Application of Natural Neutrophil Products for Stimulation of Monocyte-Derived Macrophages Obtained before and after Osteochondral or Bone Injury
Abstract
1. Introduction
2. Materials and Methods
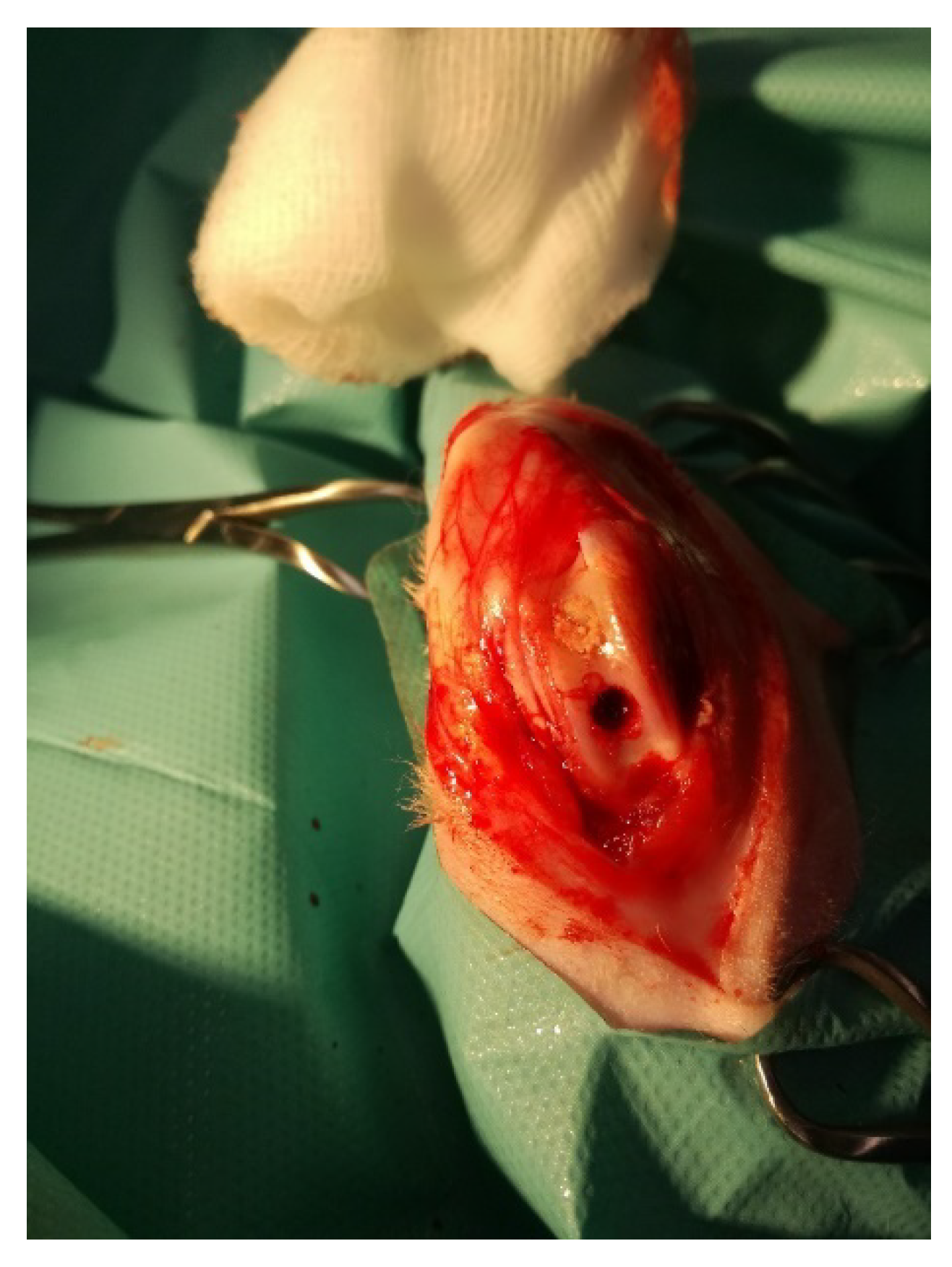
2.1. Rabbit Model of Osteochondral Defect
2.1.1. Animals and Surgical Procedures
2.1.2. Preparation of Blood-Derived Products
Preparation of Rabbit Antimicrobial Neutrophil Extract (rANE)
Neutrophil Degranulation Products (DGP) and Their Activity
Preparation of Microvesicles (MVs)
2.1.3. Monocyte-Derived Macrophages (MDMs) Culture and Activity
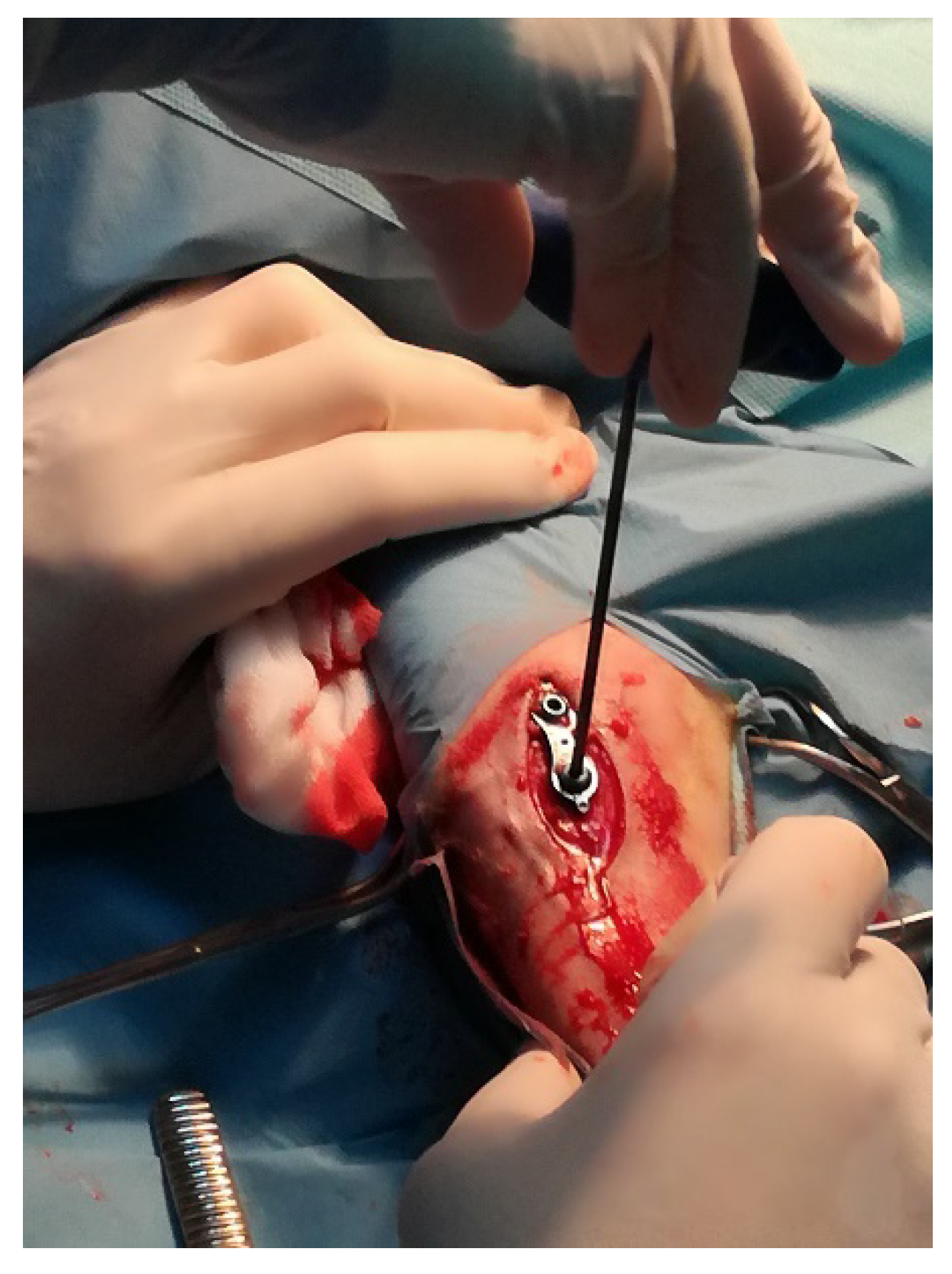
2.2. Sheep Model of Insertion of Ti Implant into the Tibial Growth Plate
2.2.1. Animals and Surgical Procedures
2.2.2. Preparation of Blood-Derived Products
Blood Sampling, Cell Cultures and Blood-Derived Products
2.2.3. MDMs Culture and Activity
2.3. Statistical Analysis
3. Results
3.1. Rabbit Model of Osteochondral Defect
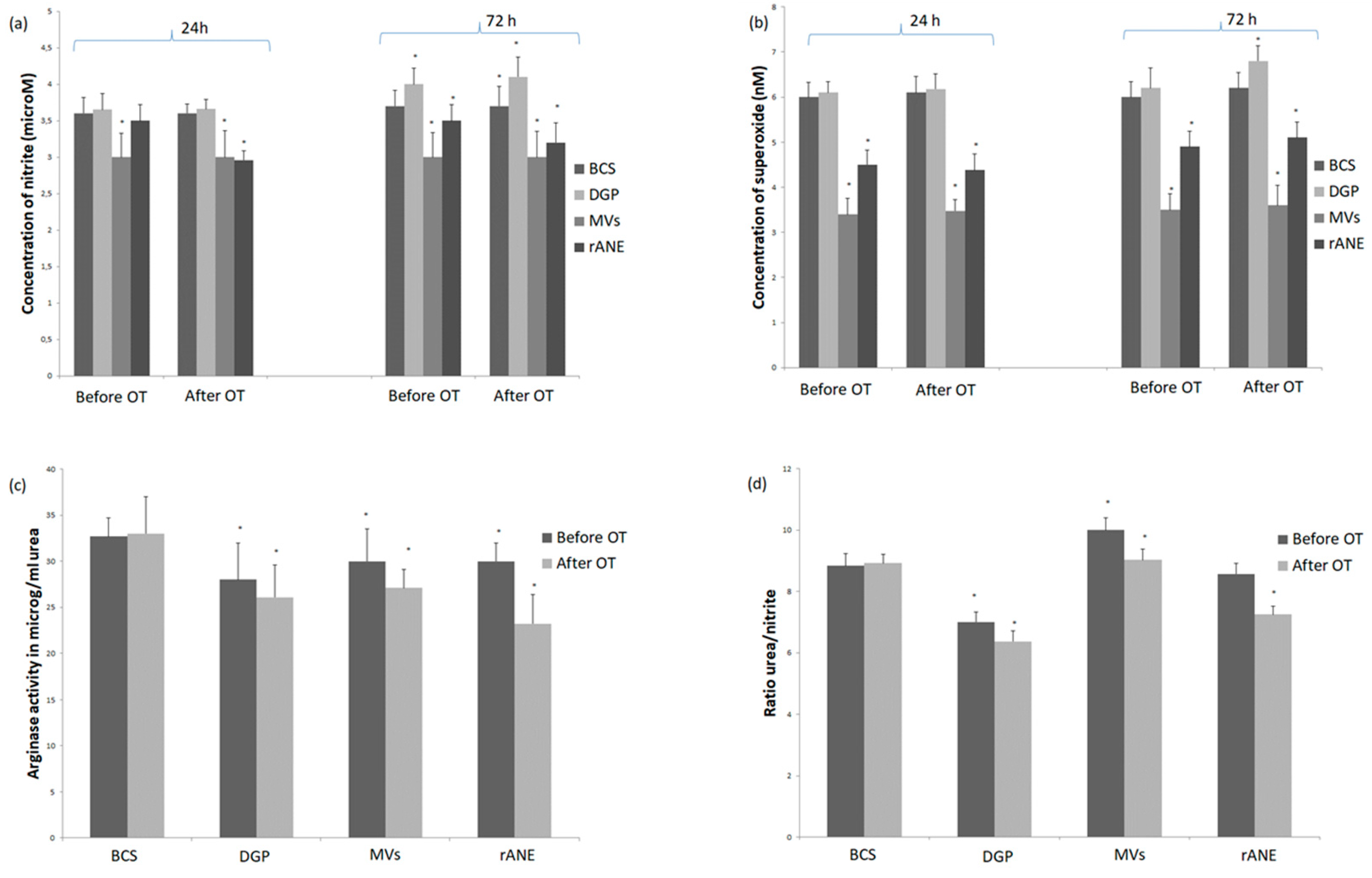
3.1.1. Neutrophils Secretory Activity
3.1.2. Rabbit MDMs Response to Neutrophil-Derived Products
3.2. Insertion of Ti Implant in the Ovine Model
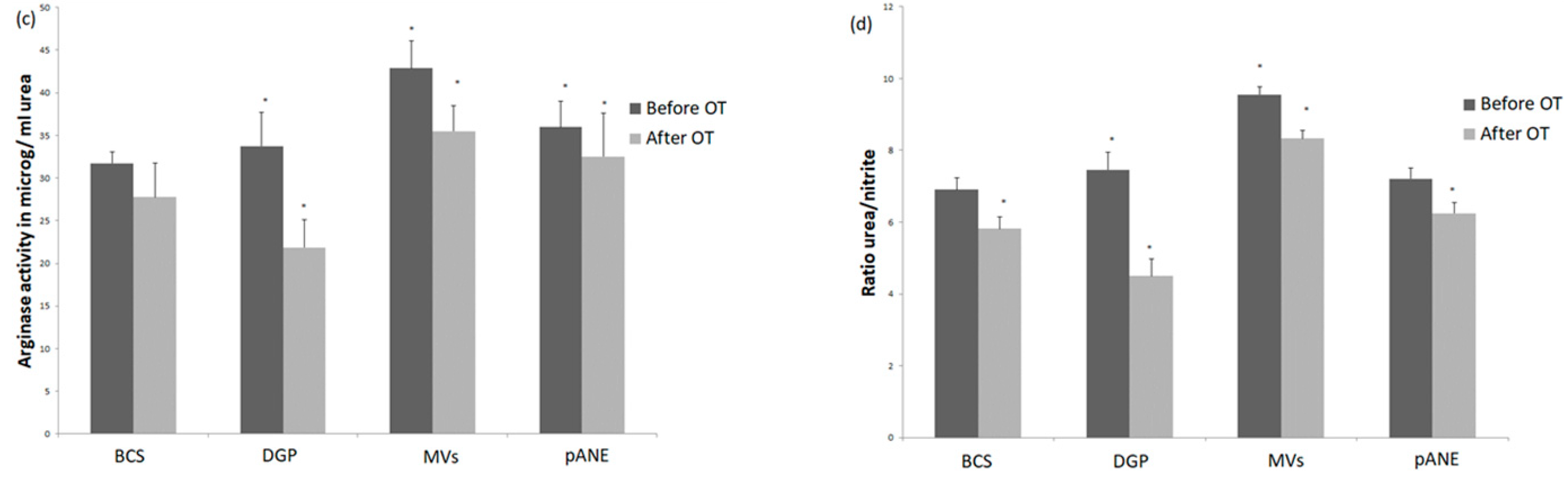
3.2.1. Neutrophils Secretory Activity
3.2.2. MDMs Response to Neutrophil-Derived Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalli, J.; Serhan, C.N. Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012, 120, e60–e72. [Google Scholar] [CrossRef] [PubMed]
- Lock, A.; Cornish, J.; Musson, D.S. The Role of In Vitro Immune Response Assessment for Biomaterials. J. Funct. Biomater. 2019, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomat. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Nemeth, T. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef]
- Gibon, E.; Lu, Y.L.; Nathan, K.; Goodman, S.G. Inflammation, ageing and bone regeneration. J. Orthop. Transl. 2017, 10, 28–35. [Google Scholar] [CrossRef]
- Kovtun, A.; Messerer, D.A.C.; Kochanek, K.S.; Lang, M.H.; Ignatius, A. Neutrophils in Tissue Trauma of the Skin, Bone, and Lung: Two Sides of the Same Coin. J. Immunol. Res. 2018, 2018, 8173983. [Google Scholar] [CrossRef]
- Chung, R.; Cool, J.C.; Scherer, M.A.; Foster, B.K.; Xian, C.J. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J. Leukoc. Biol. 2006, 80, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Németh, T.; Mócsai, A. Feedback Amplification of Neutrophil Function. Trends Immunol. 2016, 37, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From monocytes to M1/M2 macrophages: Phenotypical vs functional differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Soehnlein, O.; Zernecke, A.; Eriksson, E.E.; Rothfuchs, A.G.; Pham, C.T.; Herwald, H.; Bidzhekov, K.; Rottenberg, M.E.; Weber, C.H.; Lindbom, L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008, 112, 1461–1471. [Google Scholar] [CrossRef]
- Haney, E.F.; Hancock, R.E.W. Peptide Design for Antimicrobial and Immunomodulatory Applications. Biopolymers 2013, 100, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.E.; Szklarek, D.; Lehrer, R.I. Purification and Antibacterial Activity of Antimicrobial Peptides of Rabbit Granulocytes. Infect. Immun. 1984, 45, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Szponder, T.; Szponder, J.W.; Smolira, A. Evaluation of platelet-rich plasma and neutrophil antimicrobial extract as two autologous blood-derived agents. Tissue Eng. Regen. Med. 2017, 14, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Szponder, J.W.; Szponder, T.; Bobowiec, R. Different activation of monocyte-derived macrophages by antimicrobial peptides at a titanium tibial implantation in rabbits. Res. Vet. Sci. 2017, 115, 201–210. [Google Scholar] [CrossRef]
- Soehnlein, O.; Larsen, Y.K.; Frithiof, R.; Sorensen, O.E.; Kenne, E.; Kochanek, K.S.; Eriksson, E.E.; Herwald, H.; Agerberth, B.; Lindbom, L. Neutrophil primary granule proteins HBP and HNP1-3 boots bacterial phagocytosis by human and murine macrophages. J. Clin. Investig. 2008, 118, 3491–3502. [Google Scholar] [CrossRef]
- Soehnlein, O. Direct and alternative antimicrobial mechanisms of neutrophil-derived granule proteins. J. Mol. Med. 2009, 87, 1157–1164. [Google Scholar] [CrossRef]
- Hussen, J.; Koy, M.; Petzl, W.; Schubert, H.J. Neutrophil degranulation differentially modulates phenotype and function of bovine monocyte subsets. Innate Immun. 2016, 22, 124–137. [Google Scholar] [CrossRef]
- Rhys, H.I.; Dell’Accio, F.; Pitzalis, C.; Moore, A.; Norling, L.V.; Perretti, M. Neutrophil Microvesicles from Healthy Control and Rheumatoid Arthritis Patients Prevent the Inflammatory Activation of Macrophages. EBioMedicine 2018, 29, 60–69. [Google Scholar] [CrossRef]
- Pettersson, M.; Pettersson, J.; Thorén, M.M.; Johansson, A. Effect of cobalt ions on the interaction between macrophages and titanium. J. Biomed. Mater. Res. Part A 2018, 106, 2518–2530. [Google Scholar] [CrossRef]
- Pettersson, M.; Kelk, P.; Belibasakis, G.N.; Bylund, D.; Thorén, M.M.; Johansson, A. Titanium ions form particles that activate and execute interleukin-1b release from lipopolysaccharide-primed macrophages. J. Periodont. Res. 2017, 52, 21–32. [Google Scholar] [CrossRef]
- Katagiri, H.; Mendes, L.F.; Luyten, F.P. Definition of a Critical Size Osteochondral Knee Defect and its Negative Effect on the Surrounding Articular Cartilage in the Rat. Osteoarthr. Cartil. 2017, 25, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Utomo, L.; Bastiaansen-Jenniskens, Y.M.; Verhaar, J.A.N.; van Osch, G.J.V.M. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthr. Cartil. 2016, 24, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Szponder, J.W.; Michalska, J.; Szponder, T.; Żylińska, B.; Tarczyńska, M.; Szubstarski, M. The Role of Antimicrobial Neutrophil Extract in Modification of the Inflammatory Response During Osteochondral Autograft and Allograft Transplantation in Rabbits. J. Comp. Pathol. 2020, 175, 49–63. [Google Scholar] [CrossRef]
- Corraliza, I.M.; Campo, M.L.; Soler, G.; Modolell, M. Determination of arginase activity in macrophages: A micromethod. J. Immunol. Methods 1994, 174, 231–235. [Google Scholar] [CrossRef]
- Szponder, J.W.; Dziedzic, B.M.; Smolira, A. Analysis of antimicrobial peptides from porcine neutrophils. J. Microbiol. Methods 2010, 83, 8–12. [Google Scholar] [CrossRef]
- Soehnlein, O.; Kenne, E.; Rotzius, P.; Eriksson, E.E.; Lindbom, L. Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages. Clin. Exp. Immunol. 2007, 151, 139–145. [Google Scholar] [CrossRef]
- Karsch, A.G.; Schinnerling, K.; Conrad, K.; Friebel, J.; Allers, K.; Schneider, T.; Moos, V. Evaluation of arginine metabolism for the analysis of M1/M2 macrophage activation in human clinical specimens. Inflamm. Res. 2013, 62, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Cil. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Campbell, L.; Emmerson, E.; Williams, H.; Saville, C.R.; Krust, A.; Chambon, P.; Mace, K.A.; Hardman, J. Estrogen receptor-alpha promotes alternative macrophage activation during cutaneous repair. J. Investig. Dermatol. 2014, 134, 2447–2457. [Google Scholar] [CrossRef]
- Drożdż, A.; Stępień, E. Circulating Microvesicles in Regenerative Angiogenesis. Curr. Trends Biomed. Eng. Biosci. 2017, 3, 12–14. [Google Scholar]
- Gasser, O.; Schifferli, J.A. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004, 104, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Young, Y.J.; Shrestha, S.; Kim, J.K.; Lee, Y.B.; Lee, J.H.; Hur, K.; Mali, N.M.; Nam, S.W.; Kim, S.H.; Song, D.K.; et al. Neutrophil-derived extracellular vesicles: Proinflammatory trails and anti-inflammatory microvesicles. Cell Rep. 2019. [Google Scholar] [CrossRef]
- Gonçalo, S.C.; vanWaarde, A.; Antunes, I.F.; Dömling, A.; Elsinga, P.H. Arginase as a Potential Biomarker of Disease Progression: A Molecular Imaging Perspective. Int. J. Mol. Sci. 2020, 21, 5291. [Google Scholar]
- Shahbazi, M.-A.; Sedighi, M.; Ramos, T.B.; Kant, K.; Correia, A.; Poursina, N.; Sarmento, B.; Hirvonen, J.; Santos, H.A. Targeted Reinforcement of Macrophage Reprogramming Toward M2 Polarization by IL-4-Loaded Hyaluronic Acid Particles. ACS Omega 2018, 3, 18444–18455. [Google Scholar] [CrossRef] [PubMed]

| Neutrophil Enzyme/Reactive Oxygen Species (ROS) | Control-Cultures without N-formylmethionyl-leucyl-phenylalanine (fMLP) Stimulation | Before Implantation | After Implantation |
|---|---|---|---|
| Elastase (% activity) | 49.70 ± 0.5 | 50.00 ± 0.8 | 51.00 ± 0.69 |
| Myeloperoxidase (MPO % activity) | 23.03 ± 0.5 | 23.45 ± 0.49 | 32.51 ± 0.57 |
| Alkaline phosphatase (ALP % activity) | 22.00 ± 0.1 | 22.89 ± 2.8 | 22.59 ± 2.4 |
| Nitric oxide (NOµM of nitrite) | 2.5 ± 0.5 | 2.5 ± 0.4 | 3.0 ± 0.5 |
| Superoxide (nM) | 3.0 ± 0.25 | 3.19 ± 0.3 | 3.14 ± 0.2 |
| Neutrophil Enzyme/ROS | Control (Cultures without fMLP Stimulation) | Before Implantation | After Implantation |
|---|---|---|---|
| Elastase (% activity) | 50.72 ± 0.53 | 50.3 ± 3.88 | 54.2 ± 2.18 |
| MPO (% activity) | 24.13 ± 0.2 | 24.9 ± 2.3 | 23.3 ± 0.49 |
| ALP (% activity) | 14.80 ± 0.2 | 15.44 ± 2.31 | 14.84 ± 1.32 |
| NO (µM of nitrite) | 2.8 ± 0.5 | 3.0 ± 0.1 | 3.0 ± 0.02 |
| Superoxide (nM) | 4.5 ± 0.3 | 5.5 ± 0.11 | 4.8 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdziennicka, J.; Szponder, T.; Wessely-Szponder, J. Application of Natural Neutrophil Products for Stimulation of Monocyte-Derived Macrophages Obtained before and after Osteochondral or Bone Injury. Microorganisms 2021, 9, 124. https://doi.org/10.3390/microorganisms9010124
Zdziennicka J, Szponder T, Wessely-Szponder J. Application of Natural Neutrophil Products for Stimulation of Monocyte-Derived Macrophages Obtained before and after Osteochondral or Bone Injury. Microorganisms. 2021; 9(1):124. https://doi.org/10.3390/microorganisms9010124
Chicago/Turabian StyleZdziennicka, Joanna, Tomasz Szponder, and Joanna Wessely-Szponder. 2021. "Application of Natural Neutrophil Products for Stimulation of Monocyte-Derived Macrophages Obtained before and after Osteochondral or Bone Injury" Microorganisms 9, no. 1: 124. https://doi.org/10.3390/microorganisms9010124
APA StyleZdziennicka, J., Szponder, T., & Wessely-Szponder, J. (2021). Application of Natural Neutrophil Products for Stimulation of Monocyte-Derived Macrophages Obtained before and after Osteochondral or Bone Injury. Microorganisms, 9(1), 124. https://doi.org/10.3390/microorganisms9010124




