Parotid Space, a Different Space from Other Deep Neck Infection Spaces
Abstract
:1. Introduction
2. Methods
2.1. Statistical Analysis
2.2. Ethics Statement
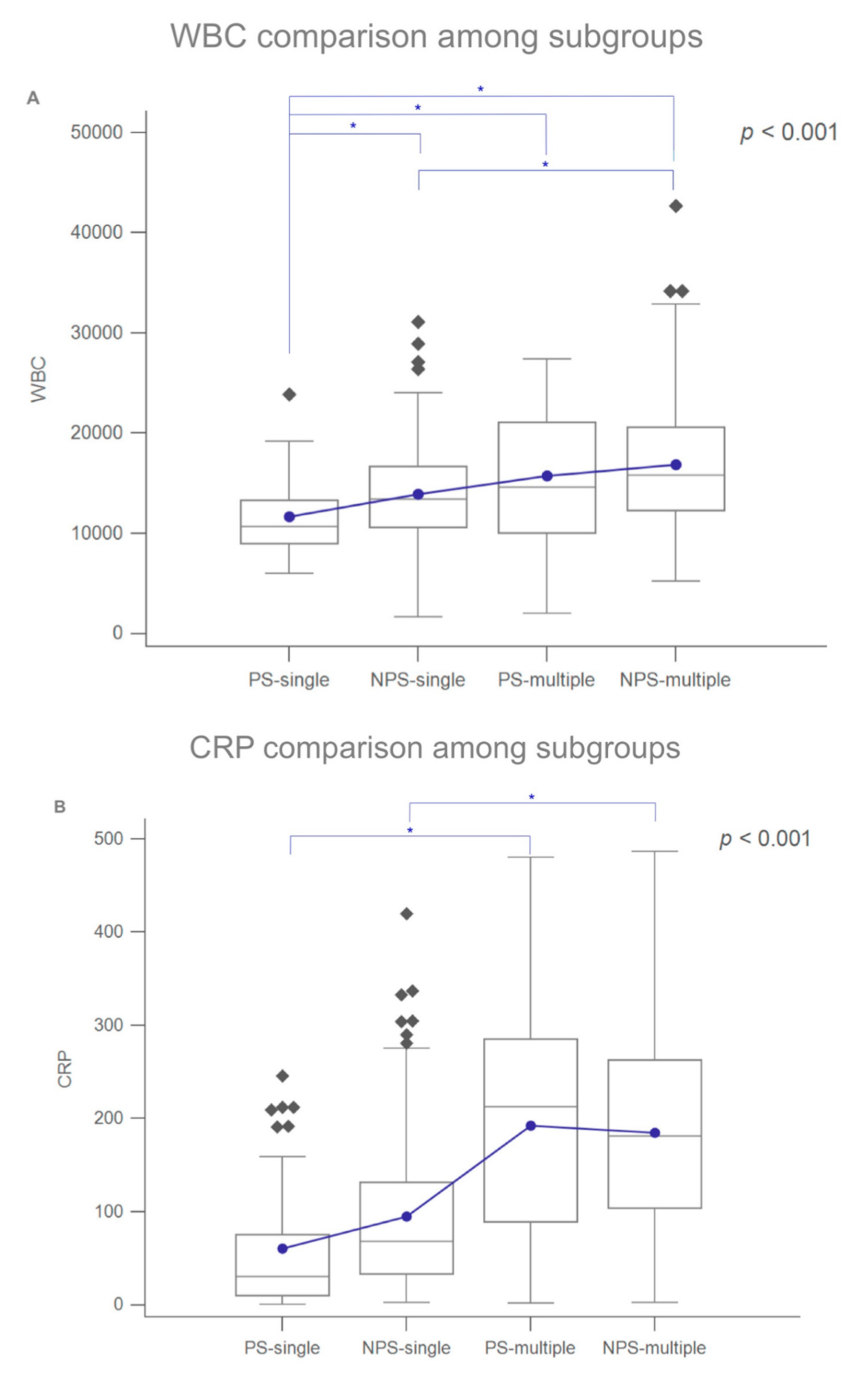
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, T.T.; Tseng, F.Y.; Yeh, T.H.; Hsu, C.J.; Chen, Y.S. Factors affecting the bacteriology of deep neck infection: A retrospective study of 128 patients. Acta Otolaryngol. 2006, 126, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.; Allen, S.M.; Stocks, R.M.; Thompson, J.W. Deep neck infection. Otolaryngol. Clin. N. Am. 2008, 41, 459–483. [Google Scholar] [CrossRef]
- Wang, L.F.; Tai, C.F.; Kuo, W.R.; Chien, C.Y. Predisposing factors of complicated deep neck infections: 12-year experience at a single institution. J. Otolaryngol. Head Neck Surg. 2010, 39, 335–341. [Google Scholar] [PubMed]
- Li, W.X.; Dong, Y.; Zhang, A.; Tian, J.; Lu, C.; Quraishi, M.S.; Liu, L. Management of deep neck infections from cervical esophageal perforation caused by foreign body: A case series study. Am. J. Otolaryngol. 2021, 42, 102870. [Google Scholar] [CrossRef] [PubMed]
- Garatea-Crelgo, J.; Gay-Escoda, C.; Bermejo, B.; Buenechea-Imaz, R. Morphological study of the parotid lymph nodes. J. Craniomaxillofac. Surg. 1993, 21, 207–209. [Google Scholar] [CrossRef]
- Marks, N.J. The anatomy of the lymph nodes of the parotid gland. Clin. Otolaryngol. Allied Sci. 1984, 9, 271–275. [Google Scholar] [CrossRef]
- Cunqueiro, A.; Gomes, W.A.; Lee, P.; Dym, R.J.; Scheinfeld, M.H. CT of the Neck: Image Analysis and Reporting in the Emergency Setting. Radiographics 2019, 39, 1760–1781. [Google Scholar] [CrossRef]
- Kauffmann, P.; Cordesmeyer, R.; Troltzsch, M.; Sommer, C.; Laskawi, R. Deep neck infections: A single-center analysis of 63 cases. Med. Oral. Patol. Oral. Cir. Bucal. 2017, 22, e536–e541. [Google Scholar] [CrossRef]
- Yang, S.W.; Lee, M.H.; See, L.C.; Huang, S.H.; Chen, T.M.; Chen, T.A. Deep neck abscess: An analysis of microbial etiology and the effectiveness of antibiotics. Infect. Drug. Resist. 2008, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.T.; Liu, T.C.; Chen, P.R.; Tseng, F.Y.; Yeh, T.H.; Chen, Y.S. Deep neck infection: Analysis of 185 cases. Head Neck 2004, 26, 854–860. [Google Scholar] [CrossRef]
- Chen, M.K.; Wen, Y.S.; Chang, C.C.; Lee, H.S.; Huang, M.T.; Hsiao, H.C. Deep neck infections in diabetic patients. Am. J. Otolaryngol. 2000, 21, 169–173. [Google Scholar] [CrossRef]
- Chi, T.H.; Yuan, C.H.; Chen, H.S. Parotid abscess: A retrospective study of 14 cases at a regional hospital in Taiwan. B-ENT 2014, 10, 315–318. [Google Scholar] [PubMed]
- Wang, L.F.; Kuo, W.R.; Tsai, S.M.; Huang, K.J. Characterizations of life-threatening deep cervical space infections: A review of one hundred ninety-six cases. Am. J. Otolaryngol. 2003, 24, 111–117. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Lee, D.H.; Yoon, T.M.; Lee, J.K.; Lim, S.C. Parotid abscess at a single institute in Korea. Medicine 2018, 97, e11700. [Google Scholar] [CrossRef] [PubMed]
- Podschun, R.; Pietsch, S.; Holler, C.; Ullmann, U. Incidence of Klebsiella species in surface waters and their expression of virulence factors. Appl. Environ. Microbiol. 2001, 67, 3325–3327. [Google Scholar] [CrossRef] [Green Version]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, R.; Leese, T. Parotid abscess in Singapore. Singapore Med. J. 2005, 46, 553–556. [Google Scholar]
- Lee, Y.Q.; Kanagalingam, J. Bacteriology of deep neck abscesses: A retrospective review of 96 consecutive cases. Singapore Med. J. 2011, 52, 351–355. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, H.D.; Lim, S.C. Predisposing factors of complicated deep neck infection: An analysis of 158 cases. Yonsei Med. J. 2007, 48, 55–62. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.J.; Snapp, K.R.; Dugan, A.J.; Westgate, P.M.; Gupta, N. Risk factors affecting length of stay in patients with deep neck space infection. Laryngoscope 2019, 130, 2133–2137. [Google Scholar] [CrossRef]
- Faden, H.; Mohmand, M. Infections Associated With Streptococcus Constellatus in Children. Pediatr. Infect. Dis. J. 2017, 36, 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.R.; Obi, N.; Epane, E.C.; Akbari, A.A.; Halpern, L.; Southerland, J.H.; Gangula, P.R. Effects of Diabetes on Salivary Gland Protein Expression of Tetrahydrobiopterin and Nitric Oxide Synthesis and Function. J. Periodontol. 2016, 87, 735–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Pintor, R.M.; Casanas, E.; Gonzalez-Serrano, J.; Serrano, J.; Ramirez, L.; de Arriba, L.; Hernandez, G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016, 2016, 4372852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Values |
|---|---|
| Males, N (%) | 278 (67.47) |
| Females, N (%) | 134 (32.53) |
| Age, years, mean ± SD | 51.14 ± 18.84 |
| Duration of symptoms, days, mean ± SD | 5.01 ± 4.51 |
| Hospital stay, days, mean ± SD | 9.48 ± 8.01 |
| WBC count, μL, mean ± SD | 15,059.94 ± 5916.45 |
| CRP, mg/L, mean ± SD | 137.95 ± 107.97 |
| Glucose, mg/dL, mean ± SD | 142.47 ± 72.10 |
| Diabetes mellitus, N (%) | 156 (37.86) |
| Surgical incision and drainage, N (%) | 188 (45.63) |
| Ultrasonography-guided drainage, N (%) | 72 (17.47) |
| Deep neck infection involving PS, N (%) | 91 (22.08) |
| PS-single, N (%) | 50 (12.13) |
| PS-multiple, N (%) | 41 (9.95) |
| Deep neck infection involving NPS, N (%) | 321 (77.92) |
| NPS-single, N (%) | 149 (36.16) |
| NPS-multiple, N (%) | 172 (41.76) |
| Tracheostomy, N (%) | 44 (10.67) |
| Culture pathogens | |
| Klebsiella pneumoniae, N (%) | 56 (13.59) |
| Streptococcus constellatus, N (%) | 49 (11.89) |
| Streptococcus anginosus, N (%) | 31 (7.52) |
| Parvimonas micra, N (%) | 29 (7.03) |
| Prevotella buccae, N (%) | 27 (6.55) |
| Prevotella intermedia, N (%) | 18 (4.36) |
| Staphylococcus aureus, N (%) | 12 (2.91) |
| Other species, N (%) | 117 (28.39) |
| No growth, N (%) | 167 (40.57) |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| PS | NPS | p-Value | OR | CI | p-Value | |
| Total, N (%) | 91 (100.0) | 321 (100.0) | ||||
| Males, N (%) | 67 (73.62) | 211 (65.73) | 0.165 | |||
| Females, N (%) | 24 (26.38) | 110 (34.27) | ||||
| Age, years, mean ± SD | 52.09 ± 19.56 | 50.87 ± 18.65 | 0.544 | |||
| Duration of symptoms, days, mean ± SD | 6.84 ± 6.43 | 4.47 ± 3.62 | 0.001 * | 1.072 | 1.013–1.134 | 0.014 * |
| Hospital stay, days, mean ± SD | 11.17 ± 9.70 | 8.97 ± 7.39 | 0.082 | |||
| WBC count, μL, mean ± SD | 13,485.71 ± 5739.79 | 15,506.23 ± 5898.16 | 0.001 * | - | - | - |
| CRP, mg/L, mean ± SD | 120.95 ± 115.31 | 143.03 ± 105.43 | 0.010 * | - | - | - |
| Glucose, mg/dL, mean ± SD | 155.13 ± 97.25 | 138.88 ± 62.92 | 0.423 | |||
| Diabetes mellitus | 0.393 | |||||
| Yes, N (%) | 38 (41.76) | 118 (36.76) | ||||
| No, N (%) | 53 (58.24) | 203 (63.24) | ||||
| Surgical incision and drainage | <0.001 * | 0.005 * | ||||
| Yes, N (%) | 19 (20.88) | 169 (52.64) | 0.384 | 0.195–0.753 | ||
| No, N (%) | 72 (79.12) | 152 (47.36) | 1.000 | |||
| Ultrasonography-guided drainage | <0.001 * | 0.001 * | ||||
| Yes, N (%) | 32 (35.17) | 40 (12.47) | 3.267 | 1.621–6.583 | ||
| No, N (%) | 59 (64.83) | 281 (87.53) | 1.000 | |||
| Tracheostomy | 0.083 | |||||
| Yes, N (%) | 5 (5.49) | 39 (12.14) | ||||
| No, N (%) | 86 (94.51) | 282 (87.86) | ||||
| Culture pathogens | ||||||
| Klebsiella pneumoniae, N (%) | 43 (47.25) | 13 (4.04) | <0.001 * | 21.885 | 10.428–45.932 | <0.001 * |
| Streptococcus constellatus, N (%) | 3 (3.29) | 46 (14.33) | 0.002 * | - | - | - |
| Streptococcus anginosus, N (%) | 2 (2.19) | 29 (9.03) | 0.025 * | - | - | - |
| Parvimonas micra, N (%) | 5 (5.49) | 24 (7.47) | 0.645 | |||
| Prevotella buccae, N (%) | 8 (8.79) | 19 (5.91) | 0.339 | |||
| Prevotella intermedia, N (%) | 3 (3.29) | 15 (4.67) | 0.773 | |||
| Staphylococcus aureus, N (%) | 5 (5.49) | 7 (2.18) | 0.141 |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| PS-Single | NPS-Single | p-Value | OR | CI | p-Value | |
| Total, N (%) | 50 (100.0) | 149 (100.0) | ||||
| Males, N (%) | 37 (74.00) | 106 (71.14) | 0.855 | |||
| Females, N (%) | 13 (26.00) | 43 (28.86) | ||||
| Age, years, mean ± SD | 49.72 ± 19.31 | 48.30 ± 17.54 | 0.665 | |||
| Duration of symptoms, days, mean ± SD | 7.00 ± 6.18 | 4.90 ± 4.29 | 0.031 * | - | - | - |
| Hospital stay, days, mean ± SD | 9.00 ± 9.05 | 6.69 ± 5.78 | 0.265 | |||
| WBC count, μL, mean ± SD | 11,630.00 ± 3867.19 | 13,932.20 ± 4990.01 | 0.001 * | - | - | - |
| CRP, mg/L, mean ± SD | 60.56 ± 68.83 | 94.94 ± 84.54 | <0.001 * | - | - | - |
| Glucose, mg/dL, mean ± SD | 149.88 ± 92.83 | 124.30 ± 42.04 | 0.910 | |||
| Diabetes mellitus | 0.596 | |||||
| Yes, N (%) | 17 (34.00) | 44 (29.53) | ||||
| No, N (%) | 33 (66.00) | 105 (70.47) | ||||
| Surgical incision and drainage | <0.001 * | 0.058 * | ||||
| Yes, N (%) | 3 (6.00) | 66 (44.29) | 0.073 | 0.011–0.468 | ||
| No, N (%) | 47 (94.00) | 83 (55.71) | 1.000 | |||
| Ultrasonography-guided drainage | <0.001 * | 0.024 * | ||||
| Yes, N (%) | 22 (44.00) | 21 (14.09) | 3.096 | 1.155–8.296 | ||
| No, N (%) | 28 (56.00) | 128 (85.91) | 1.000 | |||
| Tracheostomy | 0.068 | |||||
| Yes, N (%) | 0 (0.00) | 10 (16.71) | ||||
| No, N (%) | 50 (100.0) | 139 (93.29) | ||||
| Culture pathogens | ||||||
| Klebsiella pneumoniae, N (%) | 23 (46.00) | 4 (2.68) | <0.001 * | 62.796 | 12.687–310.808 | <0.001 * |
| Streptococcus constellatus, N (%) | 0 (0.00) | 16 (10.73) | 0.013 * | - | - | - |
| Streptococcus anginosus, N (%) | 1 (2.00) | 11 (7.38) | 0.301 | |||
| Parvimonas micra, N (%) | 2 (4.00) | 13 (8.72) | 0.364 | |||
| Prevotella buccae, N (%) | 0 (0.00) | 5 (3.35) | 0.333 | |||
| Prevotella intermedia, N (%) | 0 (0.00) | 7 (4.69) | 0.195 | |||
| Staphylococcus aureus, N (%) | 4 (8.00) | 3 (2.01) | 0.068 |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| PS-Multiple | NPS-Multiple | p-Value | OR | CI | p-Value | |
| Total, N (%) | 41 (100.0) | 172 (100.0) | ||||
| Males, N (%) | 30 (73.17) | 105 (61.04) | 0.206 | |||
| Females, N (%) | 11 (26.83) | 67 (38.96) | ||||
| Age, years, mean ± SD | 55.00 ± 19.72 | 53.11 ± 19.34 | 0.498 | |||
| Duration of symptoms, days, mean ± SD | 6.65 ± 6.80 | 4.11 ± 2.88 | 0.016 * | 1.102 | 1.005–1.209 | 0.037 * |
| Hospital stay, days, mean ± SD | 13.82 ± 9.91 | 10.95 ± 8.06 | 0.083 | |||
| WBC, μL, mean ± SD | 15,748.78 ± 6800.92 | 16,869.77 ± 6286.58 | 0.402 | |||
| CRP, mg/L, mean ± SD | 192.59 ± 119.58 | 184.69 ± 104.19 | 0.710 | |||
| Glucose, mg/dL, mean ± SD | 161.53 ± 103.18 | 151.52 ± 74.32 | 0.415 | |||
| Diabetes mellitus | 0.384 | |||||
| Yes, N (%) | 21 (51.21) | 74 (43.02) | ||||
| No, N (%) | 20 (48.79) | 98 (56.98) | ||||
| Surgical incision and drainage | 0.022 * | - | - | - | ||
| Yes, N (%) | 16 (39.02) | 103 (59.88) | ||||
| No, N (%) | 25 (60.98) | 69 (40.12) | ||||
| Ultrasonography-guided drainage | 0.039 * | 0.033 * | ||||
| Yes, N (%) | 10 (24.39) | 19 (11.05) | 3.169 | 1.093–9.188 | ||
| No, N (%) | 31 (75.61) | 153 (88.95) | 1.000 | |||
| Tracheostomy | 0.635 | |||||
| Yes, N (%) | 5 (12.19) | 29 (16.86) | ||||
| No, N (%) | 36 (87.81) | 143 (83.14) | ||||
| Culture pathogens | ||||||
| Klebsiella pneumoniae, N (%) | 20 (48.78) | 9 (5.23) | <0.001 * | 16.677 | 6.462–43.034 | <0.001 * |
| Streptococcus constellatus, N (%) | 3 (7.31) | 30 (17.44) | 0.148 | |||
| Streptococcus anginosus, N (%) | 1 (2.43) | 18 (10.46) | 0.133 | |||
| Parvimonas micra, N (%) | 3 (7.31) | 11 (6.39) | 0.735 | |||
| Prevotella buccae, N (%) | 8 (19.51) | 14 (8.13) | 0.139 | |||
| Prevotella intermedia, N (%) | 3 (7.31) | 8 (4.65) | 0.446 | |||
| Staphylococcus aureus, N (%) | 1 (2.43) | 4 (2.32) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-L.; Young, C.-K.; Liao, C.-T.; Tsai, T.-Y.; Kang, C.-J.; Huang, S.-F. Parotid Space, a Different Space from Other Deep Neck Infection Spaces. Microorganisms 2021, 9, 2361. https://doi.org/10.3390/microorganisms9112361
Chen S-L, Young C-K, Liao C-T, Tsai T-Y, Kang C-J, Huang S-F. Parotid Space, a Different Space from Other Deep Neck Infection Spaces. Microorganisms. 2021; 9(11):2361. https://doi.org/10.3390/microorganisms9112361
Chicago/Turabian StyleChen, Shih-Lung, Chi-Kuang Young, Chun-Ta Liao, Tsung-You Tsai, Chung-Jan Kang, and Shiang-Fu Huang. 2021. "Parotid Space, a Different Space from Other Deep Neck Infection Spaces" Microorganisms 9, no. 11: 2361. https://doi.org/10.3390/microorganisms9112361
APA StyleChen, S.-L., Young, C.-K., Liao, C.-T., Tsai, T.-Y., Kang, C.-J., & Huang, S.-F. (2021). Parotid Space, a Different Space from Other Deep Neck Infection Spaces. Microorganisms, 9(11), 2361. https://doi.org/10.3390/microorganisms9112361






