Abstract
Wine reflects the specificity of a terroir, including the native microbiota. In contrast to the use of Saccharomyces cerevisiae commercial starters, a way to maintain wines’ microbial terroir identities, guaranteeing at the same time the predictability and reproducibility of the wines, is the selection of autochthonous Saccharomyces and non-Saccharomyces strains towards optimal enological characteristics for the chosen area of isolation. This field has been explored but there is a lack of a compendium covering the main methods to use. Autochthonous wine yeasts from different areas of Slovakia were identified and tested, in the form of colonies grown either on nutrient agar plates or in grape must micro-fermentations, for technological and qualitative enological characteristics. Based on the combined results, Saccharomyces cerevisiae PDA W 10, Lachancea thermotolerans 5-1-1 and Metschnikowia pulcherrima 125/14 were selected as potential wine starters. This paper, as a mixture of experimental and review contributions, provides a compendium of methods used to select autochthonous wine yeasts. Thanks to the presence of images, this compendium could guide other researchers in screening their own yeast strains for wine production.
1. Introduction
Wine reflects the specificity of a terroir, including the microbial terroir [1]. This is particularly true in spontaneous fermentation by native wine yeasts that nevertheless expose winemakers to well-known complications. Most winemakers prefer to make use of commercial Saccharomyces cerevisiae starters, which guarantee predictability and reproducibility of the wines. On the other hand, the extensive use of worldwide-distributed commercial starters leads to organoleptic flattening and uniformization of the wines. Moreover, the positive contribution of non-Saccharomyces strains to must fermentation is well established. Non-Saccharpmyces species are known to modulate the wine aromatic profile in particular via esterase and β-glucosidase activities, but also to increase the glycerol content, to lower the alcohol content, and to exert proteolytic and pectinolytic activities that lead to enrichment of the aroma profile [2,3,4,5,6,7,8]. Besides S. cerevisiae commercial starters, non-Saccharomyces commercial starters have become available in recent years [9].
An alternative for preserving the role of the microbial terroir is to isolate and select autochthonous Saccharomyces and non-Saccharomyces strains towards optimal enological characteristics for use as co- or sequential inocula for wine production in the area from which they were isolated.
Enological characteristics to consider in the selection process are divided into technological and qualitative traits [10]. Technological traits (fermentation vigor, ethanol tolerance, resistance to SO2, type of growth in liquid media, growth at high and low temperatures) are characteristics useful for efficient fermentation, while qualitative traits refer to those that influence chemical and sensorial composition and properties of wine (acetic acid and sulfuric compound production, production of volatile compounds connected with pleasant notes or off-flavors, enzymatic activities).
The enological characteristics reported above are important for the selection of Saccharomyces cerevisiae strains, but they are also useful for studying non-Saccharomyces yeasts. It appears crucial to have a complete overview of the traits of yeast strains to select those possessing the best abilities for must fermentation. Moreover, when choosing strains it is necessary to consider the technology of production and the type of product (wines, sparkling wines, botrytized high-sugar wines) where the selected strains will be applied [11,12,13,14,15,16].
Isolation of autochthonous microorganisms in various countries and wine-production regions with the aim of developing region-specific wine starters continues to be attractive [17]. Such research requires sensitive, relevant, and effective scientific methods to assess genetic, biochemical and technological traits characterizing the potential of microorganisms to be used in wine production. In recent years, various new approaches for enological yeast selection have been reported, while others have been optimized or updated [18,19,20,21]. The number of tests used to screen wine strains ranges from a few to many. As examples, colony morphology [22], ethanol resistance [23,24,25,26,27,28], SO2 resistance [23,25,26,27,28,29], H2S [22,25,26,27,28,29,30,31], enzymatic properties [22,26,27,28,29,32,33], resistance to osmotic stress [24,26,27], acetic acid production and spore formation [31], growth at various temperatures [24], fermentation vigor [31], and gas production [27] have been investigated. Molecular biology approaches were also developed in order to identify and cluster isolated wine yeasts [34,35], and also to screen the yeast communities in wine-related samples through the analysis of the total extracted DNA and RNA [36,37,38]. The latest methods involve very productive scientific tools in genetics and taxonomy but have been found to be unable to replace the more traditional microbiological methods.
To the best of our knowledge, there is a lack of a digest reporting all of the main microbiological methods, including images, to use for selecting wine yeast strains. This paper provides a compendium of up-to-date, complementary, and reliable methods for this purpose, including an outline of an experimentally verified approach for results evaluation and synthesis of conclusions.
The aim of this work was to provide this compendium of methodologies and images to select wine yeasts based on studies of our own isolates. Through assessing the main technological and qualitative enological strain characteristics using media and grape must micro-fermentations, one Saccharomyces—S. cerevisiae PDA W 10—and two non-Saccharomyces—Lachancea thermotolerans 5-1-1 and Metschnikowia pulcherrima 125/14—strains were selected as potential wine starters.
2. Materials and Methods
2.1. Yeast Strains
Twenty-nine yeast strains belonging to the Culture Collection of Wine Yeasts (Food Research Institute, National Agricultural and Food Centre, Bratislava, Slovakia) were considered in this study. Twenty-six strains were previously isolated from wine-related samples from various Slovakian regions and three strains—Candida dubliniensis CCY 29-178-1, Metschnikowia pulcherrima CCY 69-2-15, and Pichia fermentans CCY 29-97-12—came from the Culture Collection of Yeasts (Institute of Chemistry, Slovak Academy of Science, Bratislava, Slovakia) (Table 1). The strains were stored in freeze-dried form until the analyses, when they were identified and then tested for technological and qualitative enological characteristics to select Saccharomyces and non-Saccharomyces strains useful for winemaking. All trials were carried out in duplicate.

Table 1.
Yeast strains used in this study.
2.2. Yeast DNA Restriction Analysis and Sequencing
The strains were grown on Yeast Peptone Dextrose (YPD) agar (HiMedia, Mumbai, India) at 25 °C and the biomass was used for molecular analyses. DNA was extracted using InstaGene Matrix (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. The 5.8S Internal Transcribed Spacer (ITS) rRNA region was amplified in a thermal cycler (Bio-Rad, iCycler) using the primers ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) under the following conditions: 25 μL reaction mixture containing 6 μL of DNA template, 1× reaction buffer, 1.2 mM MgCl2, 200 μM dNTP mix, 50 pmol of each primer (Microsynth, Balgach, Switzerland), and 1.5 U of DFS-Taq polymerase (Bioron, Ludwigshafen, Germany). The amplification program was: initial denaturation at 94 °C for 2 min, 30 cycles of 10 s at 94 °C for denaturing, annealing for 20 s at 54 °C, extension for 1 min at 72 °C, and a final extension of 8 min at 72 °C.
The ITS-PCR products on 1.5% agarose (Lonza, Basel, Switzerland) gel stained with GelRed stain (Biotium, Fremont, CA, USA) were visualized on a UV transilluminator (UVP Inc., Upland, CA, USA) and then analyzed via Restriction Fragment Length Polymorphism (RFLP) using HaeIII, HhaI (New England Biolabs, Ipswich, MA, USA) and HinfI (Thermo Fisher Scientific, Waltham, MA, USA) restriction enzymes. Restriction mixtures were incubated at 37 °C for 3 h and then analyzed on 2.5% (w/v) agarose gel at 100 V for 2 h. A representative for each PCR-RFLP profile was chosen for sequence analysis. All of the amplified products were purified using EXO-SAP-IT® (Affymetrix, Cleveland, OH, USA), according to the manufacturer’s instructions. The purified products were prepared according to the instructions of a commercial facility (Eurofins Genomics, Ebersberg, Germany) and shipped to be sequenced via the Sanger method. The sequences were compared with those available at NCBI using Blast search tool [39] and submitted to GenBank (https://submit.ncbi.nlm.nih.gov/subs/genbank/) for accession numbers.
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
The strains were grown on YPD agar at 30 °C for 2-3 days. Subsequently, a loopful from one colony of each strain was dispersed in distilled water, used to load the 96-position ZnSe plate, and dried at 37 °C for 45 min and spectra were measured with a Tensor 27 FTIR spectrometer (Bruker Optics, Ettlingen, Germany) using 32 scans per sample [40].
2.4. Yeast Screening
Depending on the test to perform, strains were grown overnight at 25 °C either on YPD agar or in YPD broth and then the cultures were used to inoculate either YPD broth or specific media. For the latter, each strain was harvested by centrifugation (5000 rpm for 10 min), washed once in NaCl 0.9% (w/v) solution, re-suspended to Optical Density (OD)600 of 1.0 in the same solution [41]. Subsequently, each strain was spotted (5 µL) in duplicate onto specific media.
2.4.1. Macroscopic and Microscopic Observation, Type of Growth, CO2 Production
Strains were grown in tubes containing YPD broth for 48 h at 25 °C to assess the modality of growth, and in YPD broth tubes with Durham tubes for 7 d at 25 °C to assess fermentation through CO2 trapped in the Durham tubes. In addition, the strains were streaked onto YPD agar in order to record the colony morphology. A small quantity of the biomass was observed under a microscope (Olympus BX53, Tokyo, Japan) to record the cell morphology.
2.4.2. Spore Formation
The yeast biomass, taken with a sterile 1 µL loop, was streaked onto sodium acetate (1 g/L) agar (20 g/L) plates to check spore formation after 10 days of incubation at 25 °C [42].
2.4.3. Growth at Various Temperatures
Each strain pre-grown overnight at 25 °C was inoculated at 1% in YPD broth and statically incubated at both 18 °C and 37 °C for 24 h to test its ability to grow at low and high temperatures [24].
2.4.4. Low pH, Ethanol, and Osmotic Tolerance
Each strain culture was spotted onto YPD agar adjusted either to pH 3.0 or supplemented with 300 g/L of glucose or ethanol content (EtOH) of 5%, 10%, 12% or 15%. The plates to test the ethanol tolerance were freshly poured and sealed with Parafilm to prevent evaporation. All inoculated plates were incubated at 30 °C and colony development was checked daily [24].
2.4.5. SO2 Resistance
Each strain culture was spotted onto YPD agar adjusted to pH 3.0 and supplemented with various concentrations of potassium metabisulphite [29]. A potassium metabisulphite stock solution was filter-sterilized (pore-size, 0.45 µm) and then added to the medium in concentrations of 80 (only for the strains selected for microvifications), 100, 200, 300, and 400 mg/L in order to correspond to half of the concentrations of SO2. The plates were incubated at 30 °C and the colony development was checked daily.
2.4.6. Catalase Activity
The yeast biomass, taken with a sterile 1 µL loop, was added to a drop of 3% (v/v) H2O2 [43]. The development of bubbles indicated positive activity.
2.4.7. Acetic Acid Production
A loopful (1 µL) of biomass of each strain was streaked onto Chalk agar (yeast extract 3 g/L, glucose 10 g/L, calcium carbonate 3 g/L, agar 15 g/L) plates and incubated for 7 d at 25 °C [44]. The presence and extent of a clear halo around the yeast biomass indicated the rate of acetic acid production.
2.4.8. H2S Production
A loopful (1 µL) of biomass of each strain was streaked onto BiGGY agar plates and incubated for 48 h at 25 °C [45]. The color intensity of the biomass indicated the rate of H2S production [20].
2.4.9. β-Glucosidase Activity
Each strain culture was spotted onto Petri plates containing arbutin (5 g/L), yeast extract (10 g/L), 40 drops/100 mL of a 1% solution of ferric ammonium citrate solution, and agar (20 g/L) according to Caridi et al. [46]. After incubation at 25 °C for 7 days, this activity was indicated by the medium changing color, from pale to dark brown.
2.4.10. Pectinase Activity
Each strain culture was spotted onto Petri plates with YNB (6.7 g/L), apple pectin (12.5 g/L), and agar (10 g/L), adjusted to pH 4 with 1 N HCl according to Sidari et al. [47]. After 10 days of incubation at 25 °C, activity was determined by measuring the diameter of the colonies and checking for the presence of a clear halo after flooding the plates with Lugol’s solution and washing with water [27].
2.4.11. Esterase Activity
Each strain culture was spotted onto Petri plates with peptone (10 g/L), NaCl (5 g/L), CaCl2·2H2O (0.1 g/L), Tween 80 (10 g/L), and agar (20 g/L) at pH 6.8 [48]. After 6 days of incubation at 25 °C, the ability to hydrolyze esters was estimated by the presence of a visible opaque precipitate around the colony.
2.4.12. Protease Activity
Each strain culture was spotted onto Petri plates with a medium prepared by mixing the two following solutions: malt extract 3 g/L, yeast extract 3 g/L, peptone 5 g/L, glucose 10 g/L, NaCl 5 g/L, agar 20 g/L (separately sterilized), adjusted to pH 3.5 with 0.1 M HCl; and a skim milk solution (10% w/v) prepared and treated at 100 °C for 10 min. After incubation for 3 days at 25 °C, the presence of a clear halo around the yeast spot indicated protease activity [29].
2.5. Micro-Fermentation Trials in Grape Must
Eleven out of 29 strains, chosen considering the results of the above-reported screening tests, were tested for fermentation vigor after 2 d and 7 d in Trauben saft—100% Direktsaft red grape must (dmBio; Drogerie Markt, Wals-Siezenheim, Austria), both with and without SO2 supplementation. The sugar content of the must was 16 °brix and the pH was 3.20. Aliquots of 100 mL of the must were distributed into flasks, 10 mL of liquid paraffin was added to avoid surface contact with oxygen, and the resulting mixtures were pasteurized at 100 °C for 20 min. Subsequently, half of the flasks were supplemented with 80 mg/L of potassium metabisulphite. All flasks were inoculated in duplicate with 5 mL of 48 h pre-cultures grown in the same red grape must incubated statically at 25 °C. Fermentation progress was monitored by recording weight loss due to the release of CO2. After 2 d and 7 d, fermentation vigor was expressed as g of CO2/100 mL of must [20].
At the end of the fermentation, the wines produced with and without SO2 were analyzed for pH, total titratable acidity (TTA), volatile acidity, ethanol, glucose and fructose, total polyphenols, total flavonoids, and volatile organic compounds (VOCs).
The pH was determined using a pH-meter (OP-208/1, Radelkis, Budapest, Hungary).
The parameters of ethanol, TTA and volatile acidity were determined according to the official methods of International Organisation of Vine and Wine (OIV) [49]. The results were expressed as the mean of four determinations ± standard deviation.
Determination of glycerol and glucose and fructose sugars was accomplished via high performance liquid chromatography (HPLC) using a PU-4003 chromatograph (Pye Unicam, Cambridge, UK) in accordance with the accredited method published by Suhaj and Belajová [50]. Chromatographic separation was performed on the Kromasil 100-5NH2amino column, 250 × 4.6 mm i.d. (EKA Chemicals AB, Sweden), using an RID-10A refractive index detector (Shimadzu, Tokyo, Japan). The RID optical unit was permanently warmed to 40 °C. All wine samples were injected into 20 µL volumes and eluted isocratically with mobile phase acetonitrile:water, 80:20 (v/v). The flow rate of the mobile phase was 1.5 mL/min. The peaks were identified by retention times and quantified via external calibration using the software QC Expert version 2.5 (TriloByte Statistical Software, Pardubice, Czech Republic). Wine samples were diluted twofold with deionized water and filtered through a 0.45 μm syringe filter with a cellulose membrane (Agilent, Waldbronn, Germany) and subsequently injected for HPLC. During the calibration measurements the correlation coefficients were higher than 0.98 for all analyzed compounds. The refractive index detector responses were linear in the range of 0.4–20 g/L for glycerol and 0.5–50 g/L for glucose and fructose.
Total polyphenols (TPC) and total flavonoids (TFC) were determined using a Shimadzu 3600 UV-VIS-NIR spectrophotometer (Shimadzu, Tokyo, Japan) with an accessory. All experiments were performed in duplicate. A 12% (v/v) ethanol was used as a reference.
TPC was determined by applying the Folin–Ciocalteu modified method [51]. Briefly, 100 µL of wine sample was appropriately diluted with 12% (v/v) ethanol, 7.9 mL of distilled water, and 500 µL of Folin–Ciocalteu reagent and mixed in a 20 mL vial. After 10 min, 1.5 mL of 20% sodium carbonate was added, and the contents mixed. Samples were incubated at room temperature in darkness for 60 min, and absorbance was measured at 765 nm. Standard solutions of gallic acid were used to construct the calibration curve (0–1500 mg/L). The results were expressed as gallic acid equivalent (GAE, mg/L).
TFC was evaluated according to the modified method with aluminum chloride [51]. Briefly, 500 µL of wine sample was added to a 10 mL vial containing 1.5 mL of 96% ethanol and 2.8 mL of distilled water. After this, 100 µL of 10% aluminum chloride and 100 µL of 1 M potassium acetate were added, and the contents mixed. After 40 min, the absorbance of the final solution was measured at 415 nm. Standard solutions of quercetin were used to construct the calibration curve. The results were expressed as quercetin equivalent (QE, mg/L).
To analyze VOCs, solid phase micro-extraction (SPME) was carried out using a polydimethylsiloxan-divinylbenzene fiber, coating thickness 65 μm (Supelco, Bellefonte, PA, USA), immersed in 10 mL of wine sample and mixed at 6 Hz on a magnetic stirrer during 30 min at 20 °C. The extracted compounds were analyzed via gas chromatography–mass spectrometry (GC-MS) using a 6890N gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) coupled to a 5973 mass spectrometric detector (Agilent Technologies). The SPME fiber was placed in the inlet of the chromatograph for 2 min at 250 °C so as to desorb the extracted compounds. The gas chromatographic separation took place in a DB-WAXetr high polarity polyethylene glycol column (length 30 m, inner diameter 0.25 mm, stationary phase thickness 0.5 μm; Agilent Technologies) using a temperature program of 35 °C for 1 min, 5 °C for 1 min and 250 °C for 1 min. The split ratio was 10:1. The average velocity of the He carrier gas was 34 cm·s-1 at constant flow. An ionization voltage of 70 eV was used. Compounds were identified by comparison of mass spectra with the NIST 14 MS library (National Institute Standards and Technology, Gaithersburg, MD, USA). Analysis was carried out solely at orientation level with relative quantification data expressed as peak area percentage.
2.6. Statistical Analysis
Data were analyzed using a one-way ANOVA and Tukey’s test at 5% probability level, using the online tool at https://www.statskingdom.com/doc_anova.html (accesed on 9 March 2021).
3. Results
3.1. Yeast Strains Grouping and Identification
Table 2 reports the grouping of strains via 5.8-ITS rRNA analysis and RFLP and their molecular identification by sequencing and comparison with the GenBank database.

Table 2.
Yeast identification by PCR-RFLP analysis of the 5.8-ITS rRNA and sequencing.
The ITS amplicons had sizes ranging from 380 to 800 bp. Eighteen RFLP patterns were observed; different profiles were also assigned to strains belonging to the same species (H. uvarum, L. thermotolerans, M. pulcherrima, P. fermentans, T. delbrueckii, S. cerevisiae).
From among the twenty-nine yeast isolates, five were identified as M. pulcherrima, five as T. delbrueckii, five as S. cerevisiae, three as H. uvarum, three as P. fermentans, two as L. thermotolerans, one as Candida dubliniensis, one as Debaryomyces hansenii, one as Metschnikowia aff. chrysoperlae, one as Meyerozyma guilliermondii, one as Pichia kluyveri, and one as Zygosaccharomyces bailii.
The accession numbers of the yeast strains sequenced and deposited to GenBank are: MZ207954 C. dubliniensis CCY 29-178-1, MZ207959 D. hansenii 5-1-6, MZ207966 H. uvarum 9-2-1, MZ207967 H. uvarum 67/14, MZ207960 L. thermotolerans 5-1-1, MZ207958 L. thermotolerans 5-1-3, MZ207961 M. aff. crysoperlae 11-1-4, MZ207962 M. pulcherrima 11-1-5, MZ207963 M. pulcherrima 11-1-7, MZ207955 M. pulcherrima 125/14, MZ207969 M. guilliermondii 12-5-1, MZ207968 P. fermentans 12-4-4, MZ207970 P. kluyveri PDA W 9, MZ207956 S. cerevisiae 60/16, MZ207957 S. cerevisiae 15-1-552, MZ207953 T. delbrueckii 3-16-1, MZ207964 T. delbrueckii 21-1-5, MZ207965 Z. bailii 24-1-25.
Figure 1 reports the clustering of the strains obtained from the FTIR analysis. The mid-infrared range of 4000–500 wavelength/cm2 (25,000–2500 nm) is used to excite atoms in molecular bonds, causing them to vibrate. A spectrum can be measured and calculated by light absorption. This specific absorption is then attributed to cell components (e.g., polysaccharides, fatty acids, proteins, mixed region, fingerprint region) [52] used for identification [53]. To enhance the resolution of complex bands and to minimize difficulties evolving from inevitable baseline shifts, the second derivations of the original spectra were calculated. This made it possible to obtain a list of the most similar spectra from the database [54], leading to identification at the species level.
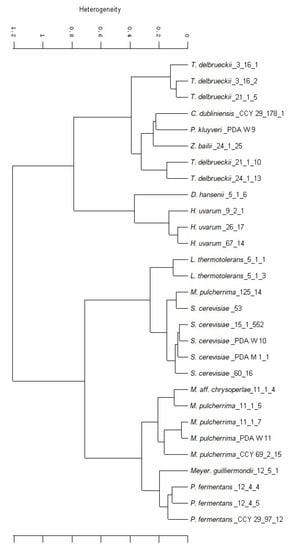
Figure 1.
Cluster analysis of the twenty-nine yeast strains as studied by Fourier-Transform Infrared Spectroscopy (FTIR).
Isolates were classified into sub-clusters by defining a spectral distance as a value for separation on the strain level. The spectral distance chosen was 0.1. The grouping reported in the figure closely corresponds to the results obtained from sequencing.
3.2. Yeast Colonies, Cells, and Spores Characteristics
Table 3 reports the morphology, color, and texture of the colonies of the different strains grown on YPD agar. The strains belonging to the Metshnikowia genus exhibited biomass turning red during the prolonged incubation time.

Table 3.
Colony characteristics of yeast strains grown on YPD agar.
The microscopic morphologies of strains belonging to the species chosen as representative are reported in Figure 2.
D.hansenii 5-1-6 exhibited conjugation tubes; T. delbrueckii 3-16-1, Z. bailii 24-1-25, S. cerevisiae PDA W 10, and H. uvarum 9-2-1 differentiated spores [55,56,57]. Figure 3 reports on the microscopic observation of selected strains grown on sodium acetate agar.
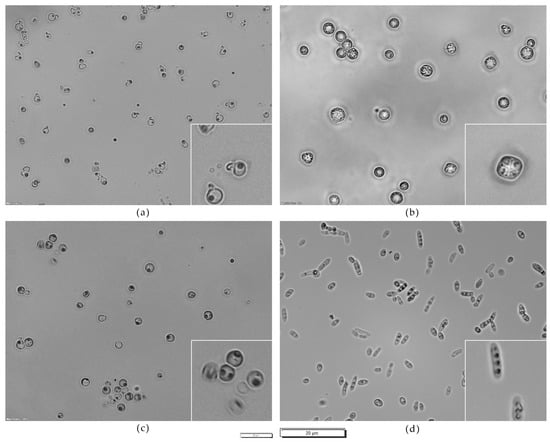
Figure 3.
Microscopic observation of (a) D. hansenii 5-1-6, (b) S. cerevisiae PDA W 10, (c) T. delbrueckii 3-16-1, (d) Z. bailii 24-1-25 grown on sodium acetate agar. Zoomed parts: (a) cells with conjugation tubes, (b–d) spores. Magnification 40×; scale 20 µm (small bar for global views; large bar for detailed views).
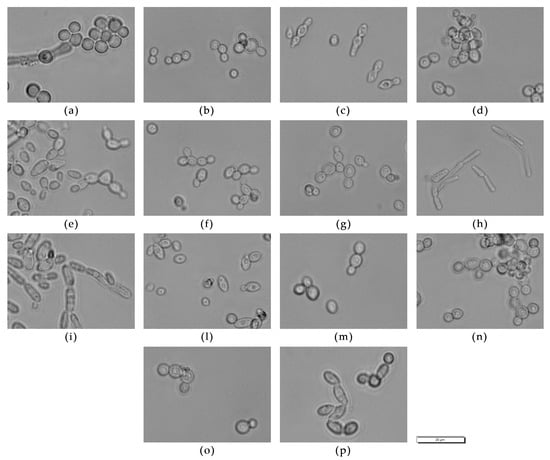
Figure 2.
Optical microscopy of representative strains: (a) C. dubliniensis CCY 29-178-1, (b) D. hansenii 5-1-6, (c) H. uvarum 9-2-1, (d) L. thermotolerans 5-1-3, (e) M. aff. chrysoperlae 11-1-4, (f) M. pulcherrima 125/14, (g) M. pulcherrima 11-1-7, (h) M. guilliermondii 12-5-1, (i) P. fermentans 12-4-4, (l) P. kluyveri PDA W 9, (m) S. cerevisiae 60/16, (n) T. delbrueckii 21-1-5, (o) T. delbrueckii 3-16-1, (p) Z. bailii 24-1-25. Magnification 40×; scale 20 µm.
3.3. Yeast Screening
All strains grew in YPD broth as dispersed cells, with the exception of the strain M. pulcherrima CCY 69-2-15, which exhibited growth with aggregated cells.
After two days of incubation in YPD broth, the quantity of CO2 produced ranged from none (empty Durham tubes) to large (almost-full Durham tubes). The strains M. guilliermondii 12-5-1 and D. hansenii 5-1-6 did not produce gas, while the strain C. dublinensis CCY 29-178-1 produced a small quantity. The strains belonging to the M. pulcherrima species (strains 11-1-7, 125/14 and CCY 69-2-15) and the strains T. delbrueckii 3-16-1 and 3-16-2, S. cerevisiae 60/16, 15-1-552, 53, PDA W 10, PDA M 1/1, L. thermotolerans 5-1-3 and 5-1-1, M. aff. chrysoperlae 11-1-4 showed moderate gas production (half-full Durham tubes), the strains M. pulcherrima 11-1-5, T. delbrueckii 21-1-5, 21-1-10, 24-1-13, Z. bailii 24-1-25, M. pulcherrima PDA W 11, H. uvarum 9-2-1 and 67/14, P. fermentans 12-4-5 and CCY 29-97-12 were characterized by above-average gas production (more than half-full Durham tubes), and the strains H. uvarum 26/17, P. fermentans 12-4-4, and P. kluyveri PDA W 9 exhibited large gas production (almost full Durham tubes). After three or more days, all strains showed a very large CO2 production (full Durham tubes) except the strains M. guilliermondii 12-5-1 and D. hansenii 5-1-6, which did not ferment. Moreover, after tube vortexing, the strains H. uvarum 67/14 and 26/17, P. fermentans 12-4-4 and 12-4-5, and P. kluyveri PDA W 9 produced thick and persistent foam.
Five out of twenty-nine strains exhibited veils: M. pulcherrima 125/14 (weak), P. fermentans 12-4-4 and 12-4-5, P. kluyveri PDA W 9 (abundant), P. fermentans CCY 29-97-12 (abundant and thick).
Concerning growth at 37 °C, the strains T. delbrueckii 3-16-1 and 3-16-2, D. hansenii 5-1-6, L. thermotolerans 5-1-1, M. aff. chrysoperlae 11-1-4, M. pulcherrima 11-1-5 and CCY 69-2-15, T. delbrueckii 21-1-5, 21-1-10, 24-1-13, Z. bailii 24-1-25, and H. uvarum 9-2-1, 26/17 and 67/14 were unable to grow. Strains showing moderate to intense growth were, in order: S. cerevisiae 53, C. dubliniensis CCY 29-178-1, M. pulcherrima 125/14, S. cerevisiae 60/16, 15-1-552, PDA W 10, and PDA M 1/1, P. fermentans 12-4-5, and PDA W 9 P. kluyveri. Strains that exhibited growth as poor pellets were M. pulcherrima PDA W 11 and P. fermentans 12-4-4, while the strains L. thermotolerans 5-1-3, M. pulcherrima 11-1-7, and M. guilliermondii 12-5-1 showed some clouding of the culture broth.
Concerning growth at 18 °C, all strains were able to grow to a certain degree; ordered from lowest to most intense growth: M. aff. chrysoperlae 11-1-4, S. cerevisiae 53, M. pulcherrima 125/14, S. cerevisiae PDA W 10, PDA M 1/1, L. thermotolerans 5-1-3, 5-1-1, H. uvarum 9-2-1, 67/14, 26/17, P. fermentans 12-4-4, 12-4-5, CCY 29-97-12, T. delbrueckii 3-16-1, 3-16-2, C. dubliniensis CCY 29-178-1, S. cerevisiae 60/16, 15-1-552, M. pulcherrima 11-1-5, 11-1-7, CCY 69-2-15 M. pulcherrima, T. delbrueckii 21-1-5, 21-1-10, 24-1-13, M. pulcherrima PDA W 11, P. kluyveri PDA W 9, D. hansenii 5-1-6, Z. bailii 24-1-25, and M. guilliermondii 12-5-1.
The ability of the strains to grow under the stressed conditions possibly occurring during must fermentation was studied to consider their potential application in vinification. All strains were able to grow well in an osmotic stress condition (300 g/L of glucose) and at pH 3.0. Table 4 reports the biochemical activities of the twenty-nine strains tested.

Table 4.
In vitro tests carried out on the twenty-nine yeast strains.
Concerning ethanol tolerance after one day of incubation (Table 4a), all strains grew well in the medium supplemented with 5% of ethanol, while differences were observed with increasing ethanol concentration. In particular, with 10% of ethanol three strains grew very well—S. cerevisiae PDA M 1/1, L. thermotolerans 5-1-3, P. kluyveri PDA W 9. The strains that tolerated 12% of ethanol to various extents were those belonging to S. cerevisiae, L. thermotolerans, M. pulcherrima 125/14, P. kluyveri PDA W 9, Z. bailii 24-1-25, and two strains of T. delbrueckii. This trend was observed also in the presence of 15% of ethanol, with the exception of the strains P. kluyveri PDA W9 and Z. bailii 24-1-25, which did not grow.
After one day of incubation in media supplemented with increasing concentrations of SO2 (Table 4a), S. cerevisiae strains showed good growth indicating full resistance (to 400 mg/L of metabisulphite), with the exception of the S. cerevisiae 60/16 which did not experience good growth with more than 200 mg/L of metabisulphite. This strain did show good growth in media supplemented with 300 and 400 mg/L of metabisulphite after two and three days of incubation, respectively. Non-Saccharomyces strains showed marked differences in a strain-dependent manner. The strains belonging to Lachancea, Debaryomyces, Hanseniaspora, and Meyerozyma genera exhibited no growth even at the lowest SO2 concentration. Some of the strains needed longer incubations to confirm their incapability to grow in presence of SO2 or to resist at different concentrations. As example, L. thermotolerans 5-1-1 grew after two days of incubation in the presence of 100 mg/L of metabisulphite, while at the highest SO2 concentration only P. kluyveri PDA W 9 and the above mentioned S. cerevisiae 60/16 improved their growth over the incubation period.
All eleven strains chosen for the microvinification trials exhibited good growth in the presence of 80 mg/L of metabisulphite.
All strains were catalase-positive, with non-Saccharomyces strains exhibiting the highest activity, especially strains belonging to the species T. delbrueckii, D. hansenii, M. pulcherrima, H. uvarum, and P. fermentans (Table 4b).
Only 6.9% of the strains were positive for acetic acid production. These belonged to the species H. uvarum (Figure 4).
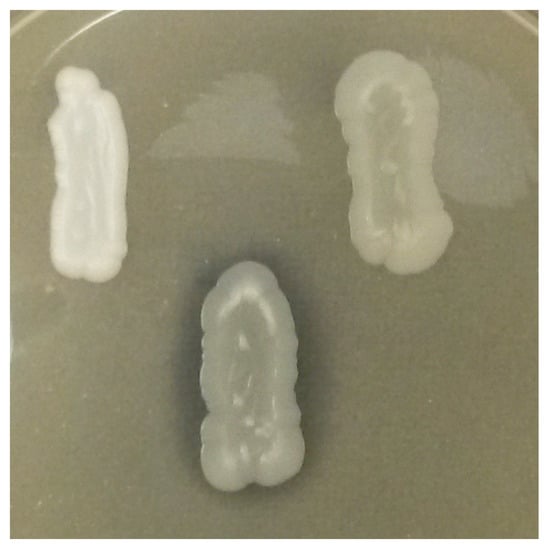
Figure 4.
Yeast strains’ acetic acid production according to the absence/presence of a halo around the biomass grew on 90 mm Petri plate. Clockwise from left: negative strain M. pulcherrima PDA W 11, positive strains H. uvarum 9-2-1 and H. uvarum 67/14 exhibiting different degrees of production.
The strains exhibited a wide range of H2S production with the biomass color ranging from white to black and passing through intermediate tints (Figure 5). The majority of the strains (41.38%) had hazel biomass followed by dark hazel (37.93%), black (13.79%), pale hazel (3.45%), and white (3.45%) (Table 4a).
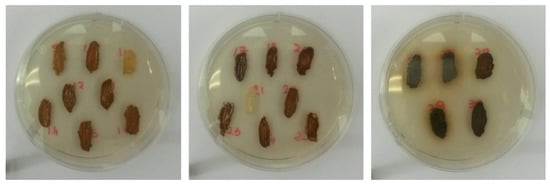
Figure 5.
Different degrees of H2S production among yeast strains according to the color of the biomass; white: low production, hazel: medium production, dark: high production.
Thirty-one percent of the strains exhibited light to strong β-glucosidase activity (Figure 6). The highest activity was recorded for strains belonging to the genus Metschnikowia and the strain P. kluyveri PDA W 9 (Table 4b).
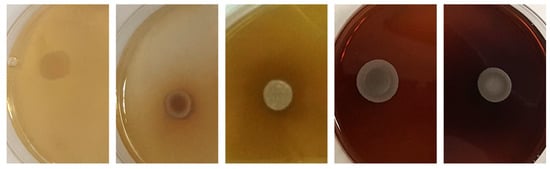
Figure 6.
Different degrees of β-glucosidase activity among yeast strains according to the absence/presence of brown color in the medium. From left to right: S. cerevisiae 60/16, D. hansenii 5-1-6, M. pulchrerrima 11-1-5, M. pulcherrima 125/14, M. pulcherrima 11-1-7.
All strains grew on the medium supplemented with pectin. The strains C. dublinensis CCY 29-178-1, P. fermentans 12-4-4 and CCY 29-97-12 exhibited wider biomass spots. Moreover, 13 out of 29 strains exhibited a halo around the biomass after flooding with Lugol’s solution (Table 4b). Shortly after washing, the strongest halos remained visible while the faint ones rapidly disappeared (Figure 7).
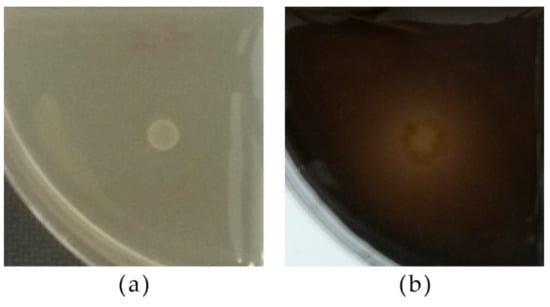
Figure 7.
Pectinase activity of yeast strains according to growth on the medium and the presence of a clear halo after washing with Lugol’s solution; (a) P. fermentans 12-4-5, (b) P. kluyveri PDA W 9.
The esterase activity of strains ranged from absent to strong (Figure 8). Only C. dubliniensis CCY 29-178-1, M. aff. chrysoperlae 11-1-4 and M. pulcherrima 11-1-5 exhibited high esterase activity while two strains—M. pulcherrima 125/14 and M. guilliermondii 12-5-1—were characterized by moderate activity (Table 4b).
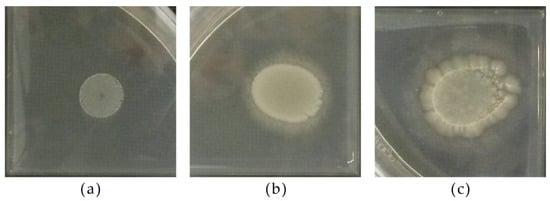
Figure 8.
Different degrees of esterase activity among yeast strains according to the presence of precipitate around the biomass: (a) no activity (S. cerevisiae 53); presence of activity in (b) M. pulchrerrima 11-1-5 and (c) C. dubliniensis CCY 29-178-1.
A total of 58.62% of strains were positive for protease activity. Those with the highest activity were among the non-Saccharomyces yeasts (Metschnikowia, Hanseniaspora, and Pichia) (Table 4b; Figure 9).

Figure 9.
Yeast strains’ protease activity according to the absence/presence of a halo around the biomass. Clockwise from left: negative strain T. delbrueckii 21-1-5, strains with different degrees of activity M. aff. chrysoperlae 11-1-4, S. cerevisiae 60/16, T. delbrueckii 21-1-10.
Eleven of the twenty-nine strains—D. hansenii 5-1-6, H. uvarum 26/17, L. thermotolerans 5-1-1, M. pulcherrima 125/14, M. pulcherrima 11-1-7, S. cerevisiae 15-1-552, S. cerevisiae 53, S. cerevisiae PDA W 10, S. cerevisiae PDA M 1/1, T. delbrueckii 3-16-1, Z. bailii 24-1-25—were chosen for micro-fermentation trials considering the results of the screening tests. The choice was made balancing the screening parameters’ results for each strain while also taking into account the possibility of further improving their characteristics through different techniques, such as hybridization. In detail, the strains chosen exhibited no or very low acetic acid production, low to medium H2S production, good ethanol and SO2 tolerance after one or two days, and a varied range of catalase, β-glucosidase, esterase, pectinase, and protease activities (Table 4).
3.4. Enological Characterization by Micro-Fermentations
The eleven strains selected through the screening process were tested using micro-fermentations in order to evaluate their fermentation performance. The non-Saccharomyces strains showed lower fermentation vigor than the S. cerevisiae strains, which exhibited the highest fermentation vigor. Similar observations were reported for M. pulcherrima 125/14. The lowest values reported were for D. hansenii 5-1-6. Similar results were reported for fermentation vigor without and with SO2, confirming the results obtained by screening on plates and indicating the resistance of the strains to the used SO2 concentration (Table 5).

Table 5.
Mean values of fermentation vigor, expressed as g CO2/100 mL, of the eleven yeast strains tested in red grape must.
Table 6, Table 7 and Table 8 show the physicochemical parameters of the wines produced using the eleven selected yeast strains. pH, TTA, and volatile acidity values for the wines produced with each strain are reported in Table 6. All produced wines had a pH higher than the un-inoculated musts, and the TTA values were linearly correlated to pH. The pH range for the trials without SO2 was 3.34–3.48, while for the trials with SO2 it was 3.29–3.44. The volatile acidity ranged from 0.12 to 1.68 g/L of acetic acid in the absence of SO2.

Table 6.
pH, total titratable acidity, and volatile acidity of the wines produced by inoculating the red must without and with SO2 for the eleven yeast strains.

Table 7.
Ethanol, glucose, and fructose content of the wines produced by inoculating the red must without and with SO2 for the eleven yeast strains.

Table 8.
Glycerol, total polyphenol, and total flavonoids content of the wines produced by inoculating the red must without and with SO2 for the eleven yeast strains.
Concerning ethanol production (Table 7), S. cerevisiae strains consumed glucose and fructose as expected, with produced ethanol percentages as high as 8.39% and 8.23% by S. cerevisiae 15-1-552 without SO2 and S. cerevisiae PDA W10 with SO2, respectively. M. pulcherrima 11-1-7 fermented 33 and 30 g/L of glucose in micro-fermentation without and with SO2, respectively, and 23 and 19 g/L of fructose in micro-fermentation without and with SO2, respectively, producing the lowest percentages of ethanol (4.15% and 4.10% without and with SO2). L. thermotolerans 5-1-1 fermented 80 g/L of glucose in trials without SO2 and consumed almost all of the glucose in trials with SO2; it fermented 70 and 81 g/L of fructose in the absence and presence of SO2, respectively, producing the highest values of ethanol (approximately 7.15–7.25%). Moreover, T. delbrueckii 3-16-1 produced wines with approximately 7% of ethanol. The lowest ethanol production was recorded for D. hansenii 5-1-6.
Among the non-Saccharomyces yeasts, M. pulcherrima 11-1-7 produced wines with the highest glycerol concentration, followed by Z. bailii 24-1-25, while the lowest concentration was produced by D. hansenii 5-1-6 (Table 8).
Comparing the total concentrations of polyphenols and flavonoids between the inoculated and un-inoculated musts (Table 8), in wines without SO2, increases or decreases in concentrations of polyphenol and flavonoid were reported in a strain-dependent manner. By contrast, in wines produced with SO2, a decrease in concentration of flavonoids was observed for all strains tested (Table 8).
During GC-MS analysis, various volatile aroma compounds were detected. Most of them are well known as contributors to wine aroma [58,59]. All fermented samples, with the exception of that fermented by D. hansenii 5-1-6, contained high amounts of 2-phenylethanol, which is an established aroma compound with a sweet, floral, rosy character. The highest amounts of this compound were produced, among samples fermented with SO2 (Table S1), by L. thermotolerans 5-1-1. Only samples fermented by S. cerevisiae strains, together with that fermented by M. pulcherrima 125/14, contained remarkable amounts of 4-vinylguaiacol, which is an aroma compound with a sweet-smoky character, typical for Traminer wines or for whisky. Various strains produced medium-chain fatty acids, a phenomenon more pronounced in samples with SO2 (Table S1). Several samples contained considerable amounts of pyran and furan derivatives, which were probably sourced or metabolized from the UHT-treated substrate. Dodecanoic acid was detected only in various fermented samples treated with SO2, while only some fermented samples without SO2 (Table S2) contained propylene glycol, 4-cyclopentene-1,3-dione, diethyleneglycol ethylether, 2-methylthiolane, hexanoic acid and nonanoic acid.
4. Discussion
This contribution aimed to give a guide as comprehensive as possible to methods for wine yeast selection while studying our own strains. We decided to test the strains for all the characteristics to obtain for each of them a complete profile in view of possible genetic improvement. The simple trials used allowed us to exclude those strains possessing the worst features (alone or in combination) for wine-making—high acetic acid and H2S production, low ethanol and SO2 tolerance, foam production, zero or low enzymatic activity—selecting the best strains to test in must fermentations.
The strains here reported as M. pulcherrima are to be considered M. pulcherrima-like strains due to the difficulty in assigning an exact taxonomic position, as a result of a lack of distinctive morphological and physiological properties among species belonging to the M. pulcherrima clade and the lack of rDNA barcode gaps [60,61,62,63]. In contrast to Sipiczki [63], our strain of M. aff. chrysoperlae is a pigmented strain, as are all of our strains of M. pulcherrima. In our study, since it is known that for taxonomical proposes it is necessary to use more gene markers in order to well classify yeast strains, we chose to use only ITS fragment sequencing as an identification tool in combination with the RFLP and FTIR approaches. The sequencing of ITS regions is suitable as a rapid and preliminary identification tool for yeasts, which can then be deeply taxonomically analyzed exploiting other molecular markers as shown by previous studies [60,61,62,63].
FTIR spectroscopy facilitates the grouping of yeasts based on the chemical composition of their cells. It is a high-throughput method requiring no chemicals to be used, and therefore is cheap and convenient. Our results presented in Figure 1 demonstrate the overall success of this method to group yeast strains similarly to the sequencing-based approach, which is much more tedious and costly. Based on this, and based on our experience and several other studies [40,53,54], we can recommend the use of FTIR spectroscopy for preliminary grouping of strains and reducing the number of strains in order to pass to further evaluation, by elimination of those that are most probably duplicates or multiplicates.
The initial yeast concentration used in the screening might differ among yeast strains and species due to different morphology and size; therefore, we compared each strain with its own control condition, avoiding the comparison of different yeast species.
The presence of sulfur off-flavor in wine as a result of yeast metabolism is negatively correlated to wine quality, as it is an undesired wine off-flavor, and it also gives rise to health concerns. The screening of yeast strains that produce zero or low H2S is particularly necessary to take into account for the production of organic and sulfite-free wines. The degree of H2S production by non-Saccharomyces yeasts and the wide intra-species variability observed by different authors are consistent with the findings of this study [28,29,64]. By contrast, our findings for H. uvarum and M. pulcherrima conflict with the results of Polizzotto et al. [22] and Belda et al. [65] who reported absent or low sulfite-reductase activity.
The ability of the non-Saccharomyces strains under study to grow under stressed conditions was assayed to understand their potential application in vinification. The strains’ ability to grow at low pH and in high concentrations of glucose makes them suitable for harsh environments; in addition, their growth at different temperatures makes them suitable for red and white vinification. Our results confirm that S. cerevisiae is the most ethanol-tolerant species compared to many non-Saccharomyces. Concerning the non-Saccharomyces strains, some of them exhibited higher ethanol tolerance (up to 12% or to a lesser extent up to 15%) compared to results from the literature [28,62,63,64,66,67,68], confirming the results of Mukherjee et al. [69] for L. thermotolerans, T. delbrueckii and Z. bailii. The use of SO2 in winemaking is mandatory to control spoilage and microorganisms and to protect wines from oxidation. Therefore, it is important for wine yeasts to be able to tolerate SO2 at the dosage commonly used for commercial wine fermentation; on the other hand, the health aspect has to be taken into account. For this reason, although all strains were tested at increasing concentrations of SO2 (100–400 mg/L), the microvinification trials were carried out using a low SO2 concentration (80 mg/L). Yeasts belonging to Torulaspora, Metschnikowia, Zygosaccharomyces, and Lachancea genera were less sensitive to SO2 than commonly considered [23] and this is consistent with other authors’ results [28,29]. Concerning the strains’ contribution to the pH of wine, our strain of L. thermotolerans confirms the existing strains’ variability in producing lactic acid and consuming malic acid [66]. The selected L. thermotolerans strain did not significantly influence pH and total acidity compared to the S. cerevisiae controls (53, PDA W 10, PDA M1/1) (Table 6). Previous studies report variability in lactic acid production from 0.2 g/L to approximately 10 g/L and reductions in pH from insignificant differences to 0.5 [70,71,72]. However, many other quality parameters can be improved by L. thermotolerans, so the choice of strain can be interesting. Results from the catalase test gave information on the ability of strains to cope with oxidative stress and to perform better during fermentation [73]. All of the strains tested in this study were catalase positive to various extents and in agreement with other authors for H. uvarum, Candida, Pichia, D. hansenii, M. pulcherrima [27,28].
The wine industry makes use of protease and pectinase to prevent wine haze and facilitate wine clarification, together with glycosidase to favor the expression of grape varietal aromas [74]. Wine yeasts can possess one or more natural enzymatic activities useful for vinification [32,61,75,76,77]. Yeast enzymes of interest include esterases, glycosidases, proteases, and cellulases able to hydrolyze structural components [78,79] that determine, based on their presence and intensity, the sensorial complexity of wines [80].
Our results regarding β-glucosidase, which breaks down glycosidic complexes releasing terpenes and other volatile compounds, in our selected yeast strains are consistent with other authors reporting high β-glucosidase incidence in Debaryomyces and Pichia [65,77,81] and especially in M. pulcherrima, mostly in a strain-dependent manner [28,29,32,65,77,81,82,83]. Our strains positive for esterase, which hydrolyzes long-chain esters, belonged to the genera Candida, Meyerozyma, and Metschnikowia, in agreement with other studies [29,84]. The protease activity was strongest in our strains of H. uvarum, L. thermotolerans, M. pulcherrima, Pichia spp., T. delbrueckii, and S. cerevisiae and these results were in agreement with other studies [27,28,32,65,85,86,87] while they were in conflict with Comitini et al. [29] who reported no strains of L. thermotolerans, M. pulcherrima, T. delbrueckii, and S. cerevisiae exhibiting any protease activity. Similar negative protease S. cerevisiae strains were reported by Charoenchai et al. [33]. Some authors reported no yeasts (including Candida, Debaryomyces, Hanseniaspora, Metschnikowia, Pichia, Saccharomyces, Torulaspora) possessing pectinolytic activity [33,77]. Indeed, pectinolytic activity is rarely found in wine-related yeasts but it is reported in Candida, M. pulcherrima, L. thermotolerans, T. delbrueckii, H. uvarum, and S. cerevisiae [21,27,82,88] and our results were in agreement with these authors.
Fermentation vigor is a good indicator of the strain’s promptness and of the progress of the fermentation. It is easy to monitor as a weight measurement, which is directly proportional to sugar consumption and ethanol synthesis, determining the fermentation power. As expected, the S. cerevisiae strains had higher fermentation vigor than the non-Saccharomyces strains, in agreement with Caridi et al. 2002 [20], with the exception of the M. pulcherrima 125/14 strain. This strain, in fact, had higher fermentation vigor than the usually reported 4.5% ethanol (v/v) [89]. However, it is reported that some strains of M. pulcherrrima can produce 9–11.5% ethanol [90], with an ethanol tolerance of at least 6% with a few exceptions above 9% [28].
Acetic acid is one of the compounds that impact the sensory profile of wine, contributing to definitions of its quality. An acetic acid concentration of 0.7–1.1 g/L is considered unpleasant; the maximum acceptable limit for volatile acidity in most wines is 1.2 g/L of acetic acid [91,92]. Values in the range 0.2–0.7 g/L are usually considered optimal [91]. Although most non-Saccharomyces are considered high acetic acid producers [93,94] other evidence indicates T. delbrueckii, L. thermotolerans, M. pulcherrima as low producers [29,95,96,97,98,99]. Our results were consistent with these reports, with all selected being within the optimal range with the exception of H. uvarum 26/17, the highest producer as reported for the species by Aponte and Blaiotta [99]. However, the behavior of this strain confirmed the utility of the visible halo as a screening test [16,77,100].
The quality of wine is also linked to the glycerol concentration, although this has recently been contested [101]. Noble and Bursick [102] indicated 5.2 g/L as the taste threshold with a maximum acceptable level of 25 g/L [103]. It is usually reported that glycerol production is higher in wines fermented with non-Saccharomyces compared to those produced with S. cerevisiae [101]. In addition, Zhu et al. [104] reported higher glycerol concentration for non-Saccharomyces than for S. cerevisiae, which was in agreement with the results of the majority of our tested strains. The role of this metabolite must be considered taking into account its relationship with ethanol and acetic acid production. In fact, higher glycerol production could result in a reduction in ethanol production [105] and a higher production of acetic acid [106].
The role of phenolics in wine is related to the sensorial and health aspects. The effect of SO2 in vinification is well known [107] as is the contribution of S. cerevisiae to the polyphenolic profile of wine [108,109,110,111,112,113]. Recently, Morata et al. [114] reported different antocyanin adsorption by non-Saccharomyces with the goal of improving the color stability of wine. The different polyphenols concentrations in wines produced by our tested yeasts could be attributable to the strain (production of metabolites and cell wall adsorption) used in wines produced without SO2 and to the concurrent role of strain and SO2 in wines produced with the addition of SO2.
Analysis of volatile compounds using GC-MS aimed to determine the production of known and described aroma-active compounds by individual yeast strains. Although the aroma character of some of the compounds has been described as “pleasant“ and their presence in wine is often appreciated, those described as “unpleasant” (or off-flavors) may be very important to achieve the required complexity, fullness and/or typicality of a wine aroma, when present at appropriate concentrations and in certain combinations. Lists of common aroma-active compounds in various types of wine are available in the literature, which facilitates their tracing in experimental samples. However, interpretation of the analytical data need not be straightforward and usually requires combination with sensorial evaluation of the wine bouquet [58,59].
Based on their performance, we propose S. cerevisiae PDA W 10, L. thermotolerans 5-1-1 and M. pulcherrima 125/14 as potential wine starters. Due to the characteristics of L. thermotolerans and M. pulcherrima, these yeasts are normally used in mixed or sequential fermentations together with S. cerevisiae [66,114] in order to complete the fermentation process, guaranteeing the quality of the wine. S. cerevisiae PDA W 10 could be used as pure inoculum. Moreover, after further studies on competitive abilities of the strains and examination of the killer trait of S. cerevisiae strains, L. thermotolerans 5-1-1, M. pulcherrima 125/14, and S. cerevisiae PDA W 10 could be used as co- or sequential inocula. It must be highlighted that the M. pulcherrima 125/14 strain has interesting properties, such as its fermentation vigor, that allows for the consideration of the use of this strain as pure inoculum as well.
The step-by-step process for screening and selecting wine yeasts is as follows: yeast isolation/revitalization of stored yeasts, yeasts identification, Petri plate screening for useful enological characteristics, evaluation of results and choice of strains, use of the chosen strains for micro-vinification, analyses of the produced wines, evaluation of results and identification of wine starter strains.
5. Conclusions
Even in the present era of modern molecular technologies, we believe it is important to maintain the know-how of classical methods for selecting wine strains useful for production of high-quality wine. The screening and testing procedures led to the selection of strains that could be the starting point for improvement by hybridization, mutagenesis, or genome engineering. Finally, we believe that this contribution reporting procedures and images could help other scientific groups in screening their own isolated yeasts for wine production.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9112223/s1: Table S1. Volatile organic compounds of wines with SO2 produced using the eleven yeast strains, Table S2. Volatile organic compounds of wines without SO2 produced using the eleven yeast strains.
Author Contributions
Conceptualization, R.S., D.P. and T.K.; methodology, R.S., D.P. and T.K.; investigation, R.S., K.Ž., B.T., E.B., T.C., M.B., A.P., M.P.; data curation, R.S., D.P.; writing—original draft preparation, R.S.; writing—review and editing, R.S., D.P. and T.K.; funding acquisition, D.P.; supervision, R.S and D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the by the Slovak Research and Development Agency [APVV-16-0264] and the VEGA project 2/0099/21.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Sidari, R.; Ženišová, K.; Tobolková, B.; Belajová, E.; Cabicarová, T.; Bučková, M.; Puškárová, A.; Planý, M.; Kuchta, T.; Pangallo, D.; Wine yeasts selection: an experimental review by images; Microorganisms and its supplementary material.
Acknowledgments
The authors wish to thank Arch. Daniela Sidari ([D] Graphics & Photography, www.danielasidari.com) for the microscopy figures realization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2013, 111, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Mateo, J.J.; Maicas, S. Application of non-Saccharomyces yeasts to wine-making process. Fermentation 2016, 2, 14. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Binati, R.L.; Lemos, J.F., Jr.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Ženišová, K.; Cabicarová, T.; Sidari, R.; Kolek, E.; Pangallo, D.; Szemes, T.; Kuchta, T. Effects of co-fermentation with Lachancea thermotolerans or Metschnikowia pulcherrima on concentration of aroma compounds in Pinot Blanc wine. J. Food Nutr. Res. 2021, 60, 87–91. [Google Scholar]
- Benito, A.; Calderón, F.; Benito, S. The influence of non-Saccharomyces species on wine fermentation quality parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Zambonelli, C. Microbiologia e Biotecnologia dei Vini, 1st ed.; Edagricole: Bologna, Italy, 1988; pp. 1–228. [Google Scholar]
- Tristezza, M.; Fantastico, L.; Vetrano, C.; Bleve, G.; Corallo, D.; Grieco, F.; Mita, G.; Grieco, F. Molecular and technological characterization of Saccharomyces cerevisiae strains isolated from natural fermentation of Susumaniello grape must in Apulia, Southern Italy. Int. J. Microbiol. 2014, 897428, 1–11. [Google Scholar] [CrossRef]
- Perpetuini, G.; Di Gianvito, P.; Arfelli, G.; Schirone, M.; Corsetti, A.; Tofalo, R.; Suzzi, G. Biodiversity of autolytic ability in flocculent Saccharomyces cerevisiae strains suitable for traditional sparkling wine fermentation. Yeast 2016, 33, 303–312. [Google Scholar] [CrossRef]
- Vigentini, I.; Barrera Cardenas, S.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Foschino, R. Use of native yeast strains for in-bottle fermentation to face the uniformity in sparkling wine production. Front. Microbiol. 2017, 8, 1225. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Berbegala, C.; Grieco, F.; Tufariello, M.; Spano, G.; Capozzi, V. Selection of indigenous yeast strains for the production of sparkling wines from native Apulian grape varieties. Int. J. Food Microbiol. 2018, 285, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Feghali, N.; Bianco, A.; Zara, G.; Tabet, E.; Ghanem, C.; Budroni, M. Selection of Saccharomyces cerevisiae starter strain for Merwah wine. Fermentation 2020, 6, 43. [Google Scholar] [CrossRef]
- Csoma, H.; Kállai, Z.; Antunovics, Z.; Czentye, K.; Sipiczki, M. Vinification without Saccharomyces: Interacting osmotolerant and “spoilage” yeast communities in fermenting and ageing botrytised high-sugar wines (Tokaj Essence). Microorganisms 2021, 9, 19. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tasting the terroir of wine yeast innovation. FEMS Yeast Res. 2020, 20, foz084. [Google Scholar] [CrossRef] [PubMed]
- Regodón, J.A.; Pérez, F.; Valdés, M.E.; De Miguel, C.; Ramírez, M. A simple and effective procedure for selection of wine yeast strains. Food Microbiol. 1997, 14, 247–254. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Carrascosa, A.V.; Barcenilla, J.M.; Pozo-Bayón, M.A.; Polo, M.C. Autolytic capacity and foam analysis as additional criteria for the selection of yeast strains for sparkling wine production. Food Microbiol. 2001, 18, 183–191. [Google Scholar] [CrossRef]
- Caridi, A.; Cufari, A.; Ramondino, D. Isolation and clonal pre-selection of enological Saccharomyces. J. Gen. Appl. Microbiol. 2002, 48, 261–267. [Google Scholar] [CrossRef]
- De Benedictis, M.; Bleve, G.; Grieco, F.; Tristezza, M.; Tufariello, M.; Grieco, F. An optimized procedure for the enological selection of non-Saccharomyces starter cultures. Antonie Van Leeuwenhoek 2011, 99, 189–200. [Google Scholar] [CrossRef]
- Polizzotto, G.; Barone, E.; Ponticello, G.; Fasciana, T.; Barbera, D.; Corona, O.; Amore, G.; Giammanco, A.; Oliva, D. Isolation, identification and oenological characterization of non-Saccharomyces yeasts in a Mediterranean island. Lett. Appl. Microbiol. 2016, 63, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Henick-Kling, T.; Edinger, W.; Daniel, P.; Monk, P. Selective effects of sulfur dioxide and yeast starter culture addition on indigenous yeast populations and sensory characteristics of wine. J. Appl. Microbiol. 1998, 84, 865–876. [Google Scholar] [CrossRef]
- Peris, D.; Pérez-Través, L.; Belloch, C.; Querol, A. Enological characterization of Spanish Saccharomyces kudriavzevii strains, one of the closest relatives to parental strains of winemaking and brewing Saccharomyces cerevisiae × S. kudriavzevii hybrids. Food Microbiol. 2016, 53, 31–40. [Google Scholar] [CrossRef]
- Francesca, N.; Chiurazzi, M.; Romano, R.; Aponte, M.; Settanni, L.; Moschetti, G. Indigenous yeast communities in the environment of “Rovello bianco” grape variety and their use in commercial white wine fermentation. World J. Microbiol. Biotechnol. 2010, 26, 337–351. [Google Scholar] [CrossRef]
- Vaudano, E.; Bertolone, E.; Petrozziello, M. Exploring the possibility of using Kazachstania exigua (ex. Saccharomyces exiguus) in wine production. In Industrial, Medical and Environmental Applications of Microorganisms: Current Status and Trends; Mendez-Vilas, A., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; pp. 304–309. [Google Scholar]
- Mestre Furlani, M.V.; Maturano, J.P.; Combina, M.; Mercado, L.A.; Toro, M.E.; Vazquez, F. Selection of non-Saccharomyces yeasts to be used in grape musts with high alcoholic potential: A strategy to obtain wines with reduced ethanol content. FEMS Yeast Res. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and phenotypic characterization of Metschnikowia pulcherrima strains from Douro wine region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Genovés, S.; Vallés, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef]
- Caridi, A.; Sidari, R.; Pulvirenti, A.; Blaiotta, G. Genetic Improvement of wine yeasts for opposite adsorption activity of phenolics and ochratoxin A during red winemaking. Food Biotechnol. 2020, 34, 352–370. [Google Scholar] [CrossRef]
- Fernández, M.; Úbeda, J.F.; Briones, A.I. Typing of non-Saccharomyces yeasts with enzymatic activities of interest in wine-making. Int. J. Food Microbiol. 2000, 59, 29–36. [Google Scholar] [CrossRef]
- Charoenchai, C.; Fleet, G.H.; Henschke, P.A.; Tood, B.E.N. Screening of non-Saccharomyces wine yeasts for the presence of extracellular hydrolytic enzymes. Aust. J. Grape Wine Res. 1997, 3, 2–8. [Google Scholar] [CrossRef]
- Brežná, B.; Ženišová, K.; Chovanová, K.; Chebeňová, V.; Kraková, L.; Kuchta, T.; Pangallo, D. Evaluation of fungal and yeast diversity in Slovakian wine-related microbial communities. Antonie Van Leeuwenhoek 2010, 98, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Kraková, L.; Chovanová, K.; Ženišová, K.; Turcovská, V.; Brežná, B.; Kuchta, T.; Pangallo, D. Yeast diversity investigation of wine-related samples from two different Slovakian wine-producing areas through a multistep procedure. LWT—Food Sci. Technol. 2012, 46, 406–411. [Google Scholar] [CrossRef]
- Ženišová, K.; Chovanová, K.; Chebeňová-Turcovská, V.; Godálová, Z.; Kraková, L.; Kuchta, T.; Pangallo, D.; Brežná, B. Mapping of wine yeast and fungal diversity in the Small Carpathian wine-producing region (Slovakia): Evaluation of phenotypic, genotypic and culture-independent approaches. Ann. Microbiol. 2014, 64, 1819–1828. [Google Scholar] [CrossRef]
- Bučková, M.; Puškárová, A.; Ženišová, K.; Kraková, L.; Piknová, Ľ.; Kuchta, T.; Pangallo, D. Novel insights into microbial community dynamics during the fermentation of Central European ice wine. Int. J. Food Microbiol. 2018, 266, 42–51. [Google Scholar] [CrossRef]
- Böhmer, M.; Smol’ak, D.; Ženišová, K.; Čaplová, Z.; Pangallo, D.; Puškárová, A.; Bučková, M.; Cabicarová, T.; Budiš, J.; Šoltýs, K.; et al. Comparison of microbial diversity during two different wine fermentation processes. FEMS Microbiol. Lett. 2020, 367, fnaa150. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSIBLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Lejková, J.; Koreňová, J.; Ženišová, K.; Valík, L.; Kuchta, T. Isolation of autochthonous lactic acid bacteria from ewes’ lump cheese, bryndza cheese and barrelled ewes’ cheese, and their characterization using Fourier transform infrared spectroscopy. J. Food Nutr. Res. 2015, 54, 308–313. [Google Scholar]
- Sherman, F. Getting started with yeast. Methods Enzymol. 1991, 194, 3–21. [Google Scholar]
- Fowell, R.R. Sodium acetate agar as a sporulation medium for yeasts. Nature 1952, 170, 578. [Google Scholar] [CrossRef] [PubMed]
- Bautista Gallego, J.; Rodríguez-Gómez, F.; Barrio, E.; Querol, A.; Garrido-Fernandez, A.; Arroyo-López, F.N. Exploring the yeast biodiversity of green table olive industrial fermentations for technological applications. Int. J. Food Microbiol. 2011, 147, 89–96. [Google Scholar] [CrossRef]
- Lemaresquier, H.; Gainvors, A.; Lequart, C.; Charlemagne, B.; Frézier, V.; Belarbi, A. Sélection de levures oenologiques á activité clarifiante. Rev. Fr. Oenol. 1995, 154, 23–29. [Google Scholar]
- Nickerson, W. Reduction of inorganic substances by yeasts. I. Extracellular reduction of sulfite by species of Candida. J. Infect. Dis. 1953, 93, 43–56. [Google Scholar] [CrossRef]
- Caridi, A.; Pulvirenti, A.; Restuccia, C.; Sidari, R. Screening for yeasts able to hydrolyse arbutin in the presence of glucose or ethanol. Ann. Microbiol. 2005, 55, 43–46. [Google Scholar]
- Sidari, R.; Martorana, A.; De Bruno, A. Effect of brine composition on yeast biota associated with naturally fermented Nocellara messinese table olives. LWT—Food Sci. Technol. 2019, 109, 163–170. [Google Scholar] [CrossRef]
- Buzzini, P.; Martini, A. Extracellular enzymatic activity profiles in yeast and yeast-like strains isolated from tropical environments. J. Appl. Microbiol. 2002, 93, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2016; Volume 2, ISBN 979-10-91799-48-5.
- Suhaj, M.; Belajová, E. Criteria, assessment methods and quality control of fruit juices in the Slovak Republic. Agriculture 2003, 49, 369–374. [Google Scholar]
- Hosu, A.; Cristea, V.-M.; Cimpoiu, C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in Romanian red wines: Prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef]
- Lee, D.C.; Chapman, D. Infrared spectroscopic studies of biomembranes and model membranes. Biosci. Rep. 1986, 6, 235–256. [Google Scholar] [CrossRef]
- Kümmerle, M.; Scherer, S.; Seiler, H. Rapid and reliable identification of food-borne yeasts by fourier-transform infrared spectroscopy. Appl. Environ. Microbiol. 1998, 64, 2207–2214. [Google Scholar] [CrossRef]
- Helm, D.; Labischinski, H.; Naumann, D. Elaboration of a procedure for identification of bacteria using Fourier-transform IR spectral libraries: A stepwise correlation approach. J. Microbiol. Methods 1991, 14, 127–132. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, V. Methods for isolation, phenotypic characterization and maintenance of yeasts. In The Yeasts, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 1, Chapter 7; pp. 87–110. [Google Scholar]
- Taxis, C.; Keller, P.; Kavagiou, Z.; Jensen, L.J.; Colombelli, J.; Bork, P.; Stelzer, E.H.K.; Knop, M. Spore number control and breeding in Saccharomyces cerevisiae: A key role for a self-organizing system. J. Cell Biol. 2005, 171, 627–640. [Google Scholar] [CrossRef]
- Kreger-van Rij, N.J.W.; Ahearn, D.G. Shape and structure of the ascospores of Hanseniaspora uvarum. Mycologia 1968, 60, 604–612. [Google Scholar]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Philipp, C.; Sari, S.; Eder, P.; Patzl-Fischerleitner, E.; Eder, R. Austrian Pinot blanc wines: Typicity, wine styles and the influence of different oenological decisions on the volatile profile of wines. In Proceedings of the 42nd World Congress of Vine and Wine, Geneva, Switzerland, 15–19 July 2019; Volume 15, p. 02005. [Google Scholar] [CrossRef]
- Sipiczki, M.; Pfliegler, W.P.; Holb, I.J. Metschnikowia species share a pool of diverse rRNA genes differing in regions that determine hairpin-loop structures and evolve by reticulation. PLoS ONE 2013, 8, e67384. [Google Scholar] [CrossRef] [PubMed]
- Brysch-Herzberg, M.; Seidel, M. Yeast diversity on grapes in two German wine growing regions. Int. J. Food Microbiol. 2015, 214, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Sipiczki, M. Overwintering of vineyard yeasts: Survival of interacting yeast communities in grapes mummified on vines. Front. Microbiol. 2016, 7, 212. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia pulcherrima and related pulcherrimin-producing yeasts: Fuzzy species boundaries and complex antimicrobial antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef]
- Garavaglia, J.; de Cassia de Souza Schneider, R.; Camargo Mendes, S.D.; Welke, J.E.; Alcaraz Zini, C.; Bastos Caramão, E.; Valente, P. Evaluation of Zygosaccharomyces bailii BCV 08 as a co-starter in wine fermentation for the improvement of ethyl esters production. Microbiol. Res. 2015, 173, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Ruiz, J.; Alastruey Izquierdo, A.; Navascues, E.; Marquina, D.; Santos, A. Unraveling the enzymatic basis of wine “flavorome”: A phylo-functional study of wine related yeast species. Front. Microbiol. 2016, 7, 12. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Ramírez, M.; Velázquez, R. The yeast Torulaspora delbrueckii: An interesting but difficult-to-use tool for winemaking. Fermentation 2018, 4, 94. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Bañuelos, M.A.; Suárez-Lepe, J.A. Isolation, selection, and identification techniques for non-Saccharomyces yeasts of oenological interest. In Biotechnological Progress and Beverage Consumption; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 19, Chapter 15; pp. 467–508. [Google Scholar]
- Mukherjee, V.; Radecka, D.; Aerts, G.; Verstrepen, K.J.; Lievens, B.; Thevelein, J.M. Phenotypic landscape of non-conventional yeast species for different stress tolerance traits desirable in bioethanol fermentation. Biotechnol. Biofuels 2017, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef]
- Viela, A. Use of nonconventional yeasts for modulating wine acidity. Fermentation 2019, 5, 27. [Google Scholar] [CrossRef]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res. Int. 2019, 119, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Bisson, L.F.; Karpel, J.E.; Ramakrishnan, V.; Joseph, L. Functional genomics of wine yeast Saccharomyces cerevisiae. Adv. Food Nutr. Res. 2007, 53, 65–121. [Google Scholar]
- van Oort, M.; Canal-Llaubères, R.-M.; Law, B.A. Enzymes in wine production. In Enzymes in Food Technology; Sheffield Academic Press: Sheffield, UK, 2002; pp. 76–90. [Google Scholar]
- Pando Bedriñana, R.; Lastra Queipo, A.; Suárez Valles, B. Screening of enzymatic activities in non-Saccharomyces cider yeasts. J. Food Biochem. 2012, 36, 683–689. [Google Scholar] [CrossRef]
- Madrigal, T.; Maicas, S.; Tolosa, J.J.M. Glucose and ethanol tolerant enzymes produced by Pichia (Wickerhamomyces) isolates from enological ecosystems. Am. J. Enol. Vitic. 2013, 64, 126–133. [Google Scholar] [CrossRef]
- Ianieva, O.; Podgorsky, V. Enological potential of non-Saccharomyces yeast strains of enological and brewery origin from Ukrainian Collection of Microorganisms. Mycology 2020, 12, 203–215. [Google Scholar] [CrossRef]
- Strauss, M.L.A.; Jolly, N.P.; Lambrechts, M.G.; van Rensburg, P. Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J. Appl. Microbiol. 2001, 91, 182–190. [Google Scholar] [CrossRef]
- Maturano, Y.P.; Rodríguez, L.A.; Toro, M.E.; Nally, M.C.; Vallejo, M.; Castellanos de Figueroa, L.I.; Combina, M.; Vazquez, F. Multi-enzyme production by pure and mixed cultures of Saccharomyces and non-Saccharomyces yeasts during wine fermentation. Int. J. Food Microbiol. 2012, 155, 43–50. [Google Scholar] [CrossRef]
- Tempère, S.; Marchal, A.; Barbe, J.-C.; Bely, M.; Masneuf-Pomarede, I.; Marullo, P.; Albertin, W. The complexity of wine: Clarifying the role of microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3995–4007. [Google Scholar] [CrossRef]
- Rosi, I.; Vinella, M.; Domizio, P. Characterizationof β-glucosidase activity in yeasts of oenological origin. J. Appl. Bacteriol. 1994, 77, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Escribano, R.; González-Arenzana, L.; Garijo, P.; Berlanas, C.; López-Alfaro, I.; López, R.; Gutiérrez, A.R.; Santamaría, P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017, 54, 1555–1564. [Google Scholar] [CrossRef]
- Grazia, A.; Pietrafesa, A.; Capece, A.; Pietrafesa, R.; Siesto, G.; Romano, P. Exploitation of technological variability among wild non-Saccharomyces yeasts to select mixed starters for the production of low alcohol wines. In Proceedings of the 42nd World Congress of Vine and Wine, Geneva, Switzerland, 15–19 July 2019. [Google Scholar] [CrossRef]
- Aktas, E.; Yigit, N.; Ayyildiz, A. Esterase activity in various Candida species. J. Int. Med. Res. 2002, 30, 322–324. [Google Scholar] [CrossRef]
- Bilinski, C.A.; Russell, I.; Stewart, G.G. Applicability of yeast extracellular proteinases in brewing: Physiological and biochemical aspects. Appl. Environ. Microbiol. 1987, 53, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Younes, B.; Cilindre, C.; Villaume, S.; Parmentier, M.; Jeandet, P.; Vasserot, Y. Evidence for an extracellular acid proteolytic activity secreted by living cells of Saccharomyces cerevisiae PlR1: Impact on grape proteins. J. Agric. Food Chem. 2011, 59, 6239–6246. [Google Scholar] [CrossRef] [PubMed]
- Binati, R.L.; Innocente, G.; Gatto, V.; Celebrin, A.; Polo, M.; Felis, G.E.; Torriani, S. Exploring the diversity of a collection of native non-Saccharomyces yeasts to develop co-starter cultures for winemaking. Food Res. Int. 2019, 122, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, M.; Úbeda, J.F.; Vasudevan, T.G.; Cordero Otero, R.R.; Briones, A.I. Evaluation of polygalacturonase activity in Saccharomyces cerevisiae wine strains. FEMS Microbiol. Lett. 2004, 237, 261–266. [Google Scholar] [CrossRef][Green Version]
- Hranilovic, A.; Gambetta, J.M.; Jeffery, D.W.; Grbin, P.R.; Jiranek, V. Lower-alcohol wines produced by Metschnikowia pulcherrima and Saccharomyces cerevisiae co-fermentations: The effect of sequential inoculation timing. Int. J. Food Microbiol. 2020, 329, 108651. [Google Scholar] [CrossRef]
- Du Plessis, H.; Du Toit, M.; Hoff, J.W.; Hart, R.S.; Ndimba, B.K.; Jolly, N.P. Characterisation of non-Saccharomyces yeasts using different methodologies and evaluation of their compatibility with malolactic fermentation. S. Afr. J. Enol. Vitic. 2017, 38, 46–63. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma—A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- OIV. International Code of Oenological Practices; Office Internationale de la Vigne et du Vin: Paris, France, 2010.
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Mendoza, L.M.; De Nadra, M.C.M.; Farías, M.E. Kinetics and metabolic behavior of a composite culture of Kloeckera apiculata and Saccharomyces cerevisiae wine related strains. Biotechnol. Lett. 2007, 29, 1057–1063. [Google Scholar] [CrossRef]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1998, 14, 199–203. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Kapaklis, A.; Spyropoulos, H. Growth and fermentation characteristics of a strain of the wine yeast Kluyveromyces thermotolerans isolated in Greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masneuf Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii Saccharomyces cerevisiae culture on high sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef]
- Vilela, A. Lachancea thermotolerans, the non-Saccharomyces yeast that reduces the volatile acidity of wines. Fermentation 2018, 4, 56. [Google Scholar] [CrossRef]
- Aponte, M.; Blaiotta, G. Potential role of yeast strains isolated from grapes in the production of Taurasi DOCG. Front. Microbiol. 2016, 7, 809. [Google Scholar] [CrossRef]
- Pulvirenti, A.; De Vero, L.; Blaiotta, G.; Sidari, R.; Iosca, G.; Gullo, M.; Caridi, A. Selection of wine Saccharomyces cerevisiae strains and their screening for the adsorption activity of pigments, phenolics and ochratoxin A. Fermentation 2020, 6, 80. [Google Scholar] [CrossRef]
- Ivit, N.N.; Longo, R.; Kemp, B. The Effect of non-Saccharomyces and Saccharomyces non-cerevisiae yeasts on ethanol and glycerol levels in wine. Fermentation 2020, 6, 77. [Google Scholar] [CrossRef]
- Noble, A.C.; Bursick, G.F. The contribution of glycerol to perceived viscosity and sweetness in white wines. Am. J. Enol. Vitic. 1984, 35, 110–112. [Google Scholar]
- Scanes, K.T.; Hohmann, S.; Prior, B.A. Glycerol production by the yeast Saccharomyces cerevisiae and its relevance to wine: A review. S. Afr. J. Enol. Vitic. 1998, 19, 17–24. [Google Scholar] [CrossRef]
- Zhu, X.; Navarro, Y.; Mas, A.; Torija, M.-J.; Beltran, G. A rapid method for selecting non-Saccharomyces strains with a low ethanol yield. Microorganisms 2020, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- Tilloy, V.; Ortiz-Julien, A.; Dequin, S. Reduction of ethanol yield and improvement of glycerol formation by adaptive evolution of the wine yeast Saccharomyces cerevisiae under hyperosmotic conditions. Appl. Environ. Microb. 2014, 80, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Eglinton, J.M.; Heinrich, A.J.; Pollnitz, A.P.; Langridge, P.; Henschke, P.A.; de Barros Lopes, M. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldaldehyde dehydrogenase gene. Yeast 2002, 19, 295–301. [Google Scholar] [CrossRef]
- Bakker, J.; Bridle, P.; Bellworthy, S.J.; Garcia-Viguera, C.; Reader, H.P.; Watkins, S.J. Effect of sulphur dioxide and must extraction on colour, phenolic composition and sensory quality of red table wine. J. Sci. Food Agric. 1998, 78, 297–307. [Google Scholar] [CrossRef]
- Manzanares, P.; Rojas, V.; Genoves, S.; Valles, S. A preliminary search for anthocyanin-b-d-glucosidase activity in non-Saccharomyces wine yeasts. Int. J. Food Sci. Tech. 2000, 35, 95–103. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Pyruvic acid and acetaldehyde production by different strains of Saccharomyces cerevisiae: Relationship with visitin A and B formation in red wines. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Caridi, A.; Sidari, R.; Solieri, L.; Cufari, A.; Giudici, P. Wine colour adsorption phenotype: An inheritable quantitative trait loci of yeasts. J. Appl. Microbiol. 2007, 103, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Caridi, A.; Sidari, R.; Kraková, L.; Kuchta, T.; Pangallo, D. Assessment of color adsorption by yeast using Grape Skin Agar and impact on red wine color. OENO One 2015, 49, 195–203. [Google Scholar] [CrossRef]
- Caridi, A.; Sidari, R.; Giuffrè, A.M.; Pellicanò, T.M.; Sicari, V.; Zappia, C.; Poiana, M. Test of four generations of Saccharomyces cerevisiae concerning their effect on antioxidant phenolic compounds in wine. Eur. Food Res. Technol. 2017, 243, 1287–1294. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in wine biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).