Environmental Drivers and Potential Distribution of Schistosoma mansoni Endemic Areas in Ethiopia
Abstract
1. Introduction
2. Materials and Methods
2.1. Schistosomiasis Occurrence Data
2.2. Environmental and Ecological Data
2.3. Ecological Niche Modeling
2.4. Model Evaluation
3. Results
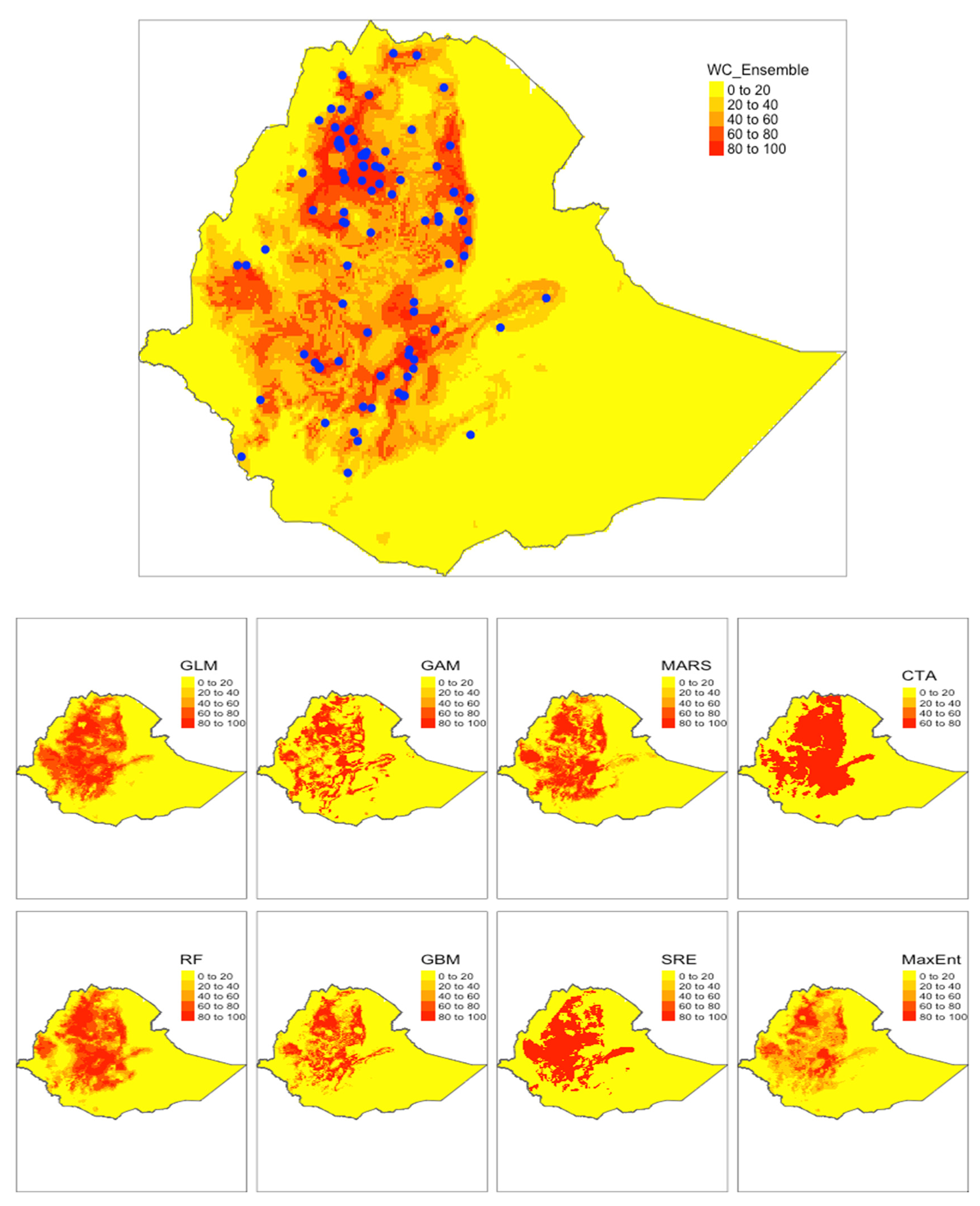
3.1. Model Performance
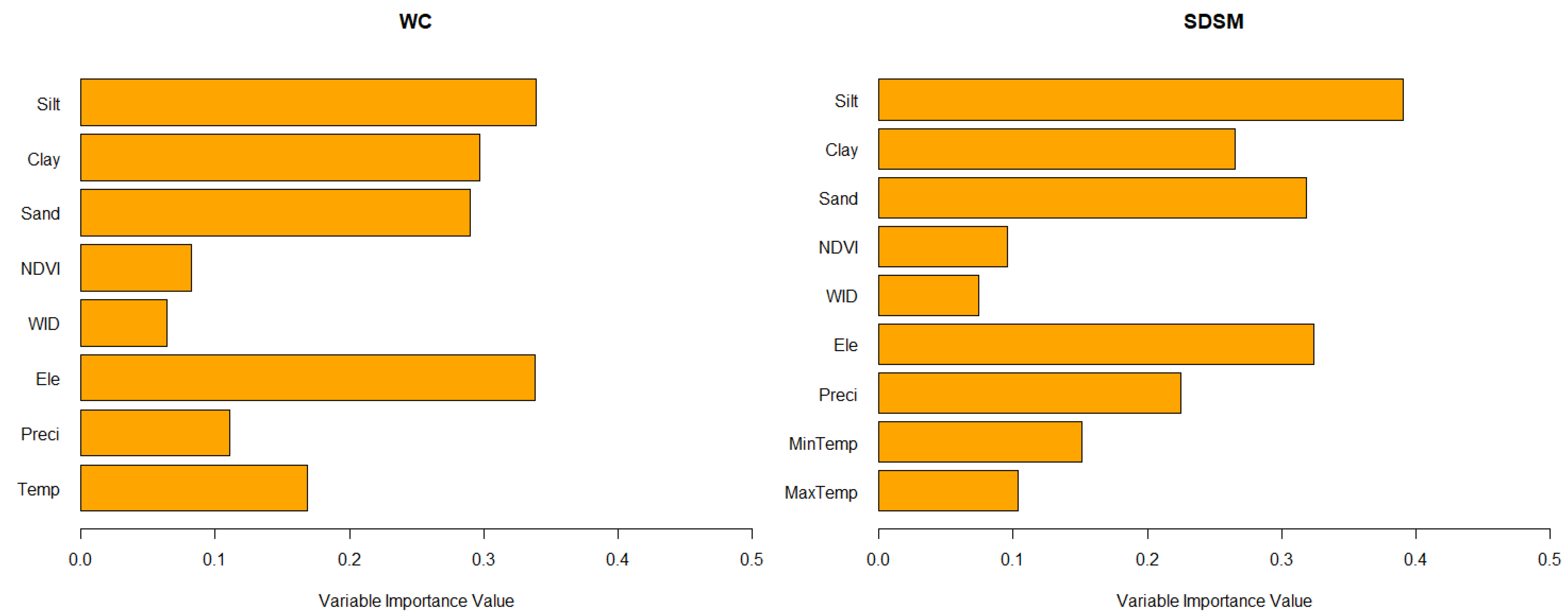
3.2. Relative Importance of Variables
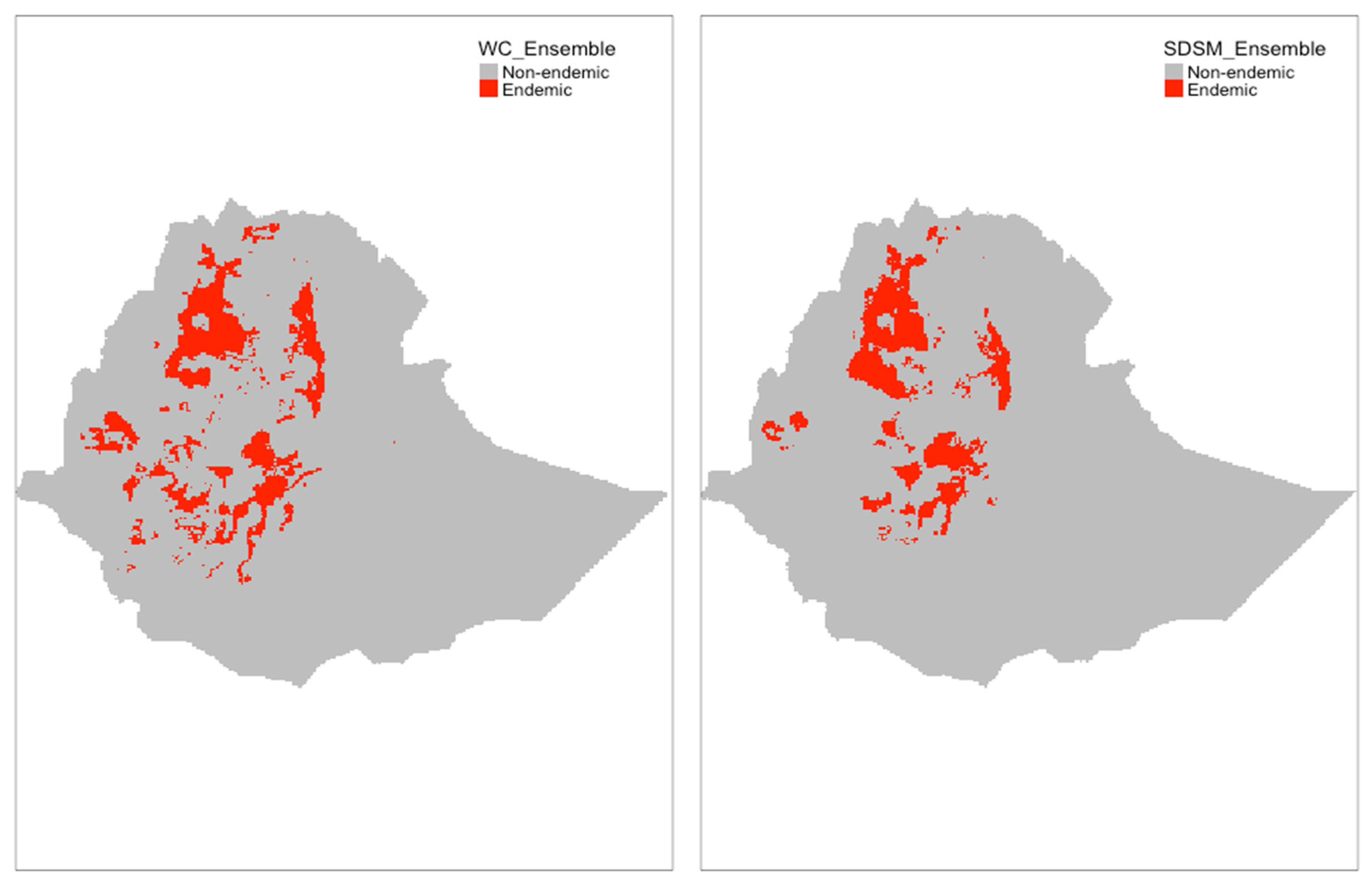
3.3. Distribution of S. mansoni Endemic Areas in Ethiopia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, R.; Feng, J.; Hong, Y.; Lv, C.; Zhao, D.; Lin, J.; Lu, K.; Li, H.; Liu, J.; Cao, X.; et al. A novel colloidal gold immunochromatography assay strip for the diagnosis of schistosomiasis japonica in domestic animals. Infect. Dis. Poverty 2017, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.; Erko, B.; Gundersen, S.G.; Medhin, G.; Berhe, N. Current status of Schistosoma mansoni infection among previously treated rural communities in the Abbey and Didessa Valleys, Western Ethiopia: Implications for sustainable control. PLoS ONE 2021, 16, e0247312. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, B.; Tomass, Z.; Wadilo, F.; Leja, D.; Liang, S.; Erko, B. Epidemiology of intestinal helminthiasis among school children with emphasis on Schistosoma mansoni infection in Wolaita zone, Southern Ethiopia. BMC Public Health 2017, 17, 587. [Google Scholar] [CrossRef] [PubMed]
- Bekana, T.; Hu, W.; Liang, S.; Erko, B. Transmission of Schistosoma mansoni in Yachi areas, southwestern Ethiopia: New foci. Infect. Dis. Poverty 2019, 8, 1. [Google Scholar] [CrossRef]
- Xue, Z.; Gebremichael, M.; Ahmad, R.; Weldu, M.L.; Bagtzoglou, A.C. Impact of temperature and precipitation on propagation of intestinal schistosomiasis in an irrigated region in Ethiopia: Suitability of satellite datasets. Trop. Med. Int. Health 2011, 16, 1104–1111. [Google Scholar] [CrossRef]
- Brooker, S. Spatial epidemiology of human schistosomiasis in Africa: Risk models, transmission dynamics and control. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 1–8. [Google Scholar] [CrossRef]
- Hussen, S.; Assegu, D.; Tadesse, B.T.; Shimelis, T. Prevalence of Schistosoma mansoni infection in Ethiopia: A systematic review and meta-analysis. Trop. Dis. Travel Med. Vaccines 2021, 7, 4. [Google Scholar] [CrossRef]
- Getachew, A.; Berhanu, E.; Mulugeta, A.; Beyene, P. Epidemiological study on Schistosoma mansoni infection in Sanja area, Amhara region, Ethiopia. Parasites Vectors 2014, 7, 15. [Google Scholar]
- Stensgaard, A.-S.; Vounatsou, P.; Sengupta, M.E.; Utzinger, J. Schistosomes, snails and climate change: Current trends and future expectations. Acta Trop. 2019, 190, 257–268. [Google Scholar] [CrossRef]
- Chala, B.; Torben, W. An epidemiological trend of urogenital schistosomiasis in Ethiopia. Front. Public Health 2018, 6, 60. [Google Scholar]
- Gordon, C.A.; Kurscheid, J.; Williams, G.M.; Clements, A.C.A.; Li, Y.; Zhou, X.-N.; Utzinger, J.; McManus, D.P.; Gray, D.J. Asian Schistosomiasis: Current Status and Prospects for Control Leading to Elimination. Trop. Med. Infect. Dis. 2019, 4, 40. [Google Scholar] [CrossRef]
- Austin, M. Spatial prediction of species distribution: An interface between ecological theory and statistical modelling. Ecol. Model. 2002, 157, 101–118. [Google Scholar] [CrossRef]
- Marcelino, V.R.; Verbruggen, H. Ecological niche models of invasive seaweeds. J. Phycol. 2015, 51, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T. Ecologic Niche Modeling and Spatial Patterns of Disease Transmission. Emerg. Infect. Dis. 2006, 12, 1822. [Google Scholar] [CrossRef] [PubMed]
- Ponpetch, K.; Erko, B.; Bekana, T.; Richards, L.; Liang, S. Biogeographical characteristics of Schistosoma mansoni endemic areas in Ethiopia: A systematic review and meta analysis. Infect. Dis. Poverty 2021, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E. Ecological Niche Modeling: An Introduction for Veterinarians and Epidemiologists. Front. Veter Sci. 2020, 7, 713. [Google Scholar] [CrossRef] [PubMed]
- Hanberry, B.; He, H.S.; Palik, B.J. Pseudoabsence Generation Strategies for Species Distribution Models. PLoS ONE 2012, 7, e44486. [Google Scholar] [CrossRef] [PubMed]
- Iturbide, M.; Bedia, J.; Herrera, S.; del Hierro, O.; Pinto, M.; Gutiérrez, J.M. A framework for species distribution modelling with improved pseudo-absence generation. Ecol. Model. 2015, 312, 166–174. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Jarvis, A.; Reuter, H.; Nelson, A.; Guevara, E. Hole-Filled Seamless SRTM Data V4: International Centre for Tropical Agriculture (CIAT). Available online: https://srtm.csi.cgiar.org/ (accessed on 31 December 2018).
- Buchhorn, M.; Smets, B.; Bertels, L.; Lesiv, M.; Tsendbazar, N. Copernicus Global Land Operations “Vegetation and Energy” CGLOPS-1. Product User Manual. 2017. Available online: https://land.copernicus.eu/global/sites/cgls.vito.be/files/products/CGLOPS1_PUM_LCC100m-V2.1_I3.10.pdf (accessed on 15 December 2018).
- Bellows, N.; Weinberger, M.; Reidy, M. Using the Demographic Health Survey wealth index to create family planning market segments based on absolute income levels. BMJ Glob. Health 2020, 5, e002450. [Google Scholar] [CrossRef]
- Rutstein, S.O. The DHS Wealth Index: Approaches for Rural and Urban Areas; Macro International Inc.: Calverton, MD, USA, 2008. [Google Scholar]
- Egede, L.E.; Voronca, D.; Walker, R.J.; Thomas, C. Rural-Urban Differences in Trends in the Wealth Index in Kenya: 1993-2009. Ann. Glob. Health 2017, 83, 248–258. [Google Scholar] [CrossRef]
- Gondwe, K.W.; Walker, R.J.; Mkandawire-Valhmu, L.; Dressel, A.; Ngui, E.M.; Kako, P.M.; Egede, L. Predictors of Wealth Index in Malawi—Analysis of Malawi Demographic Health Survey 2004-2015/16. Public Health Pract. 2020, 2, 100059. [Google Scholar] [CrossRef]
- DHS T. Ethiopia DHS 2016. 2016. Available online: https://www.dhsprogram.com/topics/wealth-index/Wealth-Index-Construction.cfm (accessed on 15 December 2018).
- Krivoruchko, K. Empirical Bayesian Kriging; Esri: Redlands, CA, USA, 2012. [Google Scholar]
- ISRIC. SoilGrids—Global Gridded Soil Information 2020. Available online: https://www.isric.org/explore/soilgrids (accessed on 20 December 2018).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Gebrechorkos, S.H.; Hülsmann, S.; Bernhofer, C. Statistically downscaled climate dataset for East Africa. Sci. Data 2019, 6, 31. [Google Scholar] [CrossRef]
- Chalghaf, B.; Chlif, S.; Mayala, B.; Ghawar, W.; Bettaieb, J.; Harrabi, M.; Benie, G.B.; Michael, E.; Ben Salah, A. Ecological Niche Modeling for the Prediction of the Geographic Distribution of Cutaneous Leishmaniasis in Tunisia. Am. J. Trop. Med. Hyg. 2016, 94, 844–851. [Google Scholar] [CrossRef]
- Molloy, S.W.; Davis, R.A.; VAN Etten, E.J.B. Species distribution modelling using bioclimatic variables to determine the impacts of a changing climate on the western ringtail possum (Pseudocheirus occidentals; Pseudocheiridae). Environ. Conserv. 2014, 41, 176–186. [Google Scholar] [CrossRef]
- Liang, R.; Lu, Y.; Qu, X.; Su, Q.; Li, C.; Xia, S.; Liu, Y.; Zhang, Q.; Cao, X.; Chen, Q.; et al. Prediction for global African swine fever outbreaks based on a combination of random forest algorithms and meteorological data. Transbound. Emerg. Dis. 2020, 67, 935–946. [Google Scholar] [CrossRef]
- Zheng, P.; Chen, Z.; Liu, Y.; Song, H.; Wu, C.-H.; Li, B.; Kraemer, M.U.; Tian, H.; Yan, X.; Zheng, Y.; et al. Association between coronavirus disease 2019 (COVID-19) and long-term exposure to air pollution: Evidence from the first epidemic wave in China. Environ. Pollut. 2021, 276, 116682. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.F.; ter Maat, H.W.; Biemans, H.; Shrestha, A.B.; Wester, P.; Immerzeel, W.W. Selecting representative climate models for climate change impact studies: An advanced envelope-based selection approach. Int. J. Clim. 2016, 36, 3988–4005. [Google Scholar] [CrossRef]
- Gebrechorkos, S.H.; Hülsmann, S.; Bernhofer, C. Regional climate projections for impact assessment studies in East Africa. Environ. Res. Lett. 2019, 14, 044031. [Google Scholar] [CrossRef]
- Escobar, L.E.; Craft, M.E. Advances and Limitations of Disease Biogeography Using Ecological Niche Modeling. Front. Microbiol. 2016, 7, 1174. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.K.; McNyset, K.M.; Curtis, A.; Hugh-Jones, M.E. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. Am. J. Trop. Med. Hyg. 2007, 77, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Edwards, T.C., Jr.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Blackburn, J.K. Integrating Geographic Information Systems and Ecological Niche Modeling into Disease Ecology: A Case Study of Bacillus anthracis in the United States and Mexico. Emerging and Endemic Pathogens; Springer: New York, NY, USA, 2010; pp. 59–88. [Google Scholar]
- Scholte, R.G.; Carvalho, O.S.; Malone, J.B.; Utzinger, J.; Vounatsou, P. Spatial distribution of Biomphalaria spp., the intermediate host snails of Schistosoma mansoni, in Brazil. Geospat. Health 2012, 6, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Murphy, M.; Holden, Z.; Cushman, S. Modeling Species Distribution and Change Using Random forest. In Predictive Species and Habitat Modeling in Landscape Ecology; Springer: New York, NY, USA, 2011; pp. 139–159. [Google Scholar]
- Li, J.; Alvarez, B.; Siwabessy, J.; Tran, M.; Huang, Z.; Przeslawski, R.; Radke, L.; Howard, F.; Nichol, S. Application of random forest, generalised linear model and their hybrid methods with geostatistical techniques to count data: Predicting sponge species richness. Environ. Model. Softw. 2017, 97, 112–129. [Google Scholar] [CrossRef]
- Čengić, M.; Rost, J.; Remenska, D.; Janse, J.H.; Huijbregts, M.A.J.; Schipper, A.M. On the importance of predictor choice, modelling technique, and number of pseudo-absences for bioclimatic envelope model performance. Ecol. Evol. 2020, 10, 12307–12317. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Assefa, A.; Tibebu, A.; Bihon, A.; Yimana, M. Global ecological niche modelling of current and future distribution of peste des petits ruminants virus (PPRv) with an ensemble modelling algorithm. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef]
- Hardy, S.M.; Lindgren, M.; Konakanchi, H.; Huettmann, F. Predicting the Distribution and Ecological Niche of Unexploited Snow Crab (Chionoecetes opilio) Populations in Alaskan Waters: A First Open-Access Ensemble Model. Integr. Comp. Biol. 2011, 51, 608–622. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Liu, S.; Sun, P.; Wang, T.; Wang, G.; Zhang, X.; Wang, L. Consensus Forecasting of Species Distributions: The Effects of Niche Model Performance and Niche Properties. PLoS ONE 2015, 10, e0120056. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble Platform for Species Distribution Modeling, R package version 3.0.3.; The R Project for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Niu, Y.; Li, R.; Qiu, J.; Xu, X.; Huang, D.; Shao, Q.; Cui, Y. Identifying and Predicting the Geographical Distribution Patterns of On-comelania hupensis. Int. J. Environ. Res. Public Health 2019, 16, 2206. [Google Scholar] [CrossRef]
- Manyangadze, T.; Chimbari, M.J.; Gebreslasie, M.; Ceccato, P.; Mukaratirwa, S. Modelling the spatial and seasonal distribution of suitable habitats of schistosomiasis intermediate host snails using Maxent in Ndumo area, KwaZulu-Natal Province, South Africa. Parasites Vectors 2016, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Poulos, H.; Chernoff, B.; Fuller, P.; Butman, D. Ensemble forecasting of potential habitat for three invasive fishes. Aquat. Invasions 2012, 7, 59–72. [Google Scholar] [CrossRef]
- Dal Maso, E.; Montecchio, L. Risk of natural spread of Hymenoscyphus fraxineus with environmental niche modelling and ensemble forecasting technique. For. Res. 2014, 3, 131. [Google Scholar] [CrossRef]
- Kloos, H.; Lo, C.T.; Birrie, H.; Ayele, T.; Tedla, S.; Tsegay, F. Schistosomiasis in Ethiopia. Soc. Sci. Med. 1988, 26, 803–827. [Google Scholar] [CrossRef]
- Kristensen, T.K.; Malone, J.B.; McCarroll, J.C. Use of satellite remote sensing and geographic information systems to model the distribution and abundance of snail intermediate hosts in Africa: A preliminary model for Biomphalaria pfeifferi in Ethiopia. Acta Trop. 2001, 79, 73–78. [Google Scholar] [CrossRef]
- Boelee, E.; Madsen, H. Irrigation and Schistosomiasis in Africa: Ecological Aspects; International Water Management Institute: Colombo, Sri Lanka, 2006. [Google Scholar]
- Chen, H.; Ponpetch, K. Satellite Imagery Data for Global Health and Epidemiology. In Statistical Methods for Global Health and Epidemiology; Springer: New York, NY, USA, 2020; pp. 25–51. [Google Scholar]


| Data | Unit | Resolution | Time Period | Source |
|---|---|---|---|---|
| Elevation | Meters Above Sea Level | 250 m | CGIAR Consortium for Spatial Information (CGIAR-CSI) [20] | |
| Wealth Index Score | 250 m | 2016 | Demographic and Health Surveys (DHS) [23] | |
| Silt | g/100 g | 250 m | 2008–2012 | International Soil Reference and Information Centre (ISRIC) [28] |
| Sand | g/100 g | 250 m | 2008–2012 | International Soil Reference and Information Centre (ISRIC) [28] |
| Clay | g/100 g | 250 m | 2008–2012 | International Soil Reference and Information Centre (ISRIC) [28] |
| NDVI * | 1 km | 1999–2017 | Copernicus Global Land Service [21] | |
| Mean Temperature | Degrees Celsius | 1 km | 1970–2000 | WorldClim [29] |
| Annual Precipitation | mm | 1 km | 1970–2000 | WorldClim [29] |
| Max Temperature | Degrees Celsius | 250 m | 1961–2005 | Gebrechorkos SH et al. [30] |
| Min Temperature | Degrees Celsius | 250 m | 1961–2005 | Gebrechorkos SH et al. [30] |
| Mean Precipitation | mm | 250 m | 1961–2005 | Gebrechorkos SH et al. [30] |
| Model | WC | SDSM | ||
|---|---|---|---|---|
| AUC | Rank | AUC | Rank | |
| GLM | 0.883 | 5 | 0.851 | 6 |
| GBM | 0.902 | 3 | 0.879 | 3 |
| GAM | 0.879 | 6 | 0.848 | 7 |
| SRE | 0.723 | 9 | 0.735 | 9 |
| CTA | 0.788 | 8 | 0.798 | 8 |
| RF | 0.929 | 2 | 0.875 | 4 |
| MARS | 0.871 | 7 | 0.880 | 2 |
| MAXENT | 0.889 | 4 | 0.872 | 5 |
| Ensemble | 0.956 | 1 | 0.960 | 1 |
| Model | WC | SDSM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACC (%) | SE (%) | SP (%) | PPV (%) | NPV (%) | BA (%) | ACC (%) | SE (%) | SP (%) | PPV (%) | NPV (%) | BA (%) | |
| GLM | 58.33 | 75.00 | 25.00 | 69.57 | 100.00 | 50.00 | 50.00 | 43.75 | 62.50 | 69.57 | 100.00 | 53.13 |
| GAM | 70.83 | 93.75 | 25.00 | 71.43 | 66.67 | 59.38 | 45.83 | 56.25 | 25.00 | 71.43 | 66.67 | 40.63 |
| MARS | 66.67 | 75.00 | 50.00 | 80.00 | 100.00 | 62.00 | 45.83 | 43.75 | 50.00 | 80.00 | 100.00 | 46.88 |
| CTA | 75.00 | 100.00 | 25.00 | 72.73 | 100.00 | 62.50 | 79.17 | 100.00 | 37.50 | 72.73 | 100.00 | 68.75 |
| RF | 41.67 | 43.75 | 37.50 | 84.21 | 100.00 | 40.63 | 83.33 | 81.25 | 87.50 | 84.21 | 100.00 | 84.38 |
| GBM | 83.33 | 93.75 | 62.50 | 78.95 | 80.00 | 78.13 | 58.33 | 50.00 | 75.00 | 78.95 | 80.00 | 62.50 |
| SRE | 54.17 | 75.00 | 12.50 | 66.67 | 33.33 | 43.75 | 58.33 | 75.00 | 25.00 | 66.67 | 33.33 | 50.00 |
| MAXENT | 37.50 | 25.00 | 62.50 | 81.82 | 46.15 | 43.75 | 29.17 | 12.50 | 62.50 | 81.82 | 46.15 | 37.50 |
| Ensemble | 83.33 | 93.75 | 62.50 | 84.21 | 100.00 | 78.13 | 41.67 | 31.25 | 62.50 | 84.21 | 100.00 | 46.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponpetch, K.; Erko, B.; Bekana, T.; Kebede, T.; Tian, D.; Yang, Y.; Liang, S. Environmental Drivers and Potential Distribution of Schistosoma mansoni Endemic Areas in Ethiopia. Microorganisms 2021, 9, 2144. https://doi.org/10.3390/microorganisms9102144
Ponpetch K, Erko B, Bekana T, Kebede T, Tian D, Yang Y, Liang S. Environmental Drivers and Potential Distribution of Schistosoma mansoni Endemic Areas in Ethiopia. Microorganisms. 2021; 9(10):2144. https://doi.org/10.3390/microorganisms9102144
Chicago/Turabian StylePonpetch, Keerati, Berhanu Erko, Teshome Bekana, Tadesse Kebede, Di Tian, Yang Yang, and Song Liang. 2021. "Environmental Drivers and Potential Distribution of Schistosoma mansoni Endemic Areas in Ethiopia" Microorganisms 9, no. 10: 2144. https://doi.org/10.3390/microorganisms9102144
APA StylePonpetch, K., Erko, B., Bekana, T., Kebede, T., Tian, D., Yang, Y., & Liang, S. (2021). Environmental Drivers and Potential Distribution of Schistosoma mansoni Endemic Areas in Ethiopia. Microorganisms, 9(10), 2144. https://doi.org/10.3390/microorganisms9102144






