Attachment of Shiga Toxin-Producing Escherichia coli (STEC) to Pre-Chill and Post-Chill Beef Brisket Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Beef Sample Preparation
2.3. Culture Preparation
2.4. Beef Tissue Attachment Assay
2.5. Statistical Analysis
3. Results
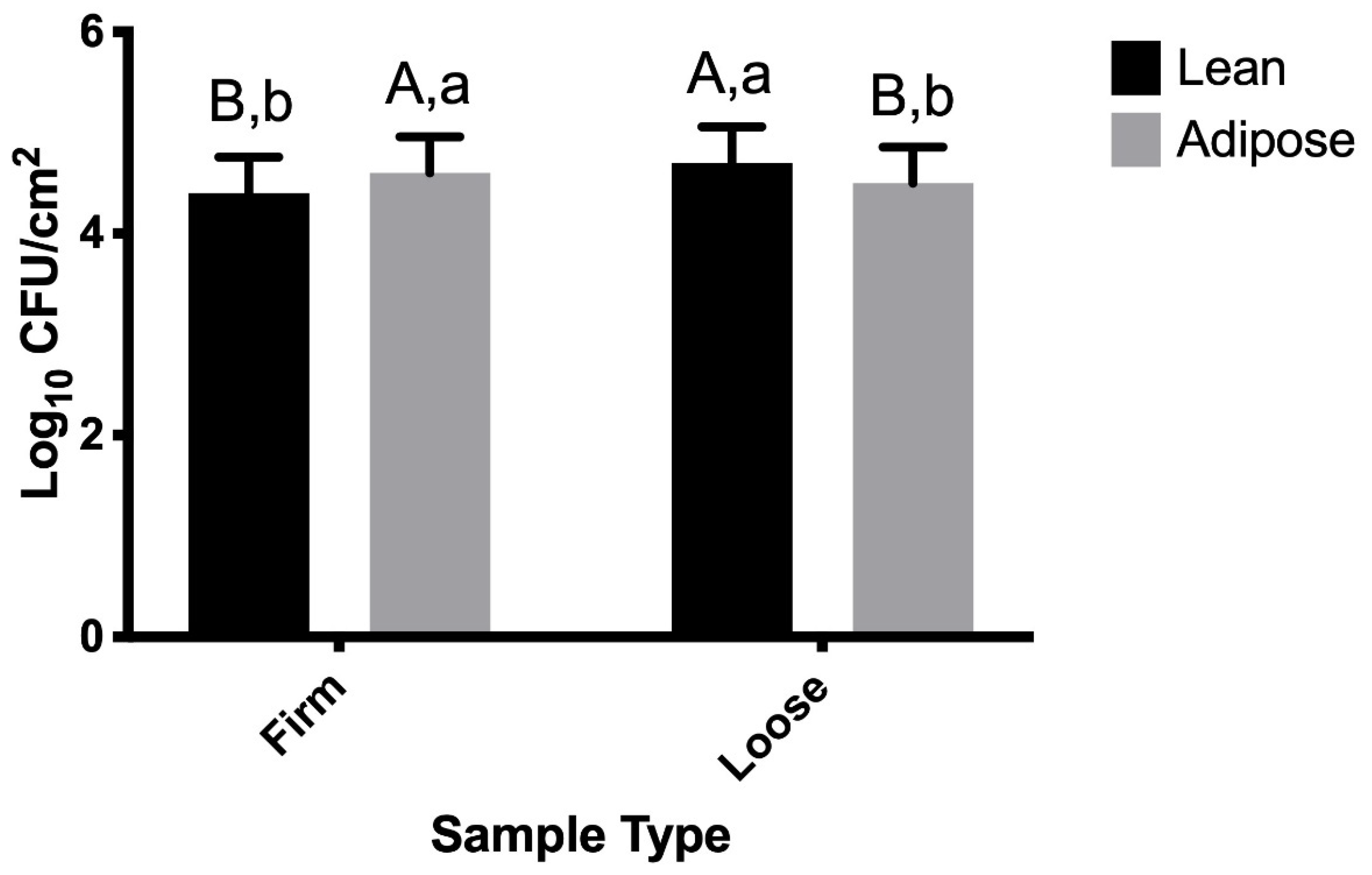
3.1. TSB-Grown STEC
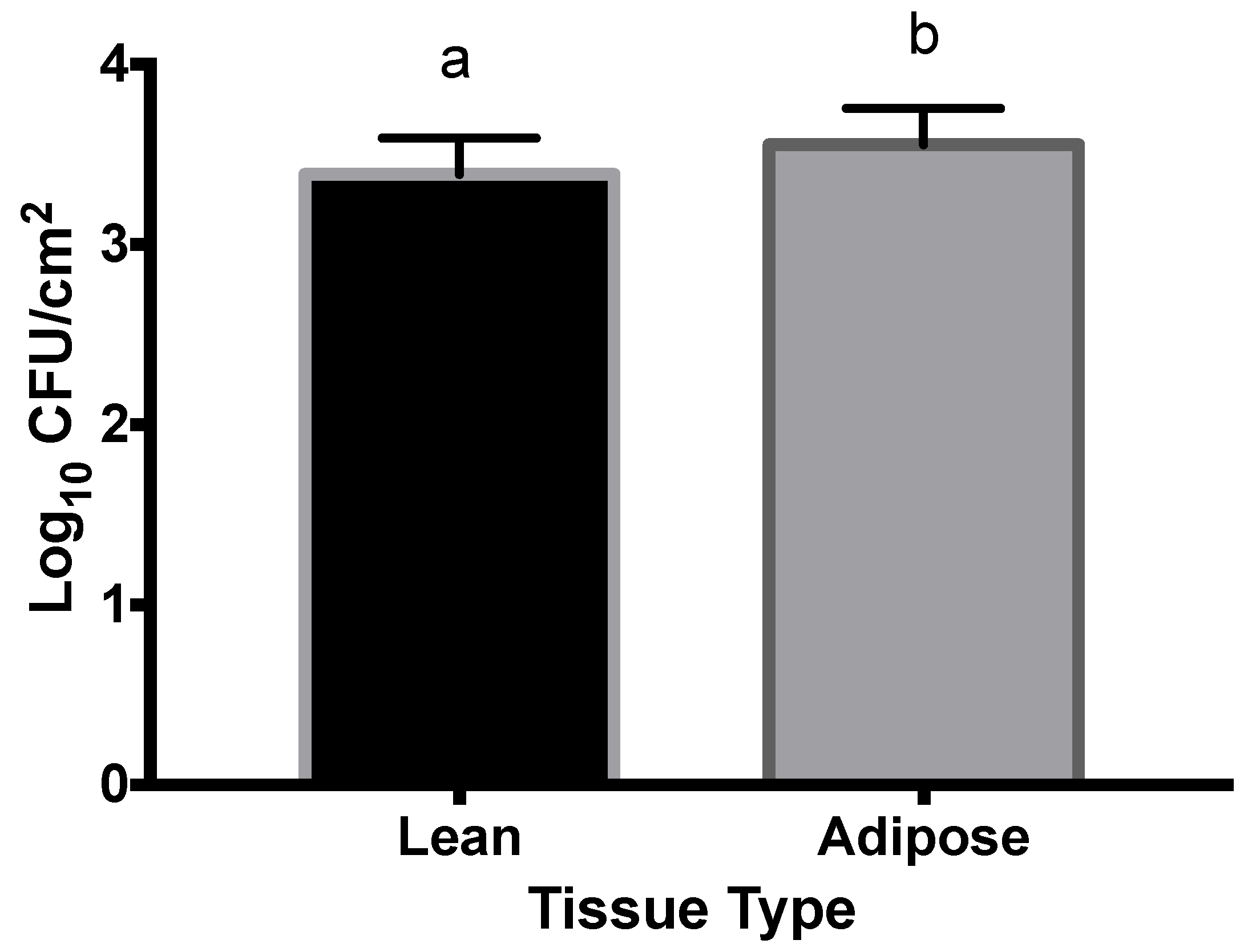
3.2. M9-Grown STEC
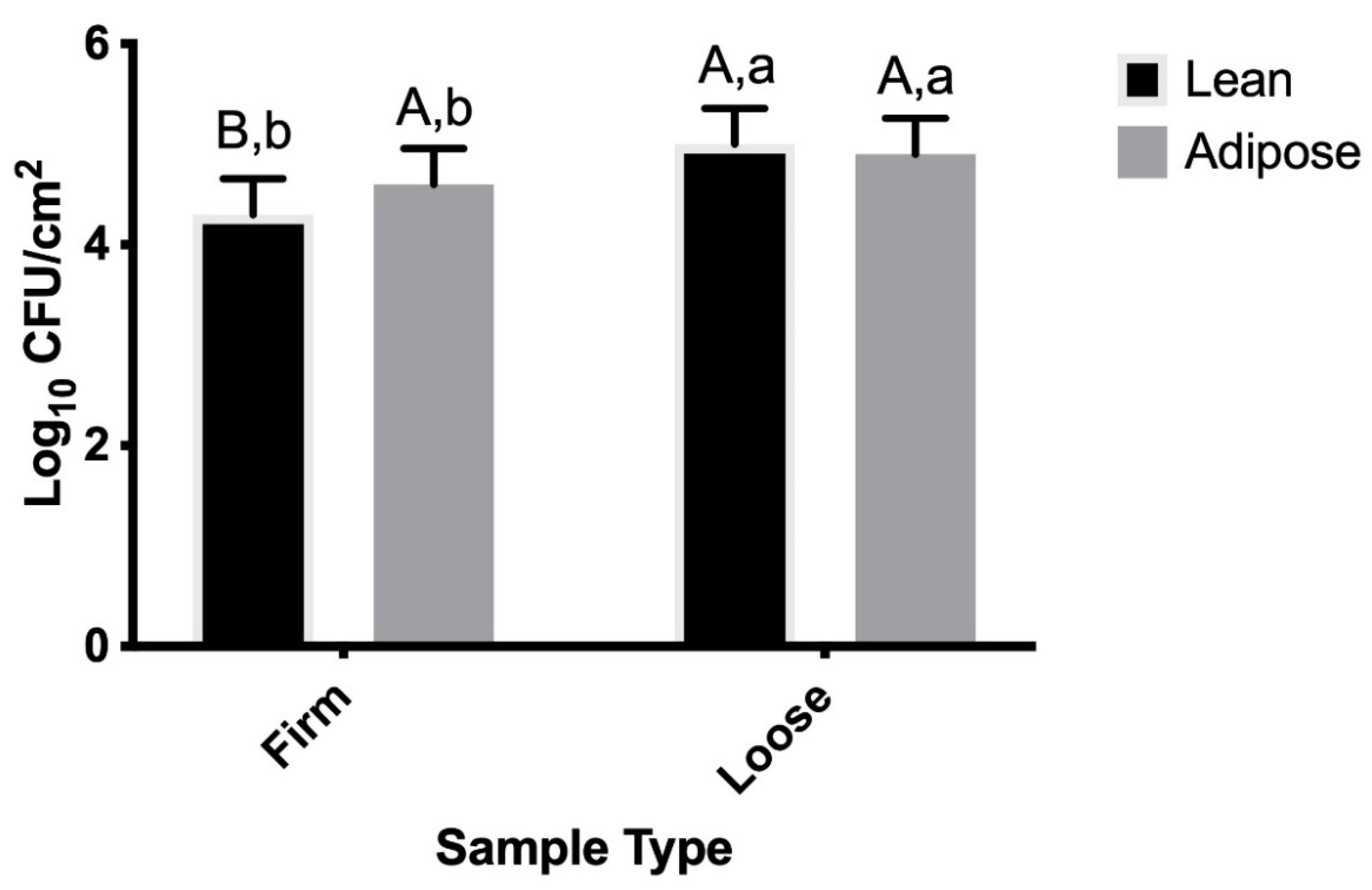
3.3. Influence of Beef Tissue Temperature on STEC Attachment
4. Discussion
4.1. TSB-Grown STEC
4.2. M9-Grown STEC
4.3. Influence of Beef Tissue Temperature on STEC Attachment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Ma, Z.; Stanford, K.; Xiao, M.B.; Niu, Y.D.; McAllister, T.A. Effects of beef juice on biofilm formation by Shiga toxin–producing Escherichia coli on stainless steel. Foodborne Pathog. Dis. 2020, 17, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Peco-Antić, A. Shiga toxin-producing Escherichia coli hemolytic uremic syndrome. Srp. Arh. Celok. Lek. 2016, 144, 664–669. [Google Scholar] [CrossRef]
- Taylor, M.R. Change and Opportunity: Harnessing Innovation to Improve the Safety of the Food Supply. Presented at the American Meat Institute Annual Convention, San Francisco, CA, USA, 29 September 1994. [Google Scholar]
- United States Department of Agriculture/Food Safety Inspection Service (USDA-FSIS). Beef products contaminated with Escherichia coli O157:H7. Fed. Reg. 1999, 64, 2803–2805. [Google Scholar]
- United States Department of Agriculture/Food Safety Inspection Service (USDA-FSIS). Shiga toxin-producing Escherichia coli in certain raw beef products. Fed. Reg. 2012, 77, 31975–31981. [Google Scholar]
- Ferens, W.A.; Hovde, C.J. Escherichia coli O157:H7: Animal reservoir and sources of human infection. Foodborne Pathog. Dis. 2011, 8, 465–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Department of Agriculture/Food Safety Inspection Service (USDA-FSIS). Industry Guideline for Minimizing the Risk of Shiga Toxin-Producing Escherichia coli (STEC) in Beef (including Veal) Slaughter Operations 2021 Guideline. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-08/FSIS-GD-2021-0008.pdf (accessed on 12 October 2021).
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Dourou, D.; Beauchamp, C.S.; Yoon, Y.; Geornaras, I.; Belk, K.E.; Smith, G.C.; Nychas, G.-J.E.; Sofos, J.N. Attachment and biofilm formation by Escherichia coli O157:H7 at different temperatures, on various food-contact surfaces encountered in beef processing. Int. J. Food Microbiol. 2011, 149, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Skandamis, P.N.; Stopforth, J.D.; Ashton, L.V.; Geornaras, I.; Kendall, P.A.; Sofos, J.N. Escherichia coli O157:H7 survival, biofilm formation and acid tolerance under simulated slaughter plant moist and dry conditions. Food Microbiol. 2009, 26, 112–119. [Google Scholar] [CrossRef]
- Tamplin, M.L.; Pauli, G.; Marmer, B.S.; Phillips, J. Models of the behavior of Escherichia coli O157:H7 in raw sterile ground beef stored at 5 °C to 46 °C. Int. J. Food Microbiol. 2005, 100, 335–344. [Google Scholar] [CrossRef]
- Parks, A.R.; Brashears, M.M. Efficacy of detergent and quaternary ammonium sanitizer on Shiga-toxin producing Escherichia coli (STEC) attached to stainless steel. Meat Sci. 2015, 101, 150. [Google Scholar] [CrossRef]
- Benedict, R.C.; Schultz, F.J.; Jones, S.B. Attachment and removal of Salmonella spp. on meat and poultry tissues. J. Food Saf. 1991, 11, 135–148. [Google Scholar] [CrossRef]
- Cabedo, L.; Sofos, J.N.; Schmidt, G.R.; Smith, G.C. Attachment of E. coli O157:H7 and other bacterial cells grown in two media to beef adipose and muscle tissues. J. Food Prot. 1997, 60, 102–106. [Google Scholar] [CrossRef]
- Chung, K.-T.; Dickson, J.S.; Crouse, J.D. Attachment and proliferation of bacteria on meat. J. Food Prot. 1989, 52, 173–177. [Google Scholar] [CrossRef]
- Dickson, J.S.; MacNeil, M.D. Contamination of beef tissue surfaces by cattle manure inoculated with Salmonella typhimurium and Listeria monocytogenes. J. Food Prot. 1991, 54, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.S.; Frank, J.F. Bacterial starvation stress and contamination of beef. Food Microbiol. 1993, 10, 215–222. [Google Scholar] [CrossRef]
- Frank, J.F. Microbial attachment to food and food contact surfaces. Adv. Food Nutr. Res. 2001, 43, 319–370. [Google Scholar]
- Piette, J.P.; Idziak, E.S. New method to study bacterial adhesion to meat. Appl. Environ. Microbiol. 1989, 55, 1531–1536. [Google Scholar] [CrossRef] [Green Version]
- Goulter, R.M.; Gentle, I.R.; Dykes, G.A. Issues in determining factors influencing bacterial attachment: A review using attachment of Escherichia coli to abiotic surfaces as an example. Lett. Appl. Microbiol. 2009, 49, 1–7. [Google Scholar] [CrossRef]
- Kirsch, K.R.; Taylor, T.M.; Griffin, D.; Castillo, A.; Marx, D.B.; Smith, L. Growth of Shiga toxin-producing Escherichia coli (STEC) and impacts of chilling and post-inoculation storage on STEC attachment to beef surfaces. Food Microbiol. 2014, 44, 236–242. [Google Scholar] [CrossRef]
- McWilliams, B.D.; Torres, A.G. Enterohemorrhagic Escherichia coli adhesins. Microbiol. Spectr. 2014, 2, EHEC-0003-2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, J.S. Attachment of Salmonella typhimurium and Listeria monocytogenes to beef tissue: Effects of inoculum level, growth temperature and bacterial culture age. Food Microbiol. 1991, 8, 143–151. [Google Scholar] [CrossRef]
- Dickson, J.S.; Koohmaraie, M. Cell surface charge characteristics and their relationship to bacterial attachment to meat surfaces. Appl. Environ. Microbiol. 1989, 55, 832–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pringle, J.H.; Fletcher, M. Influence of substratum wettability on attachment of freshwater bacteria to solid surfaces. Appl. Environ. Microbiol. 1983, 45, 811–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amézquita-López, B.A.; Soto-Beltrán, M.; Lee, B.G.; Yambao, J.C.; Quiñones, B. Isolation, genotyping and antimicrobial resistance of Shiga toxin-producing Escherichia coli. J. Microbiol. Immunol. Infect. 2018, 51, 425–434. [Google Scholar] [CrossRef]
- Pozuelo, K.C.; Vega, D.; Habib, K.; Najar-Villarreal, F.; Kang, Q.; Trinetta, V.; O’Quinn, T.G.; Phebus, R.K.; Gragg, S.E. Validation of post-harvest antimicrobial interventions to control Shiga toxin-producing Escherichia coli (STEC) on market hog carcass surfaces. Int. J. Food Microbiol. 2021, 358, 109421. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. Bad Bug Book: Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins (Second Edition). Available online: https://www.fda.gov/media/83271/download (accessed on 7 October 2021).
- Rivas, L.; Dykes, G.A.; Fegan, N. Attachment of Shiga toxigenic Escherichia coli to beef muscle and adipose tissue. J. Food Prot. 2006, 69, 999–1006. [Google Scholar] [CrossRef]
- Bouttier, S.; Linxe, C.; Ntsama, C.; Morgant, G.; Bellon-Fontaine, M.N.; Fourniat, J. Attachment of Salmonella choleraesuis choleraesuis to beef muscle and adipose tissues. J. Food Prot. 1997, 60, 16–22. [Google Scholar] [CrossRef]
- Patel, J.; Sharma, M.; Ravishakar, S. Effect of curli expression and hydrophobicity of Escherichia coli O157:H7 on attachment to fresh produce surfaces. J. Appl. Microbiol. 2010, 110, 737–745. [Google Scholar] [CrossRef]
- Rivas, L.; Fegan, N.; Dykes, G.A. Attachment of Shiga toxigenic Escherichia coli to stainless steel. Int. J. Food Microbiol. 2007, 117, 89–94. [Google Scholar] [CrossRef]
- Wang, J.; Stanford, K.; McAllister, T.A.; Johnson, R.P.; Chen, J.; Hou, H.; Zhang, G.; Niu, Y.D. Biofilm formation, virulence gene profiles, and antimicrobial resistance of nine serogroups of non-O157 Shiga toxin-producing Escherichia coli. Foodborne Pathog. Dis. 2016, 13, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Bumunang, E.W.; Stanford, K.; Bie, X.; Niu, Y.D.; McAllister, T.A. Biofilm formation by Shiga toxin-producing Escherichia coli on stainless steel coupons as affected by temperature and incubation time. Microorganisms 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample Type | ||
|---|---|---|
| Time (min) | Loose (log CFU/cm2) | Firm (log CFU/cm2) |
| 0 | 4.9 a,A | 4.4 a,B |
| 3 | 4.9 a,A | 4.6 b,c,B |
| 5 | 4.9 a,A | 4.4 a,B |
| 20 | 4.9 a,A | 4.4 a,B |
| 60 | 4.6 b,A | 4.5 a,b,A |
| 180 | 4.4 b,c,A | 4.6 a,b,A |
| 480 | 4.4 b,c,A | 4.5 a,b,A |
| 720 | 4.3 c,A | 4.5 a,b,B |
| 1440 | 4.3 c,A | 4.6 b,c,B |
| 2880 | 4.3 c,A | 4.8 c,B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unruh, D.A.; Uhl, B.C.; Phebus, R.K.; Gragg, S.E. Attachment of Shiga Toxin-Producing Escherichia coli (STEC) to Pre-Chill and Post-Chill Beef Brisket Tissue. Microorganisms 2021, 9, 2320. https://doi.org/10.3390/microorganisms9112320
Unruh DA, Uhl BC, Phebus RK, Gragg SE. Attachment of Shiga Toxin-Producing Escherichia coli (STEC) to Pre-Chill and Post-Chill Beef Brisket Tissue. Microorganisms. 2021; 9(11):2320. https://doi.org/10.3390/microorganisms9112320
Chicago/Turabian StyleUnruh, Daniel A., Bennett C. Uhl, Randall K. Phebus, and Sara E. Gragg. 2021. "Attachment of Shiga Toxin-Producing Escherichia coli (STEC) to Pre-Chill and Post-Chill Beef Brisket Tissue" Microorganisms 9, no. 11: 2320. https://doi.org/10.3390/microorganisms9112320
APA StyleUnruh, D. A., Uhl, B. C., Phebus, R. K., & Gragg, S. E. (2021). Attachment of Shiga Toxin-Producing Escherichia coli (STEC) to Pre-Chill and Post-Chill Beef Brisket Tissue. Microorganisms, 9(11), 2320. https://doi.org/10.3390/microorganisms9112320






