of the Resulting GMMA
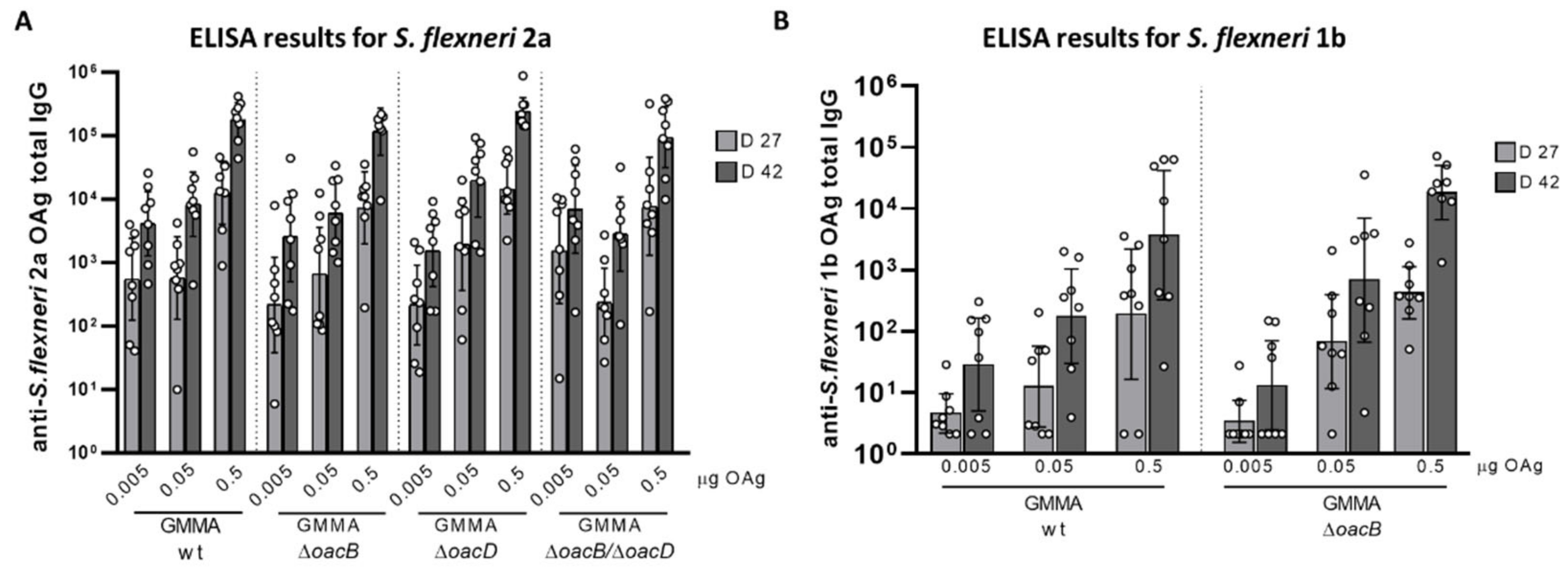
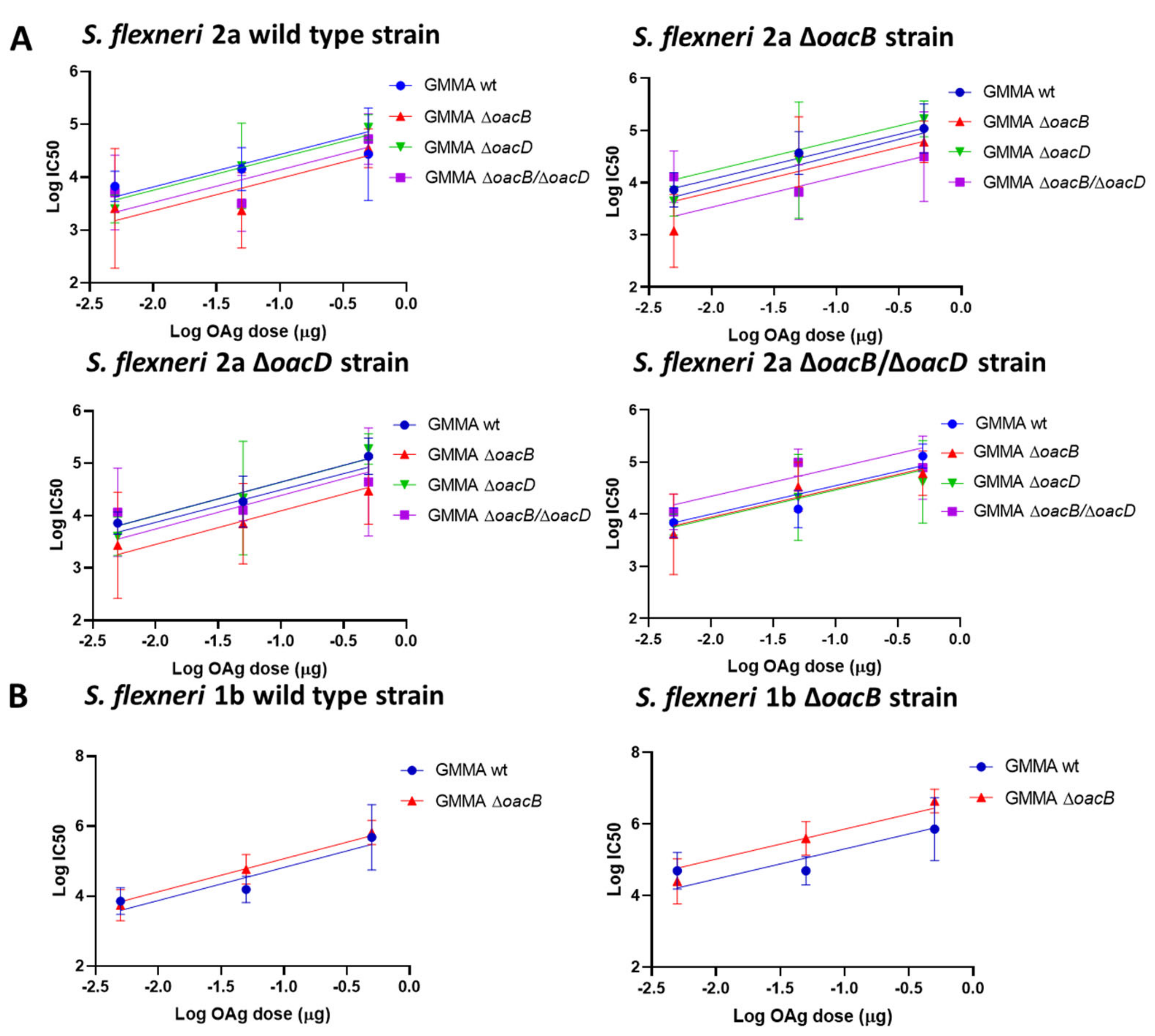
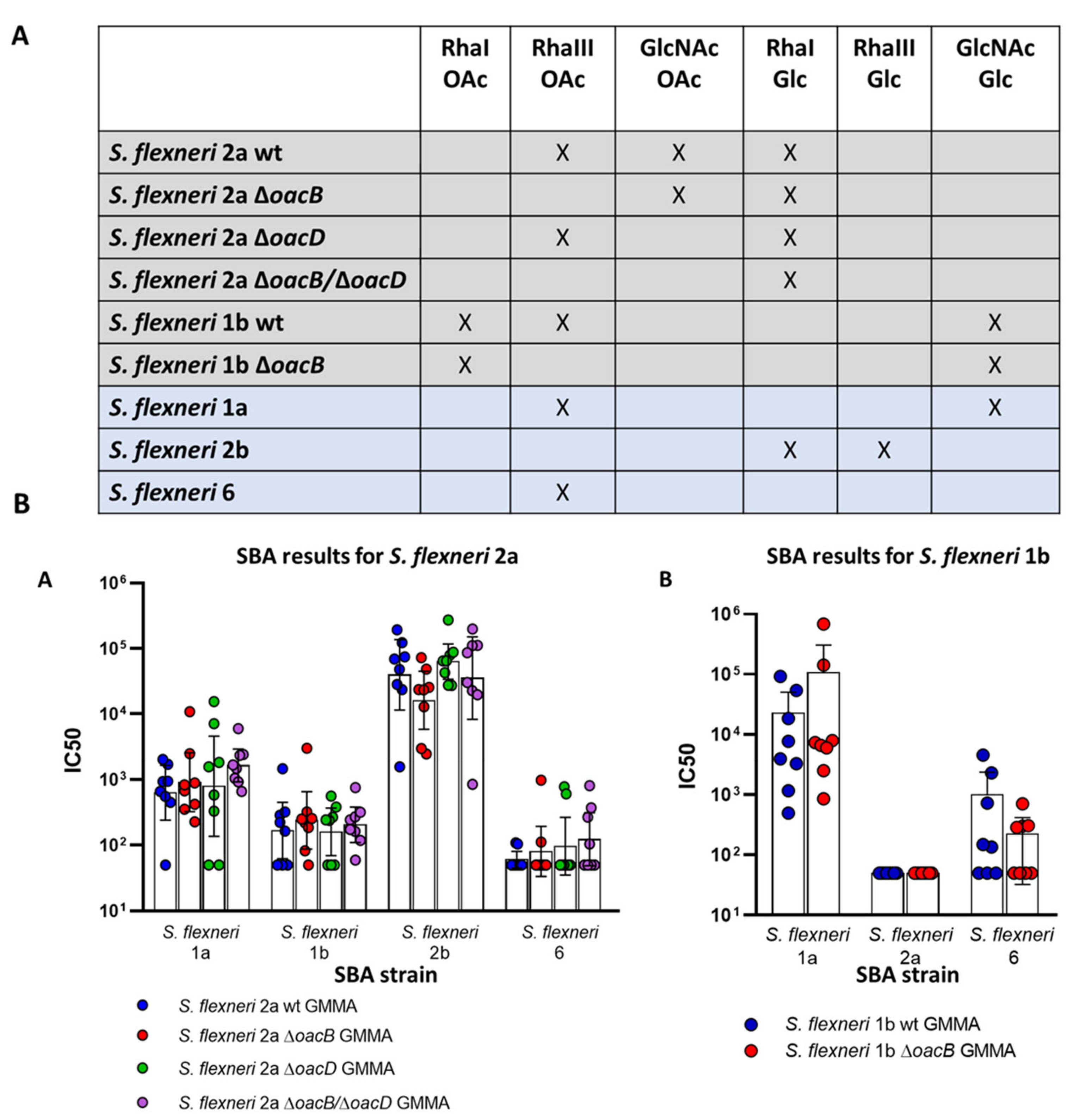
3.2. Immunogenicity Study in Mice with OAg Differing for the OAc Pattern
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef]
- Liu, B.; Knirel, Y.A.; Feng, L.; Perepelov, A.V.; Senchenkova, S.N.; Wang, Q.; Reeves, P.R.; Wang, L. Structure and genetics of Shigella O antigens. FEMS Microbiol. Rev. 2008, 32, 627–653. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Puzari, M.; Sharma, M.; Chetia, P. Emergence of antibiotic resistant Shigella species: A matter of concern. J. Infect. Public Health 2018, 11, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Shrivastava, P.S.; Ramasamy, J. Responding to the challenge of antibiotic resistance: World Health Organization. J. Res. Med Sci. 2018, 23, 21. [Google Scholar] [CrossRef]
- Camacho, A.I.; Irache, J.M.; Gamazo, C. Recent progress towards development of a Shigella vaccine. Expert Rev. Vaccines 2013, 12, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Kaminski, R.; Porter, C.; Choy, R.; White, J.; Fleckenstein, J.; Cassels, F.; Bourgeois, L. Vaccines for protecting infants from bacterial causes of diarrheal disease. Microorganisms 2021, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Wierzba, T.; Walker, R.I. Status of vaccine research and development for Shigella. Vaccine 2016, 34, 2887–2894. [Google Scholar] [CrossRef]
- West, N.P.; Sansonetti, P.; Mounier, J.; Exley, R.M.; Parsot, C.; Guadagnini, S.; Prévost, M.-C.; Prochnicka-Chalufour, A.; Delepierre, M.; Tanguy, M.; et al. Optimization of virulence functions through glucosylation of shigella LPS. Science 2005, 307, 1313–1317. [Google Scholar] [CrossRef]
- Morona, R.; Daniels, C.; Bosch, L.V.D. Genetic modulation of Shigella flexneri 2a lipopolysaccharide O antigen modal chain length reveals that it has been optimized for virulence. Microbiology 2003, 149, 925–939. [Google Scholar] [CrossRef]
- Cohen, D.; Green, M.S.; Block, C.; Rouach, T.; Ofek, I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J. Infect. Dis. 1988, 157, 1068–1071. [Google Scholar] [CrossRef]
- Cohen, D.; Orr, N.; Robin, G.; Slepon, R.; Ashkenazi, S.; Shemer, J. Detection of antibodies to Shigella lipopolysaccharide in urine after natural Shigella infection or vaccination. Clin. Diagn. Lab. Immunol. 1996, 3, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Perepelov, A.V.; Shekht, M.E.; Liu, B.; Shevelev, S.D.; Ledov, V.A.; Senchenkova, S.N.; L’Vov, V.L.; Shashkov, A.S.; Feng, L.; Aparin, P.G.; et al. Shigella flexneri O-antigens revisited: Final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol. Med. Microbiol. 2012, 66, 201–210. [Google Scholar] [CrossRef]
- Lindberg, A.A.; Karnell, A.; Weintraub, A. The lipopolysaccharide of shigella bacteria as a virulence factor. Rev. Infect. Dis. 1991, 13, S279–S284. [Google Scholar] [CrossRef] [PubMed]
- Kenne, L.; Lindberg, B.; Petersson, K. Basic structure of the oligosaccharide repeating-unit of the Shigella flexneri O-antigens. Carbohydr. Res. 1977, 56, 363–370. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Sun, Q.; Senchenkova, S.N.; Perepelov, A.V.; Shashkov, A.S.; Xu, J. O-antigen modifications providing antigenic diversity of Shigella flexneri and underlying genetic mechanisms. Biochemistry (Mosc.) 2015, 80, 901–914. [Google Scholar] [CrossRef]
- Allison, G.E.; Verma, N.K. Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri. Trends Microbiol. 2000, 8, 17–23. [Google Scholar] [CrossRef]
- Wang, J.; Knirel, Y.A.; Lan, R.; Senchenkova, S.N.; Luo, X.; Perepelov, A.V.; Wang, Y.; Shashkov, A.S.; Xu, J.; Sun, Q. Identification of an O-acyltransferase gene (oacB) that mediates 3- and 4-O-acetylation of rhamnose III in Shigella flexneri O antigens. J. Bacteriol. 2014, 196, 1525–1531. [Google Scholar] [CrossRef]
- Sun, Q.; Knirel, Y.A.; Wang, J.; Luo, X.; Senchenkova, S.N.; Lan, R.; Shashkov, A.S.; Xu, J. Serotype-converting bacteriophage SfII encodes an acyltransferase protein that mediates 6-O-acetylation of GlcNAc in Shigella flexneri O-antigens, conferring on the host a novel O-antigen epitope. J. Bacteriol. 2014, 196, 3656–3666. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Wang, J.; Luo, X.; Senchenkova, S.N.; Lan, R.; Shpirt, A.M.; Du, P.; Shashkov, A.S.; Zhang, N.; Xu, J.; et al. Genetic and structural identification of an O-acyltransferase gene (oacC) responsible for the 3/4-O-acetylation on rhamnose III in Shigella flexneri serotype 6. BMC Microbiol. 2014, 14, 266. [Google Scholar] [CrossRef] [PubMed]
- Barel, L.A.; Mulard, L.A. Classical and novel strategies to develop a Shigella glycoconjugate vaccine: From concept to efficacy in human. Hum. Vaccines Immunother. 2019, 15, 1338–1356. [Google Scholar] [CrossRef] [PubMed]
- Farzam, N.; Ramon-Saraf, R.; Banet-Levi, Y.; Lerner-Geva, L.; Ashkenazi, S.; Kubler-Kielb, J.; Vinogradov, E.; Robbins, J.B.; Schneerson, R. Vaccination with Shigella flexneri 2a conjugate induces type 2a and cross-reactive type 6 antibodies in humans but not in mice. Vaccine 2017, 35, 4990–4996. [Google Scholar] [CrossRef]
- Scorza, F.B.; Colucci, A.M.; Maggiore, L.; Sanzone, S.; Rossi, O.; Ferlenghi, I.; Pesce, I.; Caboni, M.; Norais, N.; Di Cioccio, V.; et al. High Yield Production Process for Shigella Outer Membrane Particles. PLoS ONE 2012, 7, e35616. [Google Scholar] [CrossRef]
- Rossi, O.; Pesce, I.; Giannelli, C.; D’Oro, U.; Saul, A.; Gerke, C. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: Relative activation of TLR4 and TLR2 pathways in different mutants. J. Biol. Chem. 2014, 289, 24922–24935. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Lewis, D.J.; Anemona, A.; Loulergue, P.; Leahy, J.; Sciré, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety profile and immunologic responses of a novel vaccine against shigella sonnei administered intramuscularly, intradermally and intranasally: Results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. EBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef]
- Launay, O.; Ndiaye, A.G.W.; Conti, V.; Loulergue, P.; Sciré, A.S.; Landre, A.M.; Ferruzzi, P.; Nedjaai, N.; Schütte, L.D.; Auerbach, J.; et al. Booster vaccination with GVGH Shigella sonnei 1790GAHB GMMA vaccine compared to single vaccination in unvaccinated healthy European adults: Results from a phase 1 clinical trial. Front. Immunol. 2019, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Obiero, C.W.; Ndiaye, A.G.W.; Sciré, A.S.; Kaunyangi, B.M.; Marchetti, E.; Gone, A.M.; Schütte, L.D.; Riccucci, D.; Auerbach, J.; Saul, A. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front. Immunol. 2017, 8, 1884. [Google Scholar] [CrossRef]
- Palmieri, E.; Arato, V.; Oldrini, D.; Ricchetti, B.; Aruta, M.; Pansegrau, W.; Marchi, S.; Giusti, F.; Ferlenghi, I.; Rossi, O.; et al. Stability of outer membrane vesicles-based vaccines, identifying the most appropriate methods to detect changes in vaccine potency. Vaccines 2021, 9, 229. [Google Scholar] [CrossRef]
- Micoli, F.; Rossi, O.; Conti, V.; Launay, O.; Sciré, A.S.; Aruta, M.G.; Nakakana, U.N.; Marchetti, E.; Rappuoli, R.; Saul, A.; et al. Antibodies elicited by the Shigella sonnei GMMA vaccine in adults trigger complement-mediated serum bactericidal activity: Results from a phase 1 dose escalation trial followed by a booster extension. Front. Immunol. 2021, 12, 671325. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Raso, M.M.; Gasperini, G.; Alfini, R.; Schiavo, F.; Aruta, M.G.; Carducci, M.; Forgione, M.C.; Martini, S.; Cescutti, P.; Necchi, F.; et al. GMMA and glycoconjugate approaches compared in mice for the development of a vaccine against Shigella flexneri serotype 6. Vaccines 2020, 8, 160. [Google Scholar] [CrossRef]
- Richardson, N.; Ravenscroft, N.; Arato, V.; Oldrini, D.; Micoli, F.; Kuttel, M. Conformational and immunogenicity studies of the Shigella flexneri serogroup 6 O-antigen: The effect of O-acetylation. Vaccines 2021, 9, 432. [Google Scholar] [CrossRef]
- Micoli, F.; Ravenscroft, N.; Cescutti, P.; Stefanetti, G.; Londero, S.; Rondini, S.; MacLennan, C. Structural analysis of O-polysaccharide chains extracted from different Salmonella Typhimurium strains. Carbohydr. Res. 2014, 385, 1–8. [Google Scholar] [CrossRef]
- De Benedetto, G.; Alfini, R.; Cescutti, P.; Caboni, M.; Lanzilao, L.; Necchi, F.; Saul, A.; MacLennan, C.; Rondini, S.; Micoli, F. Characterization of O-antigen delivered by generalized modules for membrane antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine 2017, 35, 419–426. [Google Scholar] [CrossRef]
- Necchi, F.; Saul, A.; Rondini, S. Development of a high-throughput method to evaluate serum bactericidal activity using bacterial ATP measurement as survival readout. PLoS ONE 2017, 12, e0172163. [Google Scholar] [CrossRef]
- DeLaine, B.C.; Wu, T.; Grassel, C.L.; Shimanovich, A.; Pasetti, M.F.; Levine, M.M.; Barry, E.M. Characterization of a multicomponent live, attenuated Shigella flexneri vaccine. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [PubMed]
- Pastor, Y.; Camacho, A.; Gil, A.G.; Ramos, R.; de Cerain, A.L.; Peñuelas, I.; Irache, J.M.; Gamazo, C. Effective protection of mice against Shigella flexneri with a new self-adjuvant multicomponent vaccine. J. Med. Microbiol. 2017, 66, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; De Ricco, R.; Rappuoli, R. Role of O-acetylation in the immunogenicity of bacterial polysaccharide vaccines. Molecules 2018, 23, 1340. [Google Scholar] [CrossRef] [PubMed]
- Konadu, E.; Shiloach, J.; Bryla, D.A.; Robbins, J.B.; Szu, S.C. Synthesis, characterization, and immunological properties in mice of conjugates composed of detoxified lipopolysaccharide of Salmonella paratyphi A bound to tetanus toxoid with emphasis on the role of O acetyls. Infect. Immun. 1996, 64, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Szu, S.C.; Li, X.R.; Stone, A.L.; Robbins, J.B. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect. Immun. 1991, 59, 4555–4561. [Google Scholar] [CrossRef]
- Chang, J.; Serrano, Y.; Garrido, R.; Rodríguez, L.M.; Pedroso, J.; Cardoso, F.; Valdés, Y.; García, D.; Fernández-Santana, V.; Verez-Bencomo, V. Relevance of O-acetyl and phosphoglycerol groups for the antigenicity of Streptococcus pneumoniae serotype 18C capsular polysaccharide. Vaccine 2012, 30, 7090–7096. [Google Scholar] [CrossRef]
- Micoli, F.; MacLennan, C.A. Outer membrane vesicle vaccines. Semin. Immunol. 2020, 50, 101433. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, G.; Raso, M.; Arato, V.; Aruta, M.; Cescutti, P.; Necchi, F.; Micoli, F. Effect of O-antigen chain length regulation on the immunogenicity of Shigella and Salmonella generalized modules for membrane antigens (GMMA). Int. J. Mol. Sci. 2021, 22, 1309. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Chassagne, P.; Theillet, F.-X.; Guerreiro, C.; Thouron, F.; Nato, F.; Delepierre, M.; Sansonetti, P.J.; Phalipon, A.; Mulard, L.A. Non-stoichiometric O-acetylation of Shigella flexneri 2a O-specific polysaccharide: Synthesis and antigenicity. Org. Biomol. Chem. 2014, 12, 4218–4232. [Google Scholar] [CrossRef] [PubMed]
- Hlozek, J.; Ravenscroft, N.; Kuttel, M.M. Effects of glucosylation and O-acetylation on the conformation of Shigella flexneri serogroup 2 O-antigen vaccine targets. J. Phys. Chem. B 2020, 124, 2806–2814. [Google Scholar] [CrossRef]
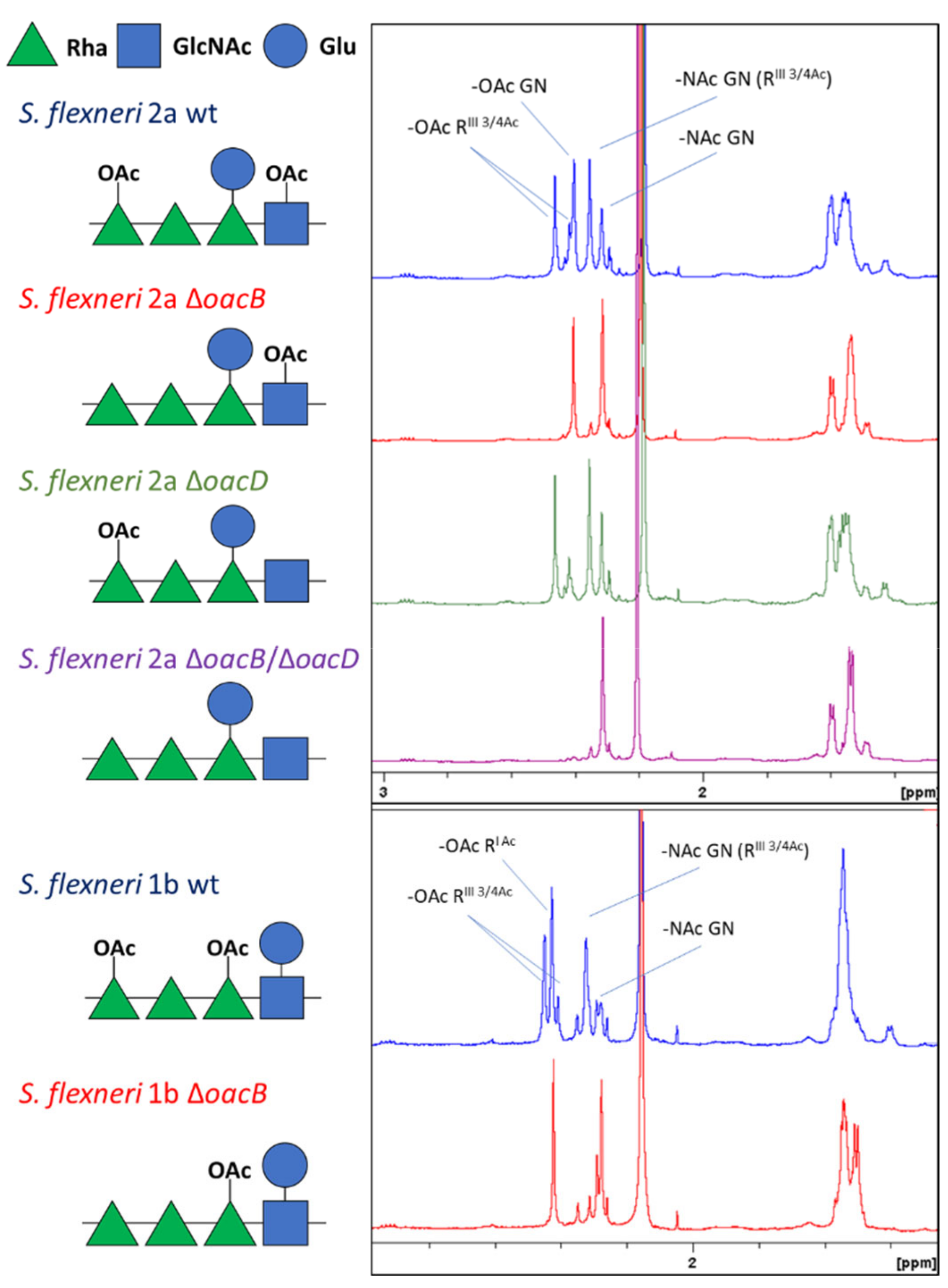
| GMMA | Mutation | OAg/Proteins w/w Ratio | OAg OAc | OAg MW |
|---|---|---|---|---|
| S. flexneri 2a | ΔtolR | 0.61 | 71% RhaIII 56% GlcNAc | 15% HMW (59.4 kDa) 50% MMW (13.7 kDa) 35% LMW (1.8 kDa) |
| ΔtolR ΔoacB | 0.62 | 65% GlcNAc | ||
| ΔtolR ΔoacD | 0.65 | 66% RhaIII | ||
| ΔtolR ΔoacB ΔoacD | 0.61 | 0 | ||
| S. flexneri 1b | ΔtolR | 0.84 | 63% RhaI 80% RhaIII | 65% MMW (13.8 kDa) 35% LMW (1.7 kDa) |
| ΔtolR ΔoacB | 0.67 | 79% RhaI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arato, V.; Oldrini, D.; Massai, L.; Gasperini, G.; Necchi, F.; Micoli, F. Impact of O-Acetylation on S. flexneri 1b and 2a O-Antigen Immunogenicity in Mice. Microorganisms 2021, 9, 2360. https://doi.org/10.3390/microorganisms9112360
Arato V, Oldrini D, Massai L, Gasperini G, Necchi F, Micoli F. Impact of O-Acetylation on S. flexneri 1b and 2a O-Antigen Immunogenicity in Mice. Microorganisms. 2021; 9(11):2360. https://doi.org/10.3390/microorganisms9112360
Chicago/Turabian StyleArato, Vanessa, Davide Oldrini, Luisa Massai, Gianmarco Gasperini, Francesca Necchi, and Francesca Micoli. 2021. "Impact of O-Acetylation on S. flexneri 1b and 2a O-Antigen Immunogenicity in Mice" Microorganisms 9, no. 11: 2360. https://doi.org/10.3390/microorganisms9112360
APA StyleArato, V., Oldrini, D., Massai, L., Gasperini, G., Necchi, F., & Micoli, F. (2021). Impact of O-Acetylation on S. flexneri 1b and 2a O-Antigen Immunogenicity in Mice. Microorganisms, 9(11), 2360. https://doi.org/10.3390/microorganisms9112360







