Impact of Guidelines Publication on Acute Bronchiolitis Management: 10-Year Experience from a Tertiary Care Center in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Case Definition
2.3. Data Collection and Participants
2.4. Inclusion and Exclusion Criteria
2.5. Diagnostic Tests
2.6. Statistical Analysis
2.7. Ethics Statement
3. Results
3.1. Primary End-Point
3.2. Secondary End-Points
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakellaropoulou, A.; Emporiadou, M.; Aivazis, V.; Mauromixalis, J.; Hatzistilianou, M. Acute bronchiolitis in a paediatric emer-gency department of Northern Greece. Comparisons between two decades. AOMS 2012, 3, 509–514. [Google Scholar] [CrossRef]
- Bronchiolitis in Children: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng9 (accessed on 10 September 2021).
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korsun, N.; Angelova, S.; Trifonova, I.; Georgieva, I.; Voleva, S.; Tzotcheva, I.; Mileva, S.; Ivanov, I.; Tcherveniakova, T.; Perenovska, P. Viral pathogens associated with acute lower respiratory tract infections in children younger than 5 years of age in Bulgaria. Braz. J. Microbiol. 2019, 50, 117–125. [Google Scholar] [CrossRef]
- Učakar, V.; Sočan, M.; Trilar, K.P. The impact of influenza and respiratory syncytial virus on hospitalizations for lower respiratory tract infections in young children: Slovenia, 2006–2011. Influenza Other Respir. Viruses 2013, 7, 1093–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagusic, M.; Slovic, A.; Ivancic-Jelecki, J.; Ljubin-Sternak, S.; Vilibić-Čavlek, T.; Tabain, I.; Forcic, D. Molecular epidemiology of human respiratory syncytial virus and human metapneumovirus in hospitalized children with acute respiratory infections in Croatia, 2014–2017. Infect. Genet. Evol. 2019, 76, 104039. [Google Scholar] [CrossRef]
- Vandini, S.; Biagi, C.; Lanari, M. Respiratory Syncytial Virus: The Influence of Serotype and Genotype Variability on Clinical Course of Infection. Int. J. Mol. Sci. 2017, 18, 1717. [Google Scholar] [CrossRef] [PubMed]
- Jevšnik, M.; Steyer, A.; Pokorn, M.; Mrvič, T.; Grosek, Š.; Strle, F.; Lusa, L.; Petrovec, M. The Role of Human Coronaviruses in Children Hospitalized for Acute Bronchiolitis, Acute Gastroenteritis, and Febrile Seizures: A 2-Year Prospective Study. PLoS ONE 2016, 11, e0155555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagi, C.; Rocca, A.; Poletti, G.; Fabi, M.; Lanari, M. Rhinovirus Infection in Children with Acute Bronchiolitis and Its Impact on Recurrent Wheezing and Asthma Development. Microorganisms 2020, 8, 1620. [Google Scholar] [CrossRef]
- Bosis, S.; Esposito, S.; Niesters, H.G.M.; Zuccotti, G.V.; Marseglia, G.; Lanari, M.; Zuin, G.; Pelucchi, C.; Osterhaus, A.D.M.E.; Principi, N. Role of respiratory pathogens in infants hospitalized for a first episode of wheezing and their impact on recurrences. Clin. Microbiol. Infect. 2008, 14, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Schuh, S.; Babl, F.E.; Dalziel, S.R.; Freedman, S.B.; Macias, C.G.; Stephens, D.; Steele, D.W.; Fernandes, R.M.; Zemek, R.; Plint, A.C.; et al. Practice Variation in Acute Bronchiolitis: A Pediatric Emergency Research Networks Study. Pediatrics 2017, 140, e20170842. [Google Scholar] [CrossRef] [Green Version]
- Baraldi, E.; Lanari, M.; Manzoni, P.; Rossi, G.A.; Vandini, S.; Rimini, A.; Romagnoli, C.; Colonna, P.; Biondi, A.; Biban, P.; et al. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital. J. Pediatr. 2014, 40, 65. [Google Scholar] [CrossRef]
- Zipursky, A.; Kuppermann, N.; Finkelstein, Y.; Zemek, R.; Plint, A.C.; Babl, F.E.; Dalziel, S.R.; Freedman, S.B.; Steele, D.W.; Fernandes, R.; et al. International Practice Patterns of Antibiotic Therapy and Laboratory Testing in Bronchiolitis. Pediatrics 2020, 146, e20193684. [Google Scholar] [CrossRef] [PubMed]
- De Brasi, D.; Pannuti, F.; Antonelli, F.; de Seta, F.; Siani, P.; de Seta, L. Therapeutic approach to bronchiolitis: Why pediatricians continue to overprescribe drugs? Italy J. Pediatr. 2010, 36, 67. [Google Scholar] [CrossRef] [Green Version]
- Barben, J.; Kuehni, C.E.; Trachsel, D.; Hammer, J.; on behalf of the Swiss Paediatric Respiratory Research Group. Management of acute bronchiolitis: Can evidence based guidelines alter clinical practice? Thorax 2008, 63, 1103–1109. [Google Scholar] [CrossRef] [Green Version]
- Lieberthal, A.S.; Bauchner, H.; Hall, C.B.; Johnson, D.W.; Kotagal, U.; Light, M.J.; Mason, W.; Meissner, H.C.; Phelan, K.J.; Zorc, J.J.; et al. Diagnosis and Management of Bronchiolitis. Pediatrics 2006, 118, 1774–1793. [Google Scholar]
- Stera, G.; Pierantoni, L.; Masetti, R.; Leardini, D.; Biagi, C.; Buonsenso, D.; Pession, A.; Lanari, M. Impact of SARS-CoV-2 Pandemic on Bronchiolitis Hospitalizations: The Experience of an Italian Tertiary Center. Children 2021, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- Gharabaghi, F.; Hawan, A.; Drews, S.; Richardson, S. Evaluation of multiple commercial molecular and conventional diagnostic assays for the detection of respiratory viruses in children. Clin. Microbiol. Infect. 2011, 17, 1900–1906. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, E.; Cantarutti, A.; Cavagnis, S.; Cantarutti, L.; Baraldi, E.; Giaquinto, C.; Donà, D. Impact of bronchiolitis guidelines publication on primary care prescriptions in the Italian pediatric population. NPJ Prim. Care Respir. Med. 2021, 31, 15. [Google Scholar] [CrossRef] [PubMed]
- Florin, T.A.; Plint, A.C.; Zorc, J.J. Viral bronchiolitis. Lancet 2017, 389, 211–224. [Google Scholar] [CrossRef]
- Meissner, H.C. Viral Bronchiolitis in Children. N. Engl. J. Med. 2016, 374, 62–72. [Google Scholar] [CrossRef]
- Schuh, S.; Lalani, A.; Allen, U.; Manson, D.; Babyn, P.; Stephens, D.; MacPhee, S.; Mokanski, M.; Khaikin, S.; Dick, P. Evaluation of the Utility of Radiography in Acute Bronchiolitis. J. Pediatr. 2007, 150, 429–433. [Google Scholar] [CrossRef]
- Ecochard-Dugelay, E.; Beliah, M.; Perreaux, F.; De Laveaucoupet, J.; Bouyer, J.; Epaud, R.; Labrune, P.; Ducou-Lepointe, H.; Gajdos, V. Clinical predictors of radiographic abnormalities among infants with bronchiolitis in a paediatric emergency department. BMC Pediatr. 2014, 14, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henao-Villada, R.; Sossa-Briceño, M.P.; Rodríguez-Martínez, C.E. Impact of the implementation of an evidence-based guideline on diagnostic testing, management, and clinical outcomes for infants with bronchiolitis. Ther. Adv. Respir. Dis. 2016, 10, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCulloh, R.J.; Smitherman, S.E.; Koehn, K.L.; Alverson, B.K. Assessing the impact of national guidelines on the management of children hospitalized for acute bronchiolitis. Pediatr. Pulmonol. 2014, 49, 688–694. [Google Scholar] [CrossRef]
- Burstein, B.; Plint, A.C.; Papenburg, J. Use of Radiography in Patients Diagnosed as Having Acute Bronchiolitis in US Emergency Departments, 2007–2015. JAMA 2018, 320, 1598–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papenburg, J.; Fontela, P.S.; Freitas, R.R.; Burstein, B. Inappropriate Antibiotic Prescribing for Acute Bronchiolitis in US Emergency Departments, 2007–2015. J. Pediatr. Infect. Dis. Soc. 2019, 8, 567–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- House, S.A.; Marin, J.R.; Hall, M.; Ralston, S.L. Trends Over Time in Use of Nonrecommended Tests and Treatments Since Publication of the American Academy of Pediatrics Bronchiolitis Guideline. JAMA Netw. Open 2021, 4, e2037356. [Google Scholar] [CrossRef]
- Biagi, C.; Pierantoni, L.; Baldazzi, M.; Greco, L.; Dormi, A.; Dondi, A.; Faldella, G.; Lanari, M. Lung ultrasound for the diagnosis of pneumonia in children with acute bronchiolitis. BMC Pulm. Med. 2018, 18, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.S.; Hughes, N.; Tat, S.; Chamberlain, J.M.; Teach, S.J.; Boniface, K. The Utility of Bedside Lung Ultrasound Findings in Bronchiolitis. Pediatr. Emerg. Care 2017, 33, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.A.; Chavez, M.A.; Hooper-Miele, C.C.; Gilman, R.H.; Steinhoff, M.C.; Ellington, L.E.; Gross, P.; Price, C.; Tielsch, J.M.; Checkley, W. Lung Ultrasound for the Diagnosis of Pneumonia in Children: A Meta-analysis. Pediatrics 2015, 135, 714–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, B.P.; Tay, E.T.; Elikashvili, I.; Sanders, J.E.; Paul, A.Z.; Nelson, B.P.; Spina, L.A.; Tsung, J.W. Feasibility and Safety of Substituting Lung Ultrasonography for Chest Radiography When Diagnosing Pneumonia in Children: A Randomized Controlled Trial. Chest 2016, 150, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.R.; Semple, M.G. Stemming the tide of hospital admissions for bronchiolitis. Arch. Dis. Child. 2016, 101, 118–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beggs, S.; Wong, Z.H.; Kaul, S.; Ogden, K.; Walters, J.A.E. High-flow nasal cannula therapy for infants with bronchiolitis. Cochrane Database Syst. Rev. 2014, CD009609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Zhang, Y.; Xiong, L.; Liu, S.; Gong, C.; Dai, J. High-flow nasal cannula therapy for children with bronchiolitis: A systematic review and meta-analysis. Arch. Dis. Child. 2019, 104, 564–576. [Google Scholar] [CrossRef]
- Griffiths, B.; Riphagen, S.; Lillie, J. Management of severe bronchiolitis: Impact of NICE guidelines. Arch. Dis. Child. 2018, 105, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Palmu, S.; Mecklin, M.; Heikkilä, P.; Backman, K.; Peltola, V.; Renko, M.; Korppi, M. National treatment guidelines decreased the use of racemic adrenaline for bronchiolitis in four Finnish university hospitals. Acta Paediatr. 2018, 107, 1966–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, K.; Hall, M.; Teach, S.J. Bronchiolitis Management Before and After the AAP Guidelines. Pediatrics 2013, 133, e1–e7. [Google Scholar] [CrossRef]
- Levin, D.; Tribuzio, M.; Green-Wrzesinki, T.; Ames, B.; Radwan, S.; Jarvis, J.D.; Vaccaro, T.; Modlin, J.F. Empiric antibiotics are justified for infants with respiratory syncytial virus lower respiratory tract infection presenting with respiratory failure: A prospective study and evidence review. Pediatr. Crit. Care Med. 2009, 11, 390–395. [Google Scholar] [CrossRef]
- Lopez, A.A.; Aslanova, R.; Bridger, N.; Chafe, R. Antibiotic Use for Inpatient Bronchiolitis: Did National Guidelines Impact Practice at a Pediatric Hospital? Hosp. Pediatr. 2020, 10, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Fontoura-Matias, J.; Moreira-Sousa, D.; Freitas, A.; Azevedo, I. Management of bronchiolitis in Portugal, 2000–2015: Do guidelines have an impact? Pediatr. Pulmonol. 2020, 55, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Mansbach, J.M.; Piedra, P.A.; Stevenson, M.D.; Sullivan, A.F.; Forgey, T.F.; Clark, S.; Espinola, J.A.; Camargo, C.A. Prospective Multicenter Study of Children with Bronchiolitis Requiring Mechanical Ventilation. Pediatrics 2012, 130, e492–e500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Mild | Moderate | Severe | |
|---|---|---|---|
| Respiratory rate | Normal to slightly increased | Increased | Markedly increased compared to normal values |
| Respiratory effort | Mild chest wall retraction | Tracheal tug Nasal Flare Moderate chest wall retraction | Marked chest wall retraction Nasal flare Grunting |
| Oxygen saturations | No supplemental oxygen requirement, O2 saturations > 95% | Saturations 90–95% | Saturations < 90%, may not be corrected by O2 |
| Feeding | Normal to slightly decreased | 50–75% of normal feeds | <50% of feeds, unable to feed |
| Apnoea | Nil | May have brief episodes | May have increasing episodes |
| Whole Population (n = 1249) | Group 1 (n = 538) | Group 2 (n = 711) | p-Value | |
|---|---|---|---|---|
| Age, median (IQR), months | 3.13 (1.76–5.46) | 3.00 (1.50–5.84) | 3.13 (1.83–5.34) | 0.093 * |
| Age under 6 months, n (%) | 990 (79.3) | 424 (78.8) | 566 (79.6) | 0.695 ° |
| Sex, n (%), male | 717 (57.4) | 309 (57.4) | 408 (57.4) | 0.986 ° |
| Prematurity, n (%) | 201 (16.1) | 88 (16.4) | 113 (15.9) | 0.831 ° |
| Other risk factors $, n (%) | 258 (20.6) | 95 (17.7) | 163 (23.0) | 0.023 ° |
| Lactation, n (%): | <0.001 ° | |||
| Breastfeeding | 444 (35.5) | 240 (45) | 204 (28.9) | |
| Mixed Lactation | 577 (46.2) | 203 (38.1) | 374 (52.9) | |
| Artificial feeding | 219 (17.3) | 90 (16.9) | 129 (18.2) | |
| Feeding difficulties, n (%) | 854 (68.4) | 345 (64.1) | 509 (71.8) | 0.004 ° |
| Time between the onset of symptoms and the hospital admission, median (IQR), days | 3 (2–5) | 3 (2–5) | 3 (2–5) | 0.304 * |
| Respiratory rate, median (IQR) | 55 (45–60) | 50 (45–60) | 55 (45–60) | <0.001 * |
| Oxygen saturations, median (SD) | 97 (95–98) | 97 (95–98) | 95 (95–98) | 0.201 * |
| Bronchiolitis severity +, n (%): | 0.654 ° | |||
| Mild | 721 (57.8) | 313 (58.2) | 408 (57.3) | |
| Moderate | 489 (39.1) | 212 (39.4) | 277 (38.9) | |
| Severe | 39 (3.1) | 13 (2.4) | 26 (3.6) | |
| RSV detection, n (%) | 812 (65) | 331 (62.3%) | 481 (68.3%) | 0.028 ° |
| WBC count, median (IQR), mmc: | 10,940 (8.425–14,090) | 10,215 (7875–13,227.5) | 11,280 (8707.5–14,910) | <0.001 * |
| Neutrophil count, median (IQR), % | 37.4 (25.4–49.05) | 36.6 (25.35–48.6) | 37.6 (25.4–49.55) | 0.334 * |
| Lymphocytes count, median (IQR), % | 48.5 (37.4–59.7) | 47.5 (36.4–57.95) | 49.1 (38.1–60.7) | 0.028 * |
| CRP, mean (SD), mg/dL | 0.48 (0.11–1.55) | 0.31 (0.07–1.23) | 0.67 (0.17–1.71) | <0.001 * |
| Group 1 (n = 538) | Group 2 (n= 711) | p-Value | |
|---|---|---|---|
| Intravenous hydration, n (%) | 422 (78.4%) | 605 (85.1%) | 0.002 ° |
| Oxygen therapy, n (%) | 224 (41.6%) | 309 (43.5%) | 0.519 ° |
| Time of oxygen therapy, hour | 0.285 * | ||
| Median (IQR) | 0 (0–48) | 0 (0–48) | |
| Mean (SD) | 29.70 (58.52) | 33.68 (60.57) | |
| Type of Oxygen therapy | <0.001 ° | ||
| NC, n (%) | 137 (61.2%) | 264 (85.4%) | |
| HT, n (%) | 79 (35.2%) | 2 (0.6%) | |
| HFNC, n (%) | 4 (1.7%) | 32 (10.4%) | |
| n-CPAP/NIV, n (%) | 2 (0.9%) | 6 (1.9%) | |
| MV, n (%) | 2 (0.9%) | 5 (1.6%) |
| Group 1 (n = 538) | Group 2 (n= 711) | p-Value | |
|---|---|---|---|
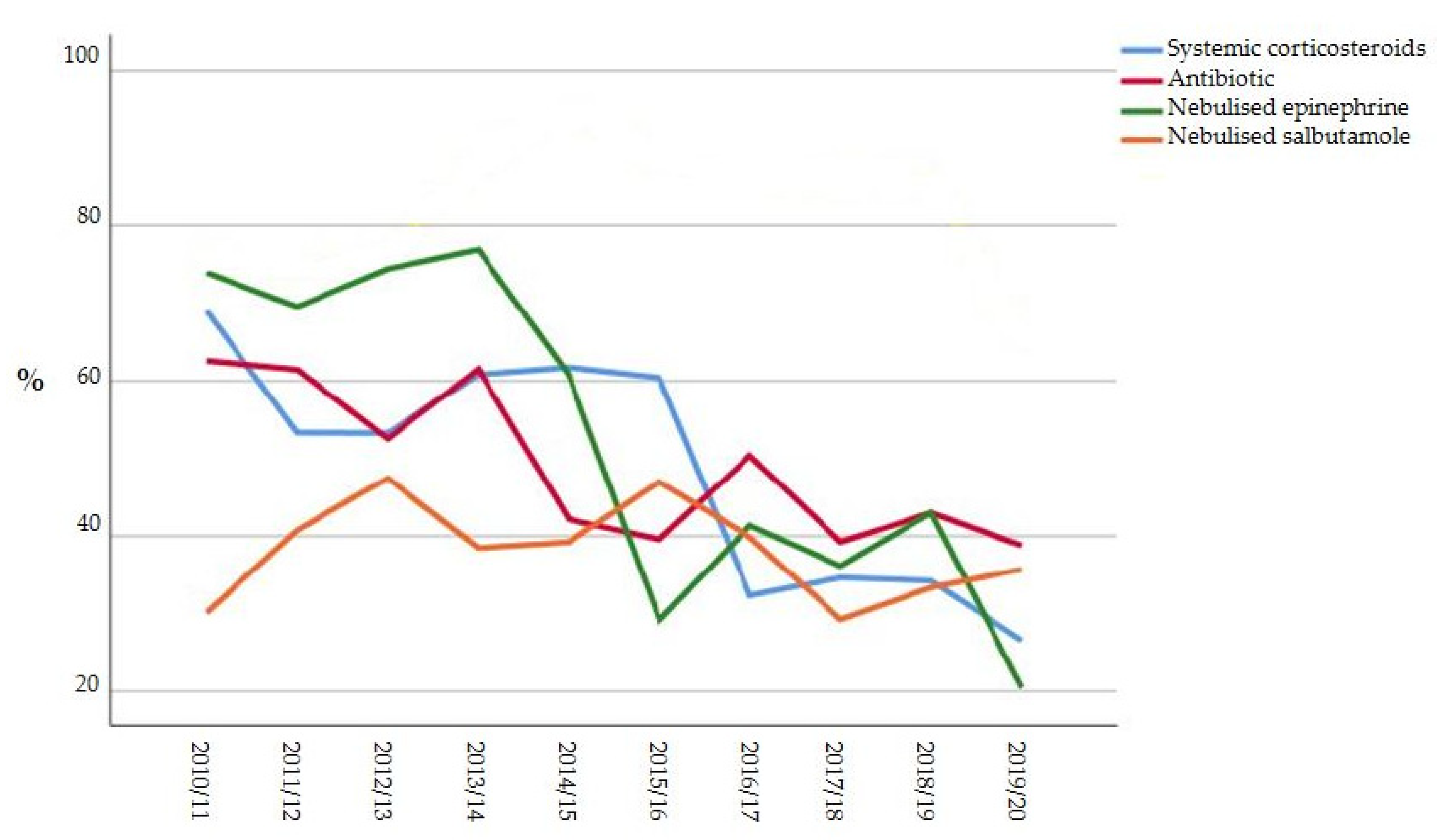
| Systemic corticosteroid, n (%) | 217 (58.9%) | 297 (41.8%) | <0.001 |
| Antibiotic, n (%) | 320 (59.5%) | 301 (42.3%) | <0.001 |
| Nebulized epinephrine, n (%) | 397 (73.8%) | 272 (38.3%) | <0.001 |
| Nebulized salbutamol, n (%) | 212 (39.4%) | 267 (37.6%) | 0.505 |
| Chest X-ray, n (%) | 496 (92.2%) | 387 (54.4%) | <0.001 |
| Group 1 (n = 538) | Group 2 (n = 711) | p-Value | |
|---|---|---|---|
| Concomitant pneumonia, n (%) | 250 (46.5) | 121 (17.0) | <0.001 * |
| Other complications, n (%) | 2 (0.37) | 12 (1.68) | 0.43 ° |
| • Sepsis Urinary tract infection | 1 (0.18) | 2 (0.28) | |
| • Acute otitis media | 1 (0.18) | 1 (0.14) | |
| • Pneumothorax | 0 (0) | 5 (0.70) | |
| • Pleural effusion | 0 (0) | 3 (0.42) | |
| Time of oxygen therapy, hour | 0.285 * | ||
| Median (IQR) | 0 (0–48) | 0 (0–48) | |
| Mean (SD) | 29.70 (58.52) | 33.68 (60.57) | |
| Length of hospital stay, mean (SD), days | 5.5 (4–8) | 4.0 (3–6) | <0.001 * |
| ICU admission, n (%) | 7 (1.3) | 16 (2.3%) | 0.217 ° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biagi, C.; Scarpini, S.; Paleari, C.; Fabi, M.; Dondi, A.; Gabrielli, L.; Gennari, M.; Lanari, M.; Pierantoni, L. Impact of Guidelines Publication on Acute Bronchiolitis Management: 10-Year Experience from a Tertiary Care Center in Italy. Microorganisms 2021, 9, 2221. https://doi.org/10.3390/microorganisms9112221
Biagi C, Scarpini S, Paleari C, Fabi M, Dondi A, Gabrielli L, Gennari M, Lanari M, Pierantoni L. Impact of Guidelines Publication on Acute Bronchiolitis Management: 10-Year Experience from a Tertiary Care Center in Italy. Microorganisms. 2021; 9(11):2221. https://doi.org/10.3390/microorganisms9112221
Chicago/Turabian StyleBiagi, Carlotta, Sara Scarpini, Camilla Paleari, Marianna Fabi, Arianna Dondi, Liliana Gabrielli, Monia Gennari, Marcello Lanari, and Luca Pierantoni. 2021. "Impact of Guidelines Publication on Acute Bronchiolitis Management: 10-Year Experience from a Tertiary Care Center in Italy" Microorganisms 9, no. 11: 2221. https://doi.org/10.3390/microorganisms9112221
APA StyleBiagi, C., Scarpini, S., Paleari, C., Fabi, M., Dondi, A., Gabrielli, L., Gennari, M., Lanari, M., & Pierantoni, L. (2021). Impact of Guidelines Publication on Acute Bronchiolitis Management: 10-Year Experience from a Tertiary Care Center in Italy. Microorganisms, 9(11), 2221. https://doi.org/10.3390/microorganisms9112221










